Excimer and exciplex formation in a pair of bright phosphorescent isomers constructed from Cu3(pyrazolate)3 and Cu3I3 coordination luminophores†
Shun-Ze
Zhan
a,
Mian
Li
a,
Xiao-Ping
Zhou
a,
Dan
Li
*a and
Seik
Weng Ng
b
aDepartment of Chemistry, Shantou University, Guangdong, 515063, P. R. China. E-mail: dli@stu.edu.cn
bDepartment of Chemistry, University of Malaya, Kuala Lumpur, 50603, Malaysia
First published on 12th October 2011
Abstract
Reported herein are a pair of supramolecular pseudo-isomers, namely, (Cu3I3)(Cu3L3)2·H2O (1) and (Cu3I3)(Cu3L3)2 (2) (L = 3-(4-pyridyl)-5-isobutyl-pyrazolate), both of which incorporate Cu3Pz3 (Pz = pyrazolate) and Cu3I3 clusters as luminophores. The two complexes show distinct yellow (570 nm) and orange (638 nm) emissions, which are ascribed to the formation of the excimer and exciplex involving the same or different copper(I)-cluster-based luminophores.
In the increasingly important field of the photofunctional d10 metal complexes,1 the cyclic, trinuclear coinage-metal (Cu, Ag and Au) complexes have garnered considerable interest owing to their fundamental and applied potentials in the areas of metalloaromaticity, metallophilic and excimeric bonding, host/guest chemistry, and metal–organic optoelectronics.2–6 These applications have to link to the π-acid/base property of the metal cyclotrimers that leads to the formation of extended binary stacking, and more specifically, to the formation of an excimer and exciplex when photoluminescence is present.3,5,6
Omary and coworkers once computationally assessed the π-acidity/basicity of M3Pz3 (M = Cu, Ag or Au),3a and indeed experimentally implemented the manipulation of their π-acidity/basicity via multiple cooperative supramolecular interactions, warranting their luminescence tuning and sensing functions.3b,c,d Our group has been investigating the structural and luminescent features of copper(I) cluster-based oligomers and polymers—particular attention has been paid to copper(I)-pyrazolates4,5,7 and copper(I)-halide8 complexes. In an attempt to combine these two entities for generating multi-emissive materials, in a recently embarked project we have realized that Cu3Pz3 and CunIn may be complementary in the consideration of their π-acidity/basicity. On one hand, both Cu3Pz3 and CunIn are neutral and can be readily formed; this means that an extra electrostatic effect and additional charge balance can be excluded when inter-cluster (between the same or different clusters) interplay occurs. On the other hand, the planar Cu3Pz3 motif exhibits weak π-acidity2b,3a,6b and is usually subject to perpendicular supramolecular interactions such as Cu⋯Cu and [Cu3Pz3]⋯halide forces, etc.,2–6 whereas in CunIn aggregates there exist both electron-donor (i.e.iodide ions with lone pair electrons) and electron-acceptor (i.e.copper(I) ions with vacant coordination sites) regions that, respectively, cope with Lewis acids and bases.8 Furthermore, both Cu3Pz3 and CunInclusters are renowned coordination luminophores whose intense, low-energy and long-lived phosphorescence originates from cuprophilic interaction based triplet excited states.1,8 Therefore, coupling these two luminophores may facilitate the formation of an excimer and exciplex, and generate interesting multi-emissions.
In this work, by introducing a new pyridyl-pyrazole ligand,4b,5b,7 namely, 3-(4-pyridyl)-5-isobutyl-1H-pyrazole (HL), to react with CuI under solvothermal conditions (see ESI† for experimental details), a pair of supramolecular pseudo-isomers,9 namely, (Cu3I3)(Cu3L3)2·H2O (1) and (Cu3I3)(Cu3L3)2 (2), are yielded simultaneously. They can be mechanically separated as bright yellow block crystals for 1 and light yellow column crystals for 2 through naked eyes under natural light. Interestingly, under UV-lamp irradiation, 1 and 2 are visually distinguishable, showing bright yellow and orange colours, respectively (Fig. S4 in ESI†).
