Structurally simple lipid bilayer transport agents for chloride and bicarbonate†
Natalie J.
Andrews
a,
Cally J. E.
Haynes
a,
Mark E.
Light
a,
Stephen J.
Moore
a,
Christine C.
Tong‡
a,
Jeffery T.
Davis
b,
William A.
Harrell Jr.
b and
Philip A.
Gale
*a
aSchool of Chemistry, University of Southampton, Southampton, UK. E-mail: philip.gale@soton.ac.uk; Fax: (+44) 23 0859 6805
bDepartment of Chemistry and Biochemistry, University of Maryland, College Park, MD, USA
First published on 23rd November 2010
Abstract
A new series of structurally simple compounds containing thiourea groups have been shown by a combination of ion-selective electrode and 13C NMR techniques to be potent chloride-bicarbonate exchange agents that function at low concentration in POPC and POPC/cholesterol membranes.
Introduction
Anion transport 1 is key to many biological processes. Misregulation of the transport of chloride across cell membranes can lead to diseases such as cystic fibrosis (caused by the reduction or absence of conductance across epithelial cell membranes as a consequence of a mutation in the CFTR anion channel), Bartter's syndrome and Dent's disease (resulting from mutations in genes which encode for chloride or salt transport channels in renal cells).2 Similarly misregulation of bicarbonate transport has also been implicated in cystic fibrosis3 and other diseases.4 Consequently a number of groups have developed synthetic anion channels and discrete molecular carriers to transport chloride across lipid bilayer membranes.5 Our interests have recently focused on the development of small molecules capable of chloride/bicarbonate antiport.6,7
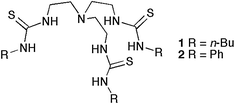
Ureas and thioureas form stable complexes with oxo-anions and have been employed in a wide variety of receptors for these species.8 We have recently shown that tris(2-aminoethyl)amine (tren) based tris-thioureas 1 and 2 function as potent chloride/bicarbonate antiport agents in POPC liposomes.9 Tren-based tris-amides were first shown to bind anions such as chloride by Reinhoudt and co-workers,10 whilst tren-based ureas have been shown by Custelcean and co-workers11 and Ghosh and co-workers12 and others to be effective hosts for oxo-anions. The transport properties of tren-based anion receptors have been investigated by D. K. Smith and co-workers in U-tube experiments,13 J. T. Davis and co-workers in lipid bilayers14 and their flippase activity in lipid bilayers by B. D. Smith and co-workers.15 In our studies we found that thioureas were significantly more effective transporters than analogous ureas. In previous work A. P. Davis and co-workers have shown that a simple 4-nitrophenylurea when pre-incorporated into a lipid bilayer functions as a chloride transporter at concentrations of transporter/lipid of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150.16 B. D. Smith and co-workers have incorporated urea groups into phospholipids and shown that these compounds function as anion transporters by a relay mechanism in which one functionalised lipid ‘hands’ the anion over to another in the opposite leaflet.17 Hence we decided to study a series of very simple thiourea based compounds and compare their anion transport activity to analogous ureas, amides and thioamides. The results show that very simple compounds containing thiourea groups, without pre-incorporation into a lipid bilayer, function as potent chloride/bicarbonate antiport agents at relatively low concentrations.
150.16 B. D. Smith and co-workers have incorporated urea groups into phospholipids and shown that these compounds function as anion transporters by a relay mechanism in which one functionalised lipid ‘hands’ the anion over to another in the opposite leaflet.17 Hence we decided to study a series of very simple thiourea based compounds and compare their anion transport activity to analogous ureas, amides and thioamides. The results show that very simple compounds containing thiourea groups, without pre-incorporation into a lipid bilayer, function as potent chloride/bicarbonate antiport agents at relatively low concentrations.
