Efficient covalent functionalisation of carbon nanotubes: the use of “click chemistry”
Guillaume
Clavé
and
Stéphane
Campidelli
*
CEA, IRAMIS, Laboratoire d'Electronique Moléculaire, SPEC (URA-2464), CEA Saclay, 91191, Gif sur Yvette, France. E-mail: stephane.campidelli@cea.fr; Fax: +33 (0)1 69 08 66 40; Tel: +33 (0)1 69 08 88 77
First published on 12th July 2011
Abstract
Amongst the different classes of nanomaterials, carbon nanotubes (CNTs) are extremely promising for applications in electronics, solar energy conversion, materials science and medicinal chemistry. However in order to use them for those applications an accurate control of the functionalisation of the nanotubes is required. Many reactions can be performed on the π-conjugated framework of CNTs (i.e. cycloadditions, radical additions or halogenations). These reactions, although very efficient, require, in general, experimental conditions which are weakly compatible with sensitive organic or biological compounds. The emergence of new or re-actualised synthetic methods (known under the term of “click chemistry”) has the potential to provide an elegant protocol to prepare carbon nanotube-based functional materials. This review will gather the recent results described in the literature using “click chemistry” to functionalise carbon nanotubes.
Introduction
The last twenty years have seen the emergence of nanoscience. This new field of research consists of the manipulation of matter at the atomic or molecular scale to create innovative materials. Carbon nanotubes (Single and Multi Walled Carbon Nanotubes: SWNTs and MWNTs) are among the most studied nano-objects because of their exceptional mechanical, optical and electronic properties. They are promising candidates for the realisation of composite materials,1,2 electronic devices and sensors,3–5 for energy conversion6–10 and for biomedical applications.11–13The major drawback of carbon nanotubes is their poor solubility in organic solvents or in aqueous media which makes their manipulation and application development difficult. In order to solve this problem, two methods of functionalisation have been developed: (i) the covalent functionalisation via chemical grafting of molecules onto the sp2 carbon atoms of the π-conjugated skeleton of the CNTs;14 (ii) the non-covalent functionalisation based on the adsorption of polycyclic aromatic compounds or surfactants via π-stacking and/or hydrophobic interactions or by the wrapping of various functional polymers around the nanotubes.15–18 Each method possesses advantages and disadvantages, and consequently, it must be carefully chosen depending on the final application.
The fabrication of nanotube-based functional materials is also limited because of the difficulty to incorporate highly engineered molecules onto the nanotube surfaces. This issue can be due to the incompatibility between the functionality of the molecules to be added and the reaction conditions required for nanotube functionalisation. Moreover, reactions on CNTs require a large excess of reagent which is difficult or impossible to recycle. Therefore there is a real need for original chemical reactions allowing the functionalisation of CNTs in an efficient, simple and reproducible way.
The past decade has seen the emergence of “click chemistry”; the term “click chemistry” defines a series of chemical reactions respecting several criteria announced in 2001 by Sharpless.19 The click reactions require simple experimental conditions, exhibiting a certain modularity, a wide scope of applications, give high yields, must generate only inoffensive by-products, be stereospecific, and be compatible with ambient condition reactions and aqueous media. Several well known reactions meet these criteria and among them oxime and hydrazone ligation20 or thiol-based 1,4-Michael addition21 have been extensively used for biomolecule modifications. Other reactions are included in the “click reactions” family, in particular, the cycloaddition reactions like Diels–Alder,22 tetrazine cycloaddition,23,24 photoclick reaction25,26 and the famous alkyne-azide 1,3-dipolar cycloaddition.27,28 The latter is the most popular reaction of “click chemistry”; the Cu(I)-catalysed azide-alkyne [3 + 2] cycloaddition (CuAAC), described simultaneously by Meldal29 and Sharpless,30 is a catalysed variation of the Huisgen 1,3-dipolar cycloaddition.31 The use of a copper(I) catalyst permits the selective access to the 1,4-regioisomer of the triazole; it allows also to perform the reaction at room temperature. The detail of the reaction mechanism is not fully understood yet, however it is well described in the recent reviews of Fokin27 and Meldal.32 The practical role and the mechanistic implications of the stabilising ligand for CuI species was discussed by Presolski et al.33 The success of the CuAAC in the past 10 years is due to the easy access of azide and alkyne derivatives from many organic or biological compounds. For bio-organic chemistry applications, the reagents of the CuAAC reaction have the important advantage of being fully bio-orthogonal regarding all the chemical functional groups present in living organisms.34,35 In addition, the 1,4-triazole ring acting as a rigid linking unit between two amino-acid can mimic the atom placement and electronic properties of a peptide bond without the same susceptibility to hydrolysis.36,37
Most of the work reported on the modification of CNTs using “click chemistry” has been performed using the CuAAC reaction and only a limited number of publications described other approaches. In this mini-review, we will try to give an exhaustive overview of the functionalisation of carbon nanotubes using “click chemistry”. It should be mentioned that “click chemistry” cannot be performed directly on carbon nanotubes due to the poor reactivity of the sp2 framework. Indeed it requires one or more chemical steps before the key reaction in order to introduce the reactive groups onto the nanotubes. This may be a limitation of this method.
Functionalisation of CNTs using Cu(I) catalyzed Huisgen [3 + 2] cycloaddition as a key step
The reaction conditions (catalytic system and temperature for the CuAAC) as well as the principal characterisation techniques used in the works discussed below are summarised in Table 1.| Hybrids | Coupling conditionsa | Characterisationb | Ref |
|---|---|---|---|
| a DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; NaAsc: sodium ascorbate; THPTA: tris-(hydroxypropyltriazolylmethyl)amine; DIPEA: diisopropylethylamine; Asc: ascorbic acid; PMDETA: N,N,N′,N′′,N′′-pentamethyldiethylenetriamine; rt: room temperature. b Abs, Em: absorption and emission spectroscopy; Magn: magnetic characterisation. | |||
| SWNT-PSS | CuI/DBU, 60 °C | IR, Raman, TGