DOI:
10.1039/C0SC00433B
(Edge Article)
Chem. Sci., 2011,
2, 363-368
A survey of electron-deficient pentacenes as acceptors in polymer bulk heterojunction solar cells†
Received
12th August 2010
, Accepted 13th October 2010
First published on 19th November 2010
Abstract
We have prepared, characterized and surveyed device performance for a series of electron deficient pentacenes for use as acceptors in polymer bulk heterojunction solar cells, using P3HT as the donor material. All of the materials reported here behaved as acceptors, and variations in the position and nature of the electron-withdrawing group on the pentacene core allowed tuning of device open-circuit voltage. Photocurrent was strongly correlated with the pentacene crystal packing motif; materials with 2D π-stacking interactions performed poorly compared with materials exhibiting 1D π-stacking interactions. The best pentacene acceptors gave repeatable device efficiency in excess of 1.2%, compared with 3.5% exhibited for PCBM-based devices.
Solar energy conversion using solution processed organic semiconductors has been a field of increasing interest, and the promise of low-cost energy generation from lightweight, flexible solar panels has led to rapid progress in device performance. Since the first polymer donor/(6,6)-phenyl C61butyric acid methyl ester (PCBM) acceptor bulk-heterojunction (BHJ) solar cell,1 dramatic advances have been made in improving power conversion efficiency in these systems.2 Most research in this field has emphasized the development of new donor polymers3 and on improvements in processing conditions to yield optimum morphologies for charge transport,4 recently leading to impressive power conversion efficiency (PCE) in polymer/fullerene devices approaching 8%.5 Due to the outstanding performance of fullerene-based materials, efforts to discover new acceptors for polymer BHJ organic photovoltaics (OPVs) have been comparatively limited.6 Although polymeric acceptors recently yielded efficiency up to 2.0% in all-polymer devices,7 small-molecule acceptors, essentially used as drop-in replacements for PCBM, have not yet reached even that level of performance. Acceptors based on chromophores such as perylene diimide,8Vinazene,9 and diketopyrrolopyrrole10,11 derivatives yielded P3HT-based BHJ solar cells with power conversion efficiencies as high as 0.55%, 0.75% and 1.0%, respectively – few other materials have shown efficiencies greater than ∼0.5%,11 although some promising new results hint at new routes to improved performance.12
The ease with which pentacene-based small-molecule acceptors can be synthetically altered to improve charge transport and film morphology makes these compounds ideal candidates for a systematic study of their structure-property relationships as OPV acceptors.13 We report here a survey of soluble pentacene derivatives with electron-withdrawing groups on the pentacene core, the impact of these groups on the open-circuit voltage of poly(3-hexylthiophene) (P3HT)/pentacene BHJ solar cells, and the optimization of top candidates to improve crystal packing and morphology. Several of the resulting materials yield photovoltaic cells with PCE greater than 1.0%, and the best performing derivative shows PCE approaching 1.3%.
In contrast with the degree of substitution required for electron transport in organic transistors,14 we recently found that only a single electron-withdrawing group – in that case a nitrile substituent – was necessary to achieve reasonable OPV performance with pentacene-based acceptors.15 For this study, we thus prepared a series of soluble pentacene derivatives with an array of electron-withdrawing groups on the pentacene core. Recent reports of the substituent positional sensitivity of electronic characteristics in pentacene derivatives16 led us to synthesize derivatives containing the electron-withdrawing group at both the 1- and 2-position on the acene ring (Fig. 1).
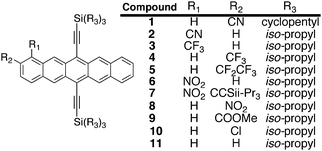 |
| | Fig. 1 Initial pentacene targets prepared for this study. | |
Results and discussion
Synthesis of the acceptors
The general synthesis of our pentacene acceptors is outlined in Schemes 1 and 2, and full synthetic details are provided in the ESI.† For most derivatives, the targets were accessed by addition of a lithium acetylide to a pentacenequinone, followed by deoxygenation with SnCl2. Quinones were prepared by either aldol condensation or Cava reaction (Scheme 1). For the cyanopentacenes and carboxymethyl derivatives, the corresponding iodopentacenes were first prepared as outlined in Scheme 1, followed by Pd-mediated coupling of the desired functional group onto the pentacene core (Scheme 2).The only di-substituted derivative presented in this study (7) was formed as a major byproduct during the synthesis of compound 6.