Results and discussion
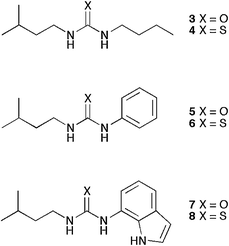
We decided to study a series of ureas and thioureas that had one common side chain and one substituent that would vary across the series. We chose the iso-pentyl side chain as the common substituent in these compounds as our previous studies had shown that 4,6-dihydroxyisophthalamides with this substituent were particularly effective membrane transporters.6 Each compound was then prepared as the urea and thio urea derivative with n-butyl-, phenyl- or 7-indolyl substituents to study the effects of changing the electronics of this substituent and also the effect of adding an additional hydrogen bond donor group. Indoles have recently been employed in a variety of effective anion receptors and anion-templated foldamers.18 Compounds 3–6 were prepared by reaction of either phenyl or butyl isocyantate or isothiocyanate in dichloromethane or chloroform with iso-pentylamine affording the compounds in 49, 49, 72 and 48% yields, respectively. Compound 7 was prepared by reduction of 7-nitroindole to the amine and then reaction with CDI to afford an activated intermediate which was then coupled to iso-pentylamine affording the product in 44% overall yield. Compound 8 was prepared by reaction of 7-aminoindole with thiophosgene to afford the isothiocyanate that was then coupled with iso-pentylamine to afford the product in 24% overall yield.Proton NMR titrations were used to determine the stability constants of compounds 3–8 with chloride and nitrate (added as tetrabutylammonium salts) and bicarbonate (added as the tetraethylammonium salt) in DMSO-d6/0.5% water solution using the WinEQNMR computer program.19 The results (shown in Table 1) show that the compounds do not detectably interact with nitrate in this solvent mixture but do bind chloride with the indole-based compounds showing the highest affinity for this anion (and in particular the urea derivative). Similarly bicarbonate is bound most strongly by the indolylurea followed by the indolylthiourea.
In order to study the chloride transport properties of compounds 3–8 we prepared a series of unilamellar 1-palmitoyl-2-oleoylphophatidylcholine (POPC) vesicles loaded with sodium chloride (489 mM) suspended in an external NaNO3 (489 mM) solution. A sample of the receptor (2% molar carrier to lipid) was added as a DMSO solution and the resultant chloride efflux monitored using a chloride selective electrode.20 After 300 s, the vesicles were lysed by addition of detergent and the final reading of the electrode was used to calibrate 100% release of chloride. The results show that the compounds containing urea groups show no significant release of chloride under these conditions (Fig. 1). Compound 6, the phenylthiourea, shows moderate activity whilst the butylthiourea and the indolylthiourea both efficiently release chloride from the vesicles. The EC50 values for the compounds are shown in Table 2. Transport experiments were repeated in vesicles composed of POPC/cholesterol 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and showed a slightly reduced rate of transport for the thiourea compounds – evidence that these species are functioning as carriers rather than forming channels (ESI Figures S29–31†). It is notable that the performance of the thiourea compounds in the POPC/cholesterol membrane is still excellent as this mixture more closely mimics biological cell membranes.
30 and showed a slightly reduced rate of transport for the thiourea compounds – evidence that these species are functioning as carriers rather than forming channels (ESI Figures S29–31†). It is notable that the performance of the thiourea compounds in the POPC/cholesterol membrane is still excellent as this mixture more closely mimics biological cell membranes.
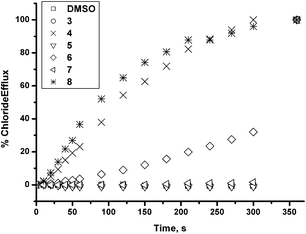 | ||
| Fig. 1 Chloride efflux promoted by 0.02 molar equiv. of receptors 3–8 from unilamellar POPC vesicles loaded with 489 mM NaCl buffered to pH 7.2 with sodium phosphate salts. The vesicles were dispersed in 489 mM NaNO3 buffered to pH 7.2 with 5 mM sodium phosphate salts. At the end of the experiment, detergent was added to lyse the vesicles and calibrate the ISE to 100% chloride release. Each point represents the average of three trials. | ||
| Compounds | EC50 at 270 s (Cl−/NO3−) | Initial rate of chloride release (Cl−/NO3−) % Cl− efflux/s at 2% carrier loading | EC50 at 270 s (Cl−/HCO3−) | Initial rate of chloride release (Cl−/HCO3−) % Cl− efflux/s at 2% carrier loading | clog P[a] | PSA/Å2[b] |
|---|---|---|---|---|---|---|