X-Ray crystallographic analysis (see ESI†) reveals the structures of 1 and 2 are closely related: they both crystallize in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with a similar chemical composition, and both contain Cu3Pz3 and Cu3I3 units that are connected viaCu–Npyridyl bonds (Fig. 1), surrounded by different supramolecular interactions (Fig. 2). The Cu3Pz3 triangles in both 1 and 2 are slightly deviated from its observed planar arrangement,2,5a,6c due to the cooperative multiple interactions surrounded. The Cu3I3 aggregates exhibit an unusual six-membered cyclic array with alternating copper and iodide sites, leaving both the Cu and I sites unoccupied and exposed for further interactions. According to our review on copper(I) halide aggregates8a and the survey in the latest version of the Cambridge Structural Database (CSD),10a among a few reports of neutral Cu3I3 aggregates, only one such cyclic Cu3I3 array was documented.10b
space group with a similar chemical composition, and both contain Cu3Pz3 and Cu3I3 units that are connected viaCu–Npyridyl bonds (Fig. 1), surrounded by different supramolecular interactions (Fig. 2). The Cu3Pz3 triangles in both 1 and 2 are slightly deviated from its observed planar arrangement,2,5a,6c due to the cooperative multiple interactions surrounded. The Cu3I3 aggregates exhibit an unusual six-membered cyclic array with alternating copper and iodide sites, leaving both the Cu and I sites unoccupied and exposed for further interactions. According to our review on copper(I) halide aggregates8a and the survey in the latest version of the Cambridge Structural Database (CSD),10a among a few reports of neutral Cu3I3 aggregates, only one such cyclic Cu3I3 array was documented.10b
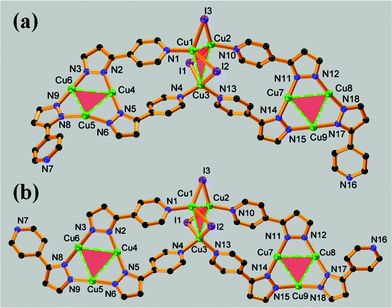 | ||
| Fig. 1 Asymmetric units of 1 (a) and 2 (b), showing different connecting orientation between Cu3Pz3 and Cu3I3 units. All H atoms, isobutyl groups and crystallized water molecules are omitted for clarity. Color codes: C in black, N in blue, Cu in green and I in purple. | ||
![Partial views of the structures of 1 (a) and 2 (b), showing the formation of [Cu3Pz3]2 excimer in 1 and [Cu3Pz3–Cu3I3] exciplex in both 1 and 2. The Cu⋯Cu and Cu⋯I interactions are depicted as green dashed bonds. Color codes: C in black, N in blue, Cu in green and I in purple.](/image/article/2011/RA/c1ra00566a/c1ra00566a-f2.gif) | ||
| Fig. 2 Partial views of the structures of 1 (a) and 2 (b), showing the formation of [Cu3Pz3]2 excimer in 1 and [Cu3Pz3–Cu3I3] exciplex in both 1 and 2. The Cu⋯Cu and Cu⋯I interactions are depicted as green dashed bonds. Color codes: C in black, N in blue, Cu in green and I in purple. | ||
As shown in Fig. 1, the linkages of Cu3Pz3 and Cu3I3 units in 1 and 2 are subtly different. The pyridyl groups are arranged asymmetrically around the Cu3Pz3 periphery, and two of the three pyridyl groups attached the same Cu3Pz3 unit point to the same direction and link with a Cu3I3 unit, which is supported by two symmetry-independent Cu3Pz3 through a total of four binding pyridyl groups. In both 1 and 2, Cu1 and Cu2 ions show planar triangular geometry and Cu3 ion adopts tetrahedral geometry, all completed by μ2-bridging iodide ions in addition to pyridyl coordination. Notably, the orientations of the third pyridyl groups in the coordination environments of 1 and 2 are different: in 1 it orients toward the vector of I3→Cu3 whereas in 2 the case is reversed, exhibiting “M” (Fig. 1a) and “W” (Fig. 1b) shapes for the fragments, respectively. Such arrangements result into the remarkably different extended structures of 1 and 2.