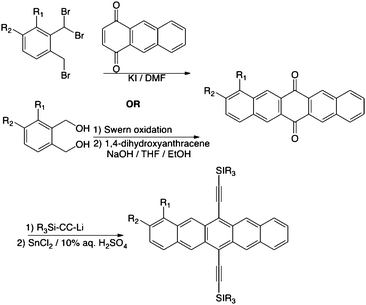 |
| | Scheme 1 General synthetic approach to electron-deficient pentacenes (for R1or R2 = CF3, Cl, I, CF2CF3, NO2). | |
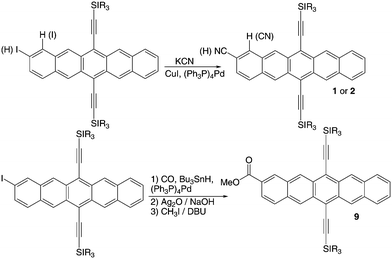 |
| | Scheme 2 Synthesis of cyanopentacenes 1 and 2, and carboxymethyl pentacene derivative 9. | |
Characterization of the acceptors
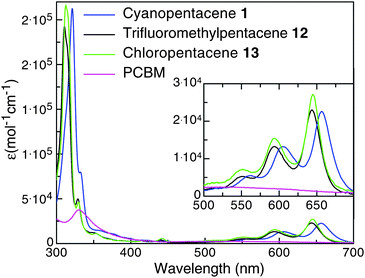
Electrochemical analysis of these pentacene derivatives showed that they all possess LUMO levels appropriate to serve as acceptors for a P3HT-based donor (Table 1). As expected, the strongly electron-withdrawing nitro substituent yielded the lowest lying LUMO level of the materials tested in this study, followed by nitrile and perfluoroalkyl substituents. We noted only small differences in the LUMO energies between pentacenes substituted on the 1-position versus those substituted on the 2-position with the same functional group. These pentacene derivatives all have similar optical absorption properties. The UV-vis spectra for typical pentacene acceptors (1, 4 and 10), along with the spectrum for PCBM, are shown in Fig. 2. The acenes absorb with good intensity in the lower energy part of the visible spectrum, which may prove useful in extending the active window of OPV devices made from larger band gap polymers such as P3HT.
Table 1 Electrochemicala data for electron-deficient pentacenes
| Compound |
HOMO/eV |
LUMO/eV |
Optical Gap/eV |
|
Differential pulse voltammetry performed at a scan rate of 20 mV s−1 with Fc/Fc+ as internal standard. Fc/Fc+ is assumed to have an absolute energy level of −4.8 to vacuum.17 Optical gap calculated from the onset of absorption in dichloromethane solution.
|
| PCBM |
−6.10 |
−3.70 |
|
|
1
|
−5.29 |
−3.50 |
1.82 |
|
2
|
−5.34 |
−3.50 |
1.81 |
|
3
|
−5.28 |
−3.41 |
1.86 |
|
4
|
−5.29 |
−3.45 |
1.83 |
|
5
|
−5.32 |
−3.42 |
1.86 |
|
6
|
−5.34 |
−3.52 |
1.73 |
|
7
|
−5.35 |
−3.61 |
1.78 |
|
8
|
−5.34 |
−3.55 |
1.73 |
|
9
|
−5.26 |
−3.49 |
1.80 |
|
10
|
−5.17 |
−3.33 |
1.85 |
Several of the pentacene acceptors yielded solution-grown single crystals of sufficient quality for at least cursory structural analysis, as described in the ESI.† Consistent with this functionalization methodology, all of the materials adopted some form of π-stacked arrangement in the solid state.18 Among these derivatives, the most common motif was a double 1-D “slipped-stack” arrangement (Fig. 3, top), adopted by 4, 5 and 7. This motif is driven by the unsymmetrical substitution pattern in these materials, leading to the un-substituted ends of the acenes residing in close proximity in the crystal. The resulting pairwise-stacked acenes are insulated from adjacent stacks by the hydrocarbon substituents of those stacks. Nitro derivative 8 adopted a 2-D “brickwork” packing similar to that of TIPS pentacene 11.19 The 1-substituted compounds (2, 3, 6) formed smaller crystals than the corresponding 2-substituted derivatives, that were too small for crystallographic analysis.