| a clog P calculated using Spartan '08 for Macintosh (Ghose-Crippen model). b Polar surface area (PSA) calculated using Spartan '08 for Macintosh. The receptors were minimised using AM1 semi-empirical methods with the two urea or thiourea NH groups parallel and the PSA and log P values calculated. In the case of the case of the indole containing species two conformations were minimised – one with the indole NH forming a convergent array with the urea NH groups and the other with the indole NH oriented towards the urea or thiourea O or S atom (hence a range of values for PSA are given). | ||||||
| 3 | — | — | — | — | 1.99 | 37.0 |
| 4 | 0.109% | 0.431 | 0.304% | 0.227 | 3.14 | 22.2 |
| 5 | — | — | — | — | 2.42 | 34.8 |
| 6 | 3.047% | 0.074 | 2.079% | 0.188 | 3.57 | 21.5 |
| 7 | — | — | — | — | 2.02 | 44.6–47.7 |
| 8 | 0.029% | 0.614 | 0.0356% | 0.386 | 3.16 | 31.3–35.5 |
We had previously shown that tren-based tris thioureas 1 and 2 were potent chloride/bicarbonate antiporters in POPC vesicles. We wished to compare the bicarbonate transport ability of the simpler mono-ureas and mono-thioureas 3–8 with the tren-based tris- ureas 1–2. Vesicles containing NaCl were prepared and suspended in a solution of Na2SO4 and a sample of receptor in DMSO solution was added to this suspension (2% molar carrier to lipid). The negligible release of chloride under these conditions rules out that these compounds function as NaCl or HCl co-transporters to a significant degree. A pulse of bicarbonate was added after 120 s to the external solution. If the compounds function as Cl−/HCO3− antiporters then efflux of chloride should be observed upon addition of the bicarbonate to the external solution. Once again no activity was observed with the urea compounds under these conditions (Fig. 2). However, both the butylthiourea 4 and phenylthiourea 6 release chloride from the vesicles under these conditions with the butylthiourea derivative 4 showing slightly faster release of chloride – however the difference in activity of these compounds is significantly less than was observed in the Cl−/NO3− antiport experiments. More active are the tris-thioureas 1 and 2 whilst the most active chloride/bicarbonate antiporter is the indolylthiourea 8. It should be noted that in the case of compounds 1 and 2 there are three thiourea groups per receptor and hence the concentration of thioureagroups in the experiments with 1 and 2 are three times higher than with compound 8.
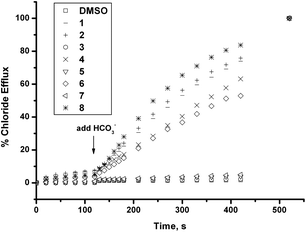 | ||
| Fig. 2 Chloride efflux promoted by 0.02 molar equiv. of receptors 1–8 from unilamellar POPC vesicles loaded with 489 mM NaCl buffered to pH 7.2 with 20 mM sodium phosphate salts upon addition of a NaHCO3 pulse to make the extravesicular bicarbonate concentration 40 mM. The vesicles were dispersed in 167 mM Na2SO4 buffered at pH 7.2 with 20 mM sodium phosphate salts. At the end of the experiment, detergent was added to lyse the vesicles and calibrate the ISE to 100% chloride release. Each point represents the average of 3 trials. | ||
The data shown in Fig. 2 provided compelling, yet indirect, evidence that the thioureas 4, 6 and 8 are able to promote the transport of bicarbonate across phospholipid membranes. We turned to 13C NMR spectroscopy to gain direct evidence that these thioureas 4, 6 and 8 facilitate transmembrane HCO3−/Cl− exchange (see Fig. 3 and ESI†).6 In these NMR experiments we monitored bicarbonate efflux from vesicles loaded with H13CO3− after the addition of compounds 3–8. EYPC vesicles filled with H13CO3− and suspended in Na2SO4 solution were aged overnight at 4 °C. Two 13C NMR signals separated by about 1 ppm (δ ∼161 and ∼160 ppm) were observed, corresponding to signals for intravesicular and extravesicular H13CO3− respectively (Fig. 3). No leakage of H13CO3− from these vesicles occurred after addition of 50 mM NaCl. A DMSO solution of the transporters was then added to give ligand-to-lipid ratios of 4 mol % for 1–8 (see Fig. S91 in the ESI†) and 13C NMR spectra were recorded 5 min after addition of compounds 1–8. For an example of the activity of the thioureas as compared to the urea analogs, see Fig. 3. Thus, thiourea 8 promoted complete Cl−/H13CO3− exchange in 5 min, as confirmed by the observation of only the NMR signal for extravesicular H13CO3− (Fig. 3). Addition of Mn2+ broadened this sharp H13CO3− signal at δ 160 ppm into the baseline, confirming that all of the intravesicular H13CO3− had been exchanged into the extravesicular solution in the 5 min period. In marked contrast, after addition of the urea analogue 7 the separate signals for intravesicular and extravesicular H13CO3− remained relatively unchanged. Addition of Mn2+ to this control sample simply erased the smaller extravesicular H13CO3− signal, whereas the major intravesicular H13CO3− signal remained intact since the paramagnetic Mn2+ cannot cross the phospholipid membrane. This 13C NMR data in Fig. 3 was consistent with the ISE data in Fig. 2, which showed that thiourea 8 is a relatively potent anion transporter, whereas the urea analogue 7 is essentially inactive as an anion transporter.