In complex 1, the asymmetric units are connected via N16–Cu5 bonds to form a 1-D coordination polymer11 with a head-to-tail fashion (Fig. S5a in ESI†). The third vacant pyridyl group (N16, Fig. 1a) attached to one Cu3Pz3 unit coordinates to one copper(I) ion (Cu5) from another Cu3Pz3 unit in neighbouring asymmetric unit, while its counterpart in the same asymmetric unit, another pyridyl group (N7), forms a hydrogen bond with one of the hydrogen atoms from crystallized water molecule (Table S4, Fig. S7a and S7b, and Hydrogen bonds discussion in ESI†). The distance of this linking N16–Cu5 bond is 2.120(5) Å, slightly larger than the normal value, and the bond angle of N6–Cu5–N8 is 152.1(2)°, causing the distortion of the locating Cu3Pz3 unit by preventing its planarity and occupying one Cu site, and hence no perpendicular supramolecular interaction is found for this type of Cu3Pz3 triangle (Cu4–Cu5–Cu6). Two adjacent 1-D chains are attracted to each other through an intertrimeric Cu⋯Cu interaction between another type of Cu3Pz3 triangle (Cu7–Cu8–Cu9, Fig. 2a and Fig. S5b in ESI†), ones that show relatively planar features to warrant an eximeric dimerization with a chair stacking conformation.2c,5 The Cu⋯Cu distance is 2.9867(10) Å, comparable with those in similar dimer of trimers of {[3,5-(Me)2Pz]Cu}3 and {[3,5-(i-Pr)2Pz]Cu}3,2,4 indicating a strong cuprophilic interaction and the formation of a [Cu3Pz3]2 excimer. Additionally, careful examination reveals these dimers are further capped from both top and bottom by I1 ions from Cu3I3 units, forming typical π-acidic [Cu3Pz3]⋯I supramolecular interaction (Fig. 2a and Fig. S5c in ESI†). The distances between the Cu and I ions in this [Cu3Pz3]⋯I force range from 3.35 to 3.85 Å, slightly longer than those reported.6b These centrosymmetric Cu3I3–Cu3Pz3–Cu3Pz3–Cu3I3 arrangements extend the 1-D chains to 2-D layers paralleling the ab crystallographic plane (Fig. S5c in ESI†).
In contrast, in complex 2 the N16–Cu5 coordination bond sustaining 1-D polymeric structure of 1 is not formed, and hence 2 is a 0-D oligomer with one asymmetric unit as a whole molecule. There are two dangling pyridyl group (N7 and N16, Fig. 1b) left, meaning both Cu3Pz3 triangles in a molecule are relatively planar (N–Cu–N bond angles range from 161.4 to 178.8°) without further coordination. Only N17 atom weakly binds to H49(C49) atom from another coordination molecule to form a hydrogen bond (Table S4, Fig. S7c, and Hydrogen bonds discussion in ESI†). However, no [Cu3Pz3]2 dimer of trimers is found in 2; instead, the supramolecular π-acidic [Cu3Pz3]⋯I interaction dominates the stacking of these oligomers, forming 1-D supramolecular chains along the c axis (Fig. 2b and Fig. S6 in ESI†). All Cu3Pz3 triangles (both Cu4–Cu5–Cu6 and Cu7–Cu8–Cu9) are subject to a [Cu3Pz3]⋯I interaction, with corresponding Cu⋯I distances ranging from 3.16 to 3.90 Å, shorter than those found in 1, suggesting a stronger π-acidic [Cu3Pz3]⋯I attraction (see Table S3 in ESI†). Given that in 2 the existence of the [Cu3Pz3]⋯I force overwhelms the formation of the Cu⋯Cu interaction, and 2 emits at even lower energy than 1 (discussed below), the formation of the [Cu3Pz3–Cu3I3] exciplex, existing in both 1 and 2, is speculated.3,6
This pair of concomitant supramolecular pseudo-isomers exhibit diverse luminescence properties. Their emissive colours are distinguishable by naked eyes under UV lamp at room temperature, being bright yellow (1) and orange (2), respectively (Fig. 3 inset photographs and Fig. S4 in ESI†). Upon excitation at 380 nm (Fig. S12a in ESI†), complex 1 shows a yellow emission with a maximum at 570 nm (Fig. 3a). The emission spectrum presents broad tailing, implying the possible coupling of a weaker, lower-energy band, which exists at all measured temperatures (Fig. S9 in ESI†). The Gaussian fitting is applied to these spectral bands (Fig. S8 in ESI†), giving, e.g. at 298 K, two Gaussian peaks (562 and 625 nm, R2 = 0.9908; χ2 = 1.04 × 10−3, Fig. 3a). When cooling down to 30 K, the emission intensity drastically increases (Fig. S9a in ESI†), especially in the range from 250 K to 200 K, and the emission peak shows a slight red shift of about 20 nm (Fig. S9b in ESI†). The decay profile fits well with a monoexponential curve at room temperature, with the lifetime being 10.34 μs (Fig. S13a in ESI†).