It is possible that the crystalline form of the material present in the P3HT blend is different from the single crystal form – analysis of the crystallites in the blend is currently underway.
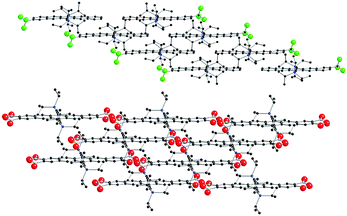 |
| | Fig. 3 Representative crystal packings. Top: double 1-D slipped-stack motif (4). Bottom: 2-D brickwork motif (8). | |
Device optimization
Because cyano derivative 1 had already exhibited some level of performance as an OPV acceptor, the test procedure for these new materials was optimized using this compound. In our previous study, we defaulted to toluene as the solvent for spin-coating blends of P3HT and 1.15 We observed that under these conditions, this pentacene derivative formed large micron-size crystals in the blend films. Since the exciton diffusion length in organic semiconductors is on the order of ∼10 nm, the donor–acceptor domains need to be of that length scale for optimum solar cell performance.20 Hence, for this study we attempted to suppress pentacene crystallization during film formation by the use of a process additive. The use of high-boiling additives such as alkanedithiols has been studied in the fabrication of traditional polymer/PCBM solar cells, and dramatically improved the performance of the resulting devices.3c,4a,b Experimentation with blends of P3HT and our acceptors showed that 1,2-dichlorobenzene (DCB) had the appropriate combination of solvating power and high boiling point to yield an improved film morphology – likely due to its ability to “solvent anneal” both P3HT and acene-based materials.1,21
Solar cells were fabricated on cleaned, pre-patterned ITO coated glass substrates coated with PEDOT:PSS. Semiconductor layers were deposited by spin-coating of 20 mg ml−1 solutions in a nitrogen-filled glovebox. Finally, 4 Å of CsF and 400 Å of Al were thermally evaporated under high vacuum to form the cathode for the devices. Solar cell current–voltage (I–V) curves were measured in the dark and under AM 1.5 100 mW cm−2 illumination.
A series of comparative studies showed that a P3HT/pentacene blend (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight) spin-cast from a toluene/DCB solvent mixture (10
1 ratio by weight) spin-cast from a toluene/DCB solvent mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
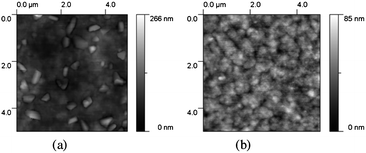
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio by volume) yielded the highest performing devices. No thermal annealing was performed on the finished devices, since this was typically found to degrade device performance by increasing the domain size of the pentacene acceptor. Clear morphological changes were observed by AFM as the proportion of DCB was increased (Fig. 4), leading to suppression of large crystal formation in one of the semiconductor components and a more uniform grain size. From toluene-cast films, large, coarse crystalline-looking features can be seen, yielding an rms roughness of 22.0 nm (Fig. 4a), whereas the film cast from a toluene/DCB mixture (Fig. 4b) possesses finer features, with a significantly lower film rms (9.1 nm), suggesting more intimate contact between the donor and acceptor phases. Under these conditions, the device performance of cyanopentacene 1 was improved to yield a Voc of 0.84 V, JSC of 3.72 mA cm−2, FF of 0.41, and a PCE of 1.29% – a representative current–voltage plot is shown in Fig. 5. We note that PCBM-based devices prepared side-by-side with the pentacene devices routinely exhibited PCE of 3.5%.