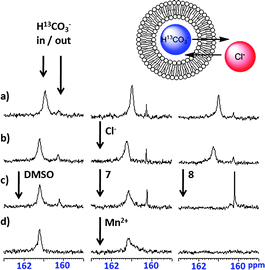 | ||
| Fig. 3 13C NMR evidence for H13CO3−/Cl− exchange promoted by 0.04 molar equiv. of thiourea transporter 8. a) before and b) after addition of a 50 mM NaCl pulse to EYPC vesicles containing 100 mM NaH13CO3 buffered to pH 7.4 with 20 mM HEPES, dispersed in 75 mM Na2SO4 buffered to pH 7.4 with 20 mM HEPES; c) 5 min after addition of a DMSO blank, urea 7, or thiourea 8; d) following addition of 0.5 mM MnCl2, a paramagnetic line broadening agent that only affects extravesicular bicarbonate. | ||
The EC50 values at 270 s were calculated for the thiourea compounds for chloride release in both the nitrate and bicarbonate antiport systems using Hill analysis (see ESI† for details). The results are shown in Table 2 together with the initial rates of chloride release for these compounds and are presented with parameters used in the absorption, distribution, metabolism, excretion and toxicity (ADMET) assessment in drug discovery21 specifically the calculated Ghose-Crippen log P values22 for compounds 3–8 and the polar surface area (PSA) of the compounds both calculated using the Spartan '08 computer program.23 We reasoned that the parameters that need to be optimised for a compound to be ‘drug-like’24 may correspond to those of an efficient carrier in that the compound would optimally possess both hydrophobic and hydrophilic properties to be capable of extracting an anion from aqueous solution and transporting it across a lipid bilayer. The results show that the thioureas have a higher clog P than the urea analogues and a lower polar surface area. The indolylurea and thiourea have the highest stability constants for bicarbonate from the members of this series and we hypothesise that the activity of the indolylthiourea compound 8 is in part due to a balance of affinity resulting from the geometry and number of hydrogen bond donors in the binding site, higher log P and moderate PSA from amongst this series of compounds. The low EC50 values for compound 8 prompted us to study the chloride transport properties of this receptor at a range of different transporter:lipid loadings in a Cl−/NO3− system. The results show chloride transport activity down to a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25000 transporter:lipid – a remarkable result for such a simple compound not pre-incorporated into the lipid bilayer membrane (Fig. 4).
25000 transporter:lipid – a remarkable result for such a simple compound not pre-incorporated into the lipid bilayer membrane (Fig. 4).
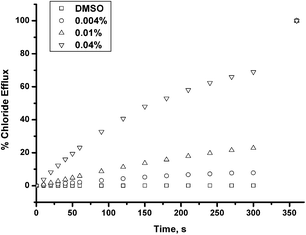 | ||
| Fig. 4 Chloride efflux promoted by 0.00004, 0.0001, and 0.0004 molar equiv. of receptor 8 from unilamellar POPC vesicles loaded with 489 mM NaCl buffered to pH 7.2 with sodium phosphate salts. The vesicles were dispersed in 489 mM NaNO3 buffered to pH 7.2 with 5 mM sodium phosphate salts. At the end of the experiment, detergent was added to lyse the vesicles and calibrate the ISE to 100% chloride release. Each point represents the average of three trials. | ||
We also prepared a series of amides and thioamides with analogous structures to the series of ureas and thioureas. The transport ability of these compounds (9–14) was studied under the same conditions as the ureas and thioureas for chloride/nitrate antiport. The results (Fig. 5) show a much diminished chloride transport ability for thioamides 10 and 14 as compared to thioureas 4 and 8.