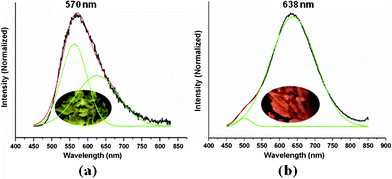 | ||
| Fig. 3 Solid-state photoluminescence spectra of 1 (a) and 2 (b) at room temperature (black) and their Gaussian fitting profiles (red and green). The inset photograph shows the visual colours of the crystals under a UV lamp with a wavelength maximum of 365 nm. | ||
Complex 2 presents an orange emission with a maximum at 638 nm (Fig. 3b) upon excitation at 380 nm (Fig. S12b in ESI†). Compared with that of 1, the emission spectra of 2 show more regular single Gaussian bands at all measured temperatures (Fig. S11 in ESI†), but with a small shoulder at the higher-energy region. The Gaussian fitting curves (Fig. S10 in ESI†) indicate, e.g. at 298 K, the emission band can be fitted into two Gaussian peaks (501 and 638 nm, R2 = 0.9943; χ2 = 6.1 × 10−4, Fig. 3b). When lowering the temperature down to 30 K, the emission intensity increases gradually (Fig. S11a in ESI†), and the peak is also red shifted by about 12 nm at 50 K (Fig. S11b in ESI†). Also the decay profile is similar, being monoexponential at 298 K and with a lifetime of 10.75 μs (Fig. S13b in ESI†).
The photoluminescence behaviors and origins can be rationalized in light of the above spectral data. The μs scale lifetimes indicate phosphorescence, consistent with the assignment of the copper(I) based triplet excited states. Several rational statements can be made: i) the emission band of 1 is separated into 562 and 625 nm; the former can be clearly assigned to the [Cu3Pz3]2 excimer,2,4,5 whereas the latter coincides with the major peak of 638 nm for 2, and hence is assigned to the [Cu3Pz3–Cu3I3] exciplex, which can be structurally identified in both 1 and 2. ii) The slight red shift (ca. 13 nm) of the exciplex band for 2 (638 nm, compared with 625 nm for 1) corresponds to shorter (ca. 0.2 Å for the shortest distance) and stronger [Cu3Pz3]⋯I contacts than those of 1. iii) Under cryogenic conditions, the increment of emission intensity is due to the reduction of non-irradiation decay processes, and the slight red shifts are reasonable because of lattice contraction, and thus the enhancement of Cu⋯Cu and [Cu3Pz3]⋯I interactions. The origin of the minor shoulder of 501 nm for 2 is unclear at this stage.
In conclusion, we have obtained two concomitant supramolecular pseudo-isomers based on copper(I) and a new pyridyl-pyrazole ligand. In both complexes, the renowned Cu3Pz3 and the unusual Cu3I3 coordination luminophores are involved, which are found to be interactive with each other, forming a [Cu3Pz3]2 excimer and [Cu3Pz3–Cu3I3] exciplex, by taking advantage of the π-acidity of the Cu3Pz3 triangle. These structural features give rise to their distinct phosphorescence properties, showing readily distinguishable bright yellow and orange emissions, respectively.
This work is financially supported by the National Natural Science Foundation for Distinguished Young Scholars of China (Grant no. 20825102) and the National Natural Science Foundation of China (Grant no. 20771072).
References
- (a) V. W.-W. Yam (ed.), Photofunctional Transition Metal Complexes (Structure and Bonding), Springer, 2007 Search PubMed; (b) N. Armaroli, G. Accorsi, F. Cardinali and A. Listorti, Top. Curr. Chem., 2007, 280, 69 CAS.
- (a) H. V. R. Dias, H. V. K. Diyabalanage, M. G. Eldabaja, O. Elbjeirami, M. A. Rawashdeh-Omary and M. A. Omary, J. Am. Chem. Soc., 2005, 127, 7489 CrossRef CAS; (b) M. A. Omary, M. A. Rawashdeh-Omary, M. W. Gonser, O. Elbjeirami, T. Grimes, T. R. Cundari, H. V. K. Diyabalanage, C. S. P. Gamage and H. V. R. Dias, Inorg. Chem., 2005, 44, 8200 CrossRef CAS; (c) T. Grimes, M. A. Omary, H. V. R. Dias and T. R. Cundari, J. Phys. Chem. A, 2006, 110, 5823 CrossRef CAS.