3 ratio by volume) yielded the highest performing devices. No thermal annealing was performed on the finished devices, since this was typically found to degrade device performance by increasing the domain size of the pentacene acceptor. Clear morphological changes were observed by AFM as the proportion of DCB was increased (Fig. 4), leading to suppression of large crystal formation in one of the semiconductor components and a more uniform grain size. From toluene-cast films, large, coarse crystalline-looking features can be seen, yielding an rms roughness of 22.0 nm (Fig. 4a), whereas the film cast from a toluene/DCB mixture (Fig. 4b) possesses finer features, with a significantly lower film rms (9.1 nm), suggesting more intimate contact between the donor and acceptor phases. Under these conditions, the device performance of cyanopentacene 1 was improved to yield a Voc of 0.84 V, JSC of 3.72 mA cm−2, FF of 0.41, and a PCE of 1.29% – a representative current–voltage plot is shown in Fig. 5. We note that PCBM-based devices prepared side-by-side with the pentacene devices routinely exhibited PCE of 3.5%.
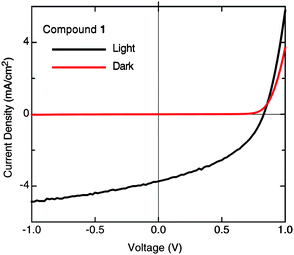 |
| | Fig. 5 Current–voltage curve for P3HT/pentacene 1 OPV. | |
The other pentacene derivatives shown in Fig. 1 were studied using the optimized fabrication procedure described above, and their device performance is presented in Table 2. All of the materials listed in Fig. 1 yielded working OPVs when blended with P3HT, and nearly all devices show higher Voc than typical P3HT:PCBM cells (Voc ∼ 0.6 V). A number of arguments have been presented in the literature to describe the impact of material parameters on device Voc, one of the most widely accepted involving the relationship between the donor HOMO and acceptor LUMO.22 Thus for example, TIPS pentacene (11), a known p-type semiconductor in OTFTs,23 has the highest lying LUMO of all the materials tested and correspondingly exhibited one of the highest Voc. However, there is also an observed relationship between Voc and film morphology,24 which may relate to the observed impact of the substitution pattern on the Voc of the solar cells. All derivatives substituted on the 1-position of the acene (2, 3, 6) showed demonstrably lower Voc than derivatives containing the same electron-withdrawing group at the 2-position (1, 4, 8). The magnitude of this difference (in all cases > 0.05 V) is significantly greater than would be estimated from the differences in LUMO energies (on the order of 0.0–0.04 eV). Contributing to this phenomenon may be the very different crystallinity of the 1-substituted vs. the 2-substituted derivatives – as mentioned previously, none of the 1-substituted derivatives presented here yielded crystals suitable even for crystallographic analysis.