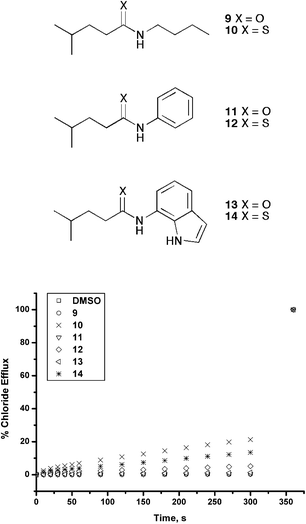 | ||
| Fig. 5 Chloride efflux promoted by 0.02 molar equiv. of receptors 9–14 from unilamellar POPC vesicles loaded with 489 mM NaCl buffered to pH 7.2 with sodium phosphate salts. The vesicles were dispersed in 489 mM NaNO3 buffered to pH 7.2 with 5 mM sodium phosphate salts. At the end of the experiment, detergent was added to lyse the vesicles and calibrate the ISE to 100% chloride release. Each point represents the average of three trials. | ||
Conclusion
A series of simple ureas and thioureas have been synthesised and their anion transport properties studied by a combination of ion-selective electrode and 13C NMR techniques. The results show that very simple thiourea compounds such as 4, 6 and 8 are capable of chloride/bicarbonate antiport with compounds 4 and 8 possessing the highest transport efficiency. Analogous amides and thioamides have greatly diminished anion transport abilities. The ‘champion’ of this series, compound 8, combines a higher clog P and lower polar surface area than its urea analogue, which is inactive as a chloride/bicarbonate antiport agent, with a convergent array of three hydrogen bond donors. This results in remarkably potent anion transport properties for such a simple compound. The goal of using small molecules as treatments for diseases caused by misregulation of chloride transport, such as cystic fibrosis, will be brought closer when compounds designed as membrane transporters have optimised ADMET properties. Conversely by optimising ADMET properties we may at the same time improve the anion transport properties of these species (with the caveat that unlike small molecule drugs, we wish the compound to remain predominantly in the membrane).Notes and references
- J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC; P. R. Brotherhood and A. P. Davis, Chem. Soc. Rev., 2010, 39, 3633–3647 RSC; A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348–357 RSC.
- F. M. Ashcroft, Ion Channels and Disease, Academic Press, San Diego and London, 2000 Search PubMed.
- J. Y. Choi, D. Muallem, K. Kiselyov, M. G. Lee, P. J. Thomas and S. Muallem, Nature, 2001, 410, 94–97 CrossRef CAS.
- A. V. Rouselle and D. Heymann, Bone, 2002, 30, 533–540 CrossRef CAS; D. Bok, G. Galbraith, I. Lopez, M. Woodruff, S. Nusinowitz, H. BeltrandelRio, W. Huang, S. Zhao, R. Geske, C. Montgomery, I. Van Sligtenhorst, C. Friddle, K. Platt, M. J. Sparks, A. Pushkin, N. Abuladze, A. Ishiyama, R. Dukkipati, W. Liu and I. Kurtz, Nat. Genet., 2003, 34, 313–319 CrossRef CAS; R. D. Vaughan-Jones, K. W. Spitzer and P. Swietach, J. Mol. Cell. Cardiol., 2009, 46, 318–331 CrossRef CAS.
- A. V. Koulov, T. N. Lambert, R. Shukla, M. Jain, J. M. Boon, B. D. Smith, H. Y. Li, D. N. Sheppard, J. B. Joos, J. P. Clare and A. P. Davis, Angew. Chem., Int. Ed., 2003, 42, 4931–4933 CrossRef CAS; P. A. Gale, M. E. Light, B. McNally, K. Navakhun, K. E. Sliwinski and B. D. Smith, Chem. Commun., 2005, 3773–3775 RSC; J. L. Sessler, L. R. Eller, W. S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989–5992 CrossRef CAS; P. A. Gale, J. Garric, M. E. Light, B. A. McNally and B. D. Smith, Chem. Commun., 2007, 1736–1738 RSC; R. I. Sáez Díaz, J. Regourd, P. V. Santacroce, J. T. Davis, D. L. Jakeman and A. Thompson, Chem. Commun., 2007, 2701–2703 RSC; P. V. Santacroce, J. T. Davis, M. E. Light, P. A. Gale, J. C. Iglesias-Sánchez, P. Prados