- (a) S. M. Tekarli, T. R. Cundari and M. A. Omary, J. Am. Chem. Soc., 2008, 130, 1669 CrossRef CAS; (b) H. V. R. Dias and C. S. P. Gamage, Angew. Chem., Int. Ed., 2007, 46, 2192 CrossRef CAS; (c) M. A. Rawashdeh-Omary, M. D. Rashdan, S. Dharanipathi, O. Elbjeirami, P. Ramesh and H. V. R. Dias, Chem. Commun., 2011, 47, 1160 RSC; (d) C. Yang, O. Elbjeirami, C. S. P. Gamage, H. V. R. Dias and M. A. Omary, Chem. Commun., 2011, 47, 7434 RSC.
- (a) J. He, Y.-G. Yin, T. Wu, D. Li and X.-C. Huang, Chem. Commun., 2006, 2845 RSC; (b) J.-X. Zhang, J. He, Y.-G. Yin, M.-H. Hu, D. Li and X.-C. Huang, Inorg. Chem., 2008, 47, 3471 CrossRef CAS.
- (a) G.-F. Gao, M. Li, S.-Z. Zhan, Z. Lv, G.-h. Chen and D. Li, Chem.–Eur. J., 2011, 17, 4113 CrossRef CAS; (b) S.-Z. Zhan, M. Li, X.-P. Zhou, J.-H. Wang, J.-R. Yang and D. Li, Chem. Commun., 2011 10.1039/C1CC14303D.
- (a) J.-P. Zhang, S. Horike and S. Kitagawa, Angew. Chem., Int. Ed., 2007, 46, 889 CrossRef CAS; (b) L. Hou, W.-J. Shi, Y.-Y. Wang, H.-H. Wang, L. Cui, P.-X. Chen and Q.-Z. Shi, Inorg. Chem., 2011, 50, 261 CrossRef CAS; (c) T. Jozak, Y. Sun, Y. Schmitt, S. Lebedkin, M. Kappes, M. Gerhards and W. R. Thiel, Chem.–Eur. J., 2011, 17, 3384 CrossRef CAS; (d) V. N. Tsupreva, A. A. Titov, O. A. Filippov, A. N. Bilyachenko, A. F. Smol'yakov, F. M. Dolgushin, D. V. Agapkin, I. A. Godovikov, L. M. Epstein and E. S. Shubina, Inorg. Chem., 2011, 50, 3325 CrossRef CAS.
- (a) S.-Z. Zhan, D. Li, X.-P. Zhou and X.-H. Zhou, Inorg. Chem., 2006, 45, 9163 CrossRef CAS; (b) S.-Z. Zhan, M. Li, J.-Z. Hou, J. Ni, D. Li and X.-C. Huang, Chem.–Eur. J., 2008, 14, 8916 CrossRef CAS; (c) S.-Z. Zhan, R. Peng, S.-H. Lin, S. W. Ng and D. Li, CrystEngComm, 2010, 12, 1385 RSC; (d) S.-Z. Zhan, M. Li, X.-P. Zhou, J. Ni, X.-C. Huang and D. Li, Inorg. Chem., 2011, 50, 8879 CAS.
- (a) R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1 CrossRef CAS and references therein; (b) M. Li, Z. Li and D. Li, Chem. Commun., 2008, 3390 RSC.
- (a) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (b) J.-P. Zhang, X.-C. Huang and X.-M. Chen, Chem. Soc. Rev., 2009, 38, 2385 RSC.
- (a) F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, B58, 380 CrossRef . Searching in CSD (version 5.32, November 2010) by cyclic Cu3I3 fragment resulted in 232 hits, among which only 9 hits for neutral Cu3I3 aggregates and only one hit for the same cyclic Cu3I3 fragment herein (Refcode: RUMNUJ, see ref. 10b); (b) R. D. Köhn, L. T. Laudo, Z. Pan, F. Speiser and G. Kociok-Köhn, Dalton Trans., 2009, 4556 RSC.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Synthetic and crystallographic details, additional structural figures and physical measurements. CCDC reference numbers 838375 (HL), 838376 (1) and 838377 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ra00566a/ |
| This journal is © The Royal Society of Chemistry 2011 |