|
Cpd. |
V
oc/V |
J
sc/mA cm−2 |
FF |
PCE (%) |
|
1
|
0.84 ± 0.01 |
3.56 ± 0.17 |
0.42 ± 0.02 |
1.27 ± 0.03 |
|
2
|
0.79 ± 0.01 |
0.24 ± 0.02 |
0.39 ± 0.01 |
0.08 ± 0.01 |
|
3
|
0.59 ± 0.02 |
0.54 ± 0.04 |
0.32 ± 0.02 |
0.10 ± 0.01 |
|
4
|
0.70 ± 0.01 |
1.86 ± 0.07 |
0.32 ± 0.01 |
0.41 ± 0.02 |
|
5
|
0.67 ± 0.01 |
2.07 ± 0.22 |
0.33 ± 0.01 |
0.46 ± 0.05 |
|
6
|
0.56 ± 0.03 |
0.27 ± 0.03 |
0.32 ± 0.01 |
0.05 ± 0.01 |
|
7
|
0.63 ± 0.03 |
1.30 ± 0.22 |
0.29 ± 0.02 |
0.24 ± 0.04 |
|
8
|
0.64 ± 0.01 |
0.49 ± 0.03 |
0.28 ± 0.02 |
0.09 ± 0.01 |
|
9
|
0.86 ± 0.01 |
1.05 ± 0.07 |
0.39 ± 0.02 |
0.35 ± 0.03 |
|
10
|
0.70 ± 0.01 |
0.94 ± 0.02 |
0.37 ± 0.01 |
0.24 ± 0.01 |
|
11
|
0.79 ± 0.01 |
0.24 ± 0.02 |
0.39 ± 0.01 |
0.08 ± 0.01 |
Structural optimization for improved device current
In general, the materials whose π-stacking motif approach 1-D π-stacking exhibit higher Jsc and hence higher PCE, consistent with our previous observations.15 In our previous study, we found that the derivative adopting a “sandwich herringbone” crystal packing arrangement (e.g.1) yielded the highest Jsc in blends with P3HT. The silylethyne route to acene functionalization allows facile tuning of crystal packing by simple changes to the silane, and a number of derivatives were screened for this desirable motif. While the carboxymethyl derivative could not be altered to yield the desired crystal packing, both the trifluoromethyl derivative 12 (requiring the tri-iso-butylsilyl substituent) and the chloro derivative 13 (requiring a tricyclopentylsilyl substituent) did yield appropriate motifs (Fig. 6). For 12, extensive disorder in the iso-butyl groups (omitted in Fig. 6) precluded refinement of the structural model, but the sandwich herringbone motif is quite apparent from the data. For 13, four-fold disorder in the position of the single chlorine substituent (apparent in the structure shown in Fig. 6) also prevented detailed structural refinement – but the packing motif is quite clear. Unlike compound 12's sandwich herringbone motif, compound 13 adopts an unusual form of the 1-D slipped-stack, where adjacent stacks interact in an edge-to-face, rather than co-planar orientation. We refer to this motif as “sandwich slipped-stack”. It is worth noting that the trifluoromethyl derivative also yielded materials with crystal motifs not previously screened for OPV performance; a simple 1-D “slipped-stack” motif18 (14, tricyclopentylsilyl substituent) and an unusual “cruciform” version of this motif, where alternate pentacene units are rotated by approximately 90° relative to their neighbors in the stack (15, tri-n-propylsilyl derivative). The apparent beneficial nature of materials with 1-D π-stacking motifs also led us to synthesize “offset” pentacene derivative 16, which we knew from prior experience would yield essentially columnar π-stacked arrays. Following the procedure optimized for derivative 1, BHJ solar cells were made from blends of 12–16 with P3HT. The device parameters for these new cells are listed in Table 3.
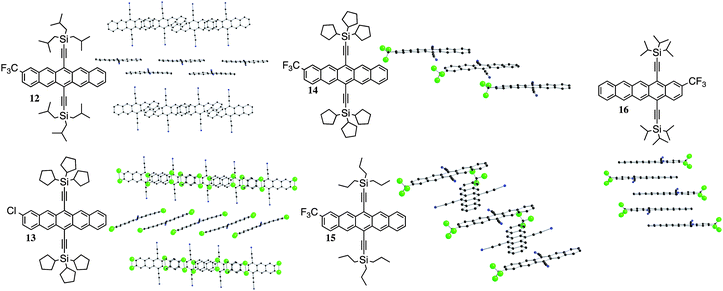 |
| | Fig. 6 Structure and crystal packing for pentacenes 12–16. Trialkylsilyl groups hidden for clarity. | |
Table 3 Device performance of pentacene acceptors 12–16
|
Cpd. |
V
oc/V |
J
sc/mA cm−2 |
FF |
PCE (%) |
|
12
|
0.80 ± 0.02 |
3.17 ± 0.03 |
0.50 ± 0.01 |
1.26 ± 0.01 |
|
13
|
0.95 ± 0.02 |
2.44 ± 0.13 |
0.43 ± 0.01 |
1.00 ± 0.08 |
|
14
|
0.78 ± 0.01 |
3.23 ± 0.06 |
0.33 ± 0.01 |
0.83 ± 0.03 |
|
15
|
0.62 ± 0.01 |
1.05 ± 0.06 |
0.27 ± 0.01 |
0.18 ± 0.01 |
|
16
|
0.32 ± 0.01 |
0.40 ± 0.02 |
0.40 ± 0.02 |
0.05 ± 0.01 |