and R. Quesada, J. Am. Chem. Soc., 2007, 129, 1886–1887 CrossRef; C. C. Tong, R. Quesada, J. L. Sessler and P. A. Gale, Chem. Commun., 2008, 6321–6323 RSC; A. Perez-Velasco, V. Gorteau and S. Matile, Angew. Chem., Int. Ed., 2008, 47, 921–923 CrossRef CAS; M. G. Fisher, P. A. Gale, J. R. Hiscock, M. B. Hursthouse, M. E. Light, F. P. Schmidtchen and C. C. Tong, Chem. Commun., 2009, 3017–3019 RSC; A. Hennig, L. Fischer, G. Guichard and S. Matile, J. Am. Chem. Soc., 2009, 131, 16889–16895 CrossRef CAS; C. R. Yamnitz, S. Negin, I. A. Carasel, R. K. Winter and G. W. Gokel, Chem. Commun., 2010, 46, 2838–2840 RSC; L. W. Judd and A. P. Davis, Chem. Commun., 2010, 46, 2227–2229 RSC; M. Yano, C. C. Tong, M. E. Light, F. P. Schmidtchen and P. A. Gale, Org. Biomol. Chem., 2010, 8, 4356–4363 RSC; S. J. Moore, M. G. Fisher, M. Yano, C. C. Tong and P. A. Gale, Chem. Commun., 2010 10.1039/c0cc04430j; R. E. Dawson, A. Hennig, D. P. Welmann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nature Chem., 2010, 2, 533–538 CrossRef CAS.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sánchez, T. Torroba and R. Quesada, Nat. Chem., 2009, 1, 138–144 CrossRef CAS.
- P. A. Gale, C. C. Tong, C. J. E. Haynes, O. Adeosun., D. E. Gross, E. Karnas, E. Sedenberg, R. Quesada and J. L. Sessler, J. Am. Chem. Soc., 2010, 132, 3240–3241 CrossRef CAS.
- For recent reviews see: V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 Search PubMed; A.-F. Li, J.-H. Wang, F. Wang and Y.-B. Jiang, Chem. Soc. Rev., 2010, 39, 3729–3745 RSC . For our contributions see: S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2005, 4696–4698 RSC; S. J. Brooks, P. R. Edwards, P. A. Gale and M. E. Light, New J. Chem., 2006, 30, 65–70 Search PubMed; S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2006, 4344–4346 RSC; S. J. Brooks, S. E. García-Garrido, M. E. Light, P. A. Cole and P. A. Gale, Chem.–Eur. J., 2007, 13, 3320–3329 RSC; S. E. García-Garrido, C. Caltagirone, M. E. Light and P. A. Gale, Chem. Commun., 2007, 1450–1452 RSC; C. Caltagirone, P. A. Gale, J. R. Hiscock, S. J. Brooks, M. B. Hursthouse and M. E. Light, Chem. Commun., 2008, 3007–3009 CrossRef.
- N. Busschaert, P. A. Gale, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and W. A. Harrell Jr, Chem. Commun., 2010, 46, 6252–6254 RSC.
- S. Valiyaveettil, J. F. L. Engbersen, W. Verboom and D. N. Reinhoudt, Angew. Chem., Int. Ed. Engl., 1993, 32, 900–901 CrossRef.
- R. Custelcean, B. A. Moyer and B. P. Hay, Chem. Commun., 2005, 5971–5973 RSC; R. Custelcean, P. Remy, P. V. Bonnesen, D.-e. Jiang and B. A. Moyer, Angew. Chem., Int. Ed., 2008, 47, 1866–1870 CrossRef; R. Custelcean and P. Remy, Cryst. Growth Des., 2009, 9, 1985–1989 CrossRef CAS; R. Custelcean, A. Bock and B. A. Moyer, J. Am. Chem. Soc., 2010, 132, 7177–7185 CrossRef CAS See also the work of: F. Y. Zhuge, B. A. Wu, J. J. Liang, J. Yang, Y. Y. Liu, C. D. Jia, C. Janiak, N. Tang and X. J. Yang, Inorg. Chem., 2009, 48, 10249–10256 Search PubMed.
- P. S. Lakshminarayanan, I. Ravikumar, E. Suresh and P. Ghosh, Chem. Commun., 2007, 5214–5216 RSC; I. Ravikumar, P. S. Lakshminarayanan, M. Arunachalam, E. Suresh and P. Ghosh, Dalton Trans., 2009, 4160–4168 RSC; I. Ravikumar and P. Ghosh, Chem. Commun., 2010, 46, 1082–1084 RSC.
- K. J. Winstanley, S. J. Allen and D. K. Smith, Chem. Commun., 2009, 4299–4301 RSC.