Yet again, we see that materials adopting a sandwich-type crystal packing arrangement (12, 13) yield the highest device currents and fill-factors (J–V plots for 12 and 13 are presented in the ESI†). The requirement of a strongly one-dimensional packing motif for best performance is further supported by the reasonably good performance of derivative 14 which, although not adopting a sandwich-type motif, still packs in a 1-D slipped-stack arrangement with a similarly insulated π-stacking channel. Compounds 15 and 16, which adopt 1-D columnar π-stacking arrangements, showed device performance roughly an order of magnitude worse than derivatives adopting other 1-D π-stacking motifs. The particularly poor performance of 16 may also be explained by the lower stability of this material – there are no substituents protecting the relatively reactive 6,13 positions of the pentacene backbone.
Further device characterization
To further investigate OPV performance of these acceptors, the EQE spectrum of derivative 1 in a blend with P3HT was acquired (Fig. 7).The peak EQE is above 25% at around 500 nm for this device, corresponding to the peak absorption of P3HT. The smaller peaks at around 330 nm and 650 nm correspond to the absorption peaks of pentacene 1, showing that it too is contributing to the generation of photocurrent outside the absorption window of P3HT, effectively extending the spectral response of the P3HT-based solar cell.
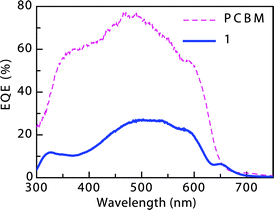 |
| | Fig. 7
EQE
spectra of BHJ solar cells based on 1:P3HT (solid) and PCBM:P3HT (dashed). | |
Finally, as a preliminary study of the stability of these new materials, their operational performance was characterized in air alongside that of a traditional P3HT/PCBM device fabricated at the same time. As shown in Fig. 8, the device lifetime of pentacene-based materials was comparable to that of the simultaneously measured PCBM-based device.
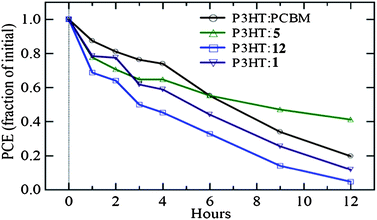 |
| | Fig. 8 Comparison of device lifetime of pentacene-based solar cellsvs. PCBM-based devices. | |
Conclusion
We have demonstrated that several silylethyne-substituted pentacene derivatives successfully serve as effective acceptors in P3HT-based BHJ solar cells. Using an improved fabrication procedure that exploits solvent mixtures to yield better film morphology, we found that both the nature and position of electron-withdrawing groups substituted onto the pentacene chromophore impact the device Voc. Both inductive and resonance electron-withdrawing groups substituted at the pentacene 2-position led to devices with voltages higher than typically observed with PCBM acceptor, with cyano, trifluoromethyl and chloro substituents yielding the best performance. After determining the aromatic ring substituents yielding the best voltage, optimization of the acceptor crystal packing by changing the alkyl groups on the silyl substituent led to improvements in device current. We found that a particular crystal packing arrangement – the “sandwich herringbone” motif – consistently yielded the best photocurrents. Studies to understand the particular reason for the improved photocurrent are currently underway. Our experiments have yielded three new acceptors all with power conversion efficiency >1.0%, with the best derivative, cyano-substituted 1, exhibiting a PCE approaching 1.3%. These results are among the best reported for non-fullerene small-molecule acceptors for polymer BHJ solar cells. The wide array of pentacene derivatives that yield efficient solar cells, the ease with which new derivatives can be prepared, and the structure/function relationships that are beginning to emerge from this study make these pentacenes a versatile platform for the development of new OPV acceptors.
Acknowledgements
J. E. A. and G. G. M. thank the Office of Naval Research for support of this research effort. Y. F. L. acknowledges a research fellowship from A*STAR (Singapore). B. P. acknowledges the National Science Foundation for support.
Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CrossRef CAS.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriaty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CAS; W. Ma, C. Yang, X. Gong, L. Lee and A. J. Heeger, Adv. Mater., 2005, 17, 1617; B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef; F. G. Brunetti, R. Kumar and F. Wudl, J. Mater. Chem., 2010, 20, 2934 RSC; W. Cai, X. Gong and Y. Cao, Sol. Energy Mater. Sol. Cells, 2010, 94, 114 CrossRef CAS.
- C. Piliego, T. W. Holcombe, J. D. Douglas, C. H. Woo, P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2010, 132, 7595 CrossRef CAS; Y. Zou, A. Najari, P. Berrouard, B. Beaupré, B. R. Aïch, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330 CrossRef CAS; Y. Liang, Y. Wu, D. Feng, S.-T. Tsai, H.-J. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS; J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS.
- Y. Yao, J. Hou, Z. Xu, G. Li and Y. Yang, Adv. Funct. Mater., 2008, 18, 1; J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS; L.-M. Chen, Z. Xu, Z. Hong and Y. Yang, J. Mater. Chem., 2010, 20, 2575 RSC.
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- J. J. Dittmer, E. A. Marseglia and R. H. Friend, Adv. Mater., 2000, 12, 1270 CrossRef CAS.
- T. Kietzke, H.-H. Horhold and D. Neher, Chem. Mater., 2005, 17, 6532 CrossRef CAS; X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS; T. W. Holcombe, C. Woo, D. F. J. Kavulak, B. C. Thompson and J. M. J. Fréchet, J. Am. Chem. Soc., 2009, 131, 14160 CrossRef CAS.
- S. Rajaram, P. B. Armstrong, B. J. Kim and J. M. J. Frechet, Chem. Mater., 2009, 21, 1775 CrossRef CAS.
- Z. E. Ooi, T. L. Tam, R. Y. C. Shin, Z. Chen, T. Kietzke, A. Sellinger, M. Baumgarten, K. Mullen and J. C. deMello, J. Mater. Chem., 2008, 18, 4619 RSC; R. Y. C. Shin, T. Kietzke, S. Sudhakar, A. Dodabalapur, Z.-K. Chen and A. Sellinger, Chem. Mater., 2007, 19, 1892 CrossRef CAS; C. H. Woo, T. W. Holcombe, D. A. Unruh, A. Sellinger and J. M. J. Fréchet, Chem. Mater., 2010, 22, 1673 CrossRef CAS; R. Y. C. Shin, P. Sonar, P. S. Siew, Z.-K. Chen and A. Sellinger, J. Org. Chem., 2009, 74, 3293 CrossRef CAS.
- P. Sonar, G.-M. Ng, T. T. Lin, A. Dodabalapur and Z.-K. Chen, J. Mater. Chem., 2010, 20, 3626 RSC.
- Y. Zhou, J. Pei, Q. Dong, X. Sun, Y. Liu and W. Tian, J. Phys. Chem. C, 2009, 113, 7882 CrossRef CAS.
- F. Brunetti, X. Gong, A. J. Heeger and F. Wudl, Angew. Chem., Int. Ed., 2010, 49, 532 CAS.
- J. E. Anthony, Angew. Chem., Int. Ed., 2008, 47, 452 CrossRef CAS.
- Y. Sakamoto, T. Suzuki, M. Kobayashi, Y. Gao, Y. Fukai, Y. Inoue, F. Sato and S. Tokito, J. Am. Chem. Soc., 2004, 126, 8138 CrossRef CAS.