- S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458–2459 CrossRef CAS.
- J. M. Boon and B. D. Smith, J. Am. Chem. Soc., 1999, 121, 11924–11925 CrossRef CAS; Y. Sasaki, R. Shukla and B. D. Smith, Org. Biomol. Chem., 2004, 2, 214–219 RSC.
- B. A. McNally, A. V. Koulov, T. N. Lambert, B. D. Smith, J. B. Joos, A. L. Sisson, J. P. Clare, V. Sgarlata, L. W. Judd, G. Magro and A. P. Davis, Chem.–Eur. J., 2008, 14, 9599–9606 CrossRef CAS.
- B. A. McNally, E. J. O'Neill, A. Nguyen and B. D. Smith, J. Am. Chem. Soc., 2008, 130, 17274–17275 CrossRef CAS.
- For overviews see: J.-m. Suk, M. K. Chae, N.-K. Kim, U. -l Kim and K.-S. Jeong, Pure Appl. Chem., 2008, 80, 599–608 Search PubMed; P. A. Gale, Chem. Commun., 2008, 4525–4540 CrossRef CAS; M. J. Chmielewski, M. Charon and J. Jurczak, Org. Lett., 2004, 6, 3501–3504 RSC . For key examples see: P. Piatek, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 2004, 126, 16073–16076 CrossRef CAS; D. Curiel, A. Cowley and P. D. Beer, Chem. Commun., 2005, 236–238 Search PubMed; K.-J. Chang, D. Moon, M. S. Lah and K. S. Jeong, Angew. Chem., Int. Ed., 2005, 44, 7926–7929 CrossRef CAS; K.-J. Chang, B.-N. Kang, M.-H. Lee and K.-S. Jeong, J. Am. Chem. Soc., 2005, 127, 12214–12215 RSC; G. W. Bates, P. A. Gale and M. E. Light, Chem. Commun., 2007, 2121–2123 CrossRef CAS; G. W. Bates, Triyanti, M. E. Light, M. Albrecht and P. A. Gale, J. Org. Chem., 2007, 72, 8921–8927 CrossRef CAS; J.-m. Suk and K.-S. Jeong, J. Am. Chem. Soc., 2008, 130, 11868–11869 RSC; C. Caltagirone, P. A. Gale, J. R. Hiscock, S. J. Brooks, M. B. Hursthouse and M. E. Light, Chem. Commun., 2008, 3007–3009 CrossRef CAS; C. Caltagirone, J. R. Hiscock, M. B. Hursthouse, M. E. Light and P. A. Gale, Chem.–Eur. J., 2008, 14, 10236–10243 CrossRef CAS; D. Makuc, M. Lenarčič, G. W. Bates, P. A. Gale and J. Plavec, Org. Biomol. Chem., 2009, 7, 3505–3511 RSC; P. A. Gale, J. R. Hiscock, C. Z. Jie, M. B. Hursthouse and M. E. Light, Chem. Sci., 2010, 1, 215–220 CrossRef CAS; P. R. Edwards, J. R. Hiscock, P. A. Gale and M. E. Light, Org. Biomol. Chem., 2010, 8, 100–106 RSC; J. Kim, H. Juwarker, X. Liu, M. S. Lah and K. S. Jeong, Chem. Commun., 2010, 46, 764–766 RSC.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- B. D. Smith and T. N. Lambert, Chem. Commun., 2003, 2261–2268 RSC.
- Virtual ADMET Assessment in Target Selection and Maturation, ed. B. Tesla and L. Turski, IOS Press, Amsterdam 2006 Search PubMed.
- A. K. Ghose, A. Pritchett and G. M. Crippen, J. Comput. Chem., 1988, 9, 80–90 CrossRef CAS.
- Spartan '08 for Macintosh, Wavefunction Inc. Irvine, CA, USA 2008 Search PubMed.
- An Introduction to Medicinal Chemistry, G. L. Patrick, Third Edition, Oxford University Press, Oxford 2005 Search PubMed; Drug-like Properties: Concepts, Structure Design and Methods, E. H. Kerns and L. Di, Academic Press, Burlington, 2008 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, anion transport studies, crystal structures of compounds 5 and 8. CCDC reference numbers 791977 & 793790. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00503g |
| ‡ Present address: Department of Chemistry, Regis University, 3333 Regis Blvd, Denver, Colorado, USA 80221-1099. |
| This journal is © The Royal Society of Chemistry 2011 |