- Y.-F. Lim, Y. Shu, S. R. Parkin, J. E. Anthony and G. G. Malliaras, J. Mater. Chem., 2009, 19, 3049 RSC.
- B. M. Medina, J. E. Anthony and J. Gierschner, ChemPhysChem, 2008, 9, 1519 CrossRef CAS.
- I. Seguy, P. Jolinat, P. Destruel, R. Mamy, H. Allouchi, C. Courseille, M. Cotrait and H. Mock, ChemPhysChem, 2001, 2, 448 CrossRef CAS.
- J. E. Anthony, D. L. Eaton and S. R. Parkin, Org. Lett., 2002, 4, 15 CrossRef CAS.
- J. E. Anthony, J. S. Brooks, D. L. Eaton and S. R. Parkin, J. Am. Chem. Soc., 2001, 123, 9482 CrossRef CAS.
- X. Yang, J. Loos, S. C. Veenstra, W. J. H. Verhees, M. M. Wienk, J. M. Kroon, M. A. J. Michels and R. A. J. Janssen, Nano Lett., 2005, 5, 579 CrossRef CAS.
- M. T. Lloyd, A. C. Mayer, S. Subramanian, D. A. Mourey, D. J. Herman, A. V. Bapat, J. E. Anthony and G. G. Malliaras, J. Am. Chem. Soc., 2007, 129, 9144 CrossRef.
- C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, T. Fromherz, M. T. Rispens, L. Sanchez and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 374 CrossRef CAS; C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, M. T. Rispens, L. Sanchez, J. C. Hummelen and T. Fromherz, Thin Solid Films, 2002, 403–404, 368 CrossRef CAS.
- S. K. Park, T. N. Jackson, J. E. Anthony and D. Mourey, Appl. Phys. Lett., 2007, 91, 063514 CrossRef.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds 1–16, and details of device fabrication. CCDC reference numbers 796836–796840. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00433b |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight) spin-cast from a toluene/DCB solvent mixture (10
1 ratio by weight) spin-cast from a toluene/DCB solvent mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio by volume) yielded the highest performing devices. No thermal annealing was performed on the finished devices, since this was typically found to degrade device performance by increasing the domain size of the pentacene acceptor. Clear morphological changes were observed by AFM as the proportion of DCB was increased (Fig. 4), leading to suppression of large crystal formation in one of the semiconductor components and a more uniform grain size. From toluene-cast films, large, coarse crystalline-looking features can be seen, yielding an rms roughness of 22.0 nm (Fig. 4a), whereas the film cast from a toluene/DCB mixture (Fig. 4b) possesses finer features, with a significantly lower film rms (9.1 nm), suggesting more intimate contact between the donor and acceptor phases. Under these conditions, the device performance of cyanopentacene 1 was improved to yield a Voc of 0.84 V, JSC of 3.72 mA cm−2, FF of 0.41, and a PCE of 1.29% – a representative current–voltage plot is shown in Fig. 5. We note that PCBM-based devices prepared side-by-side with the pentacene devices routinely exhibited PCE of 3.5%.
3 ratio by volume) yielded the highest performing devices. No thermal annealing was performed on the finished devices, since this was typically found to degrade device performance by increasing the domain size of the pentacene acceptor. Clear morphological changes were observed by AFM as the proportion of DCB was increased (Fig. 4), leading to suppression of large crystal formation in one of the semiconductor components and a more uniform grain size. From toluene-cast films, large, coarse crystalline-looking features can be seen, yielding an rms roughness of 22.0 nm (Fig. 4a), whereas the film cast from a toluene/DCB mixture (Fig. 4b) possesses finer features, with a significantly lower film rms (9.1 nm), suggesting more intimate contact between the donor and acceptor phases. Under these conditions, the device performance of cyanopentacene 1 was improved to yield a Voc of 0.84 V, JSC of 3.72 mA cm−2, FF of 0.41, and a PCE of 1.29% – a representative current–voltage plot is shown in Fig. 5. We note that PCBM-based devices prepared side-by-side with the pentacene devices routinely exhibited PCE of 3.5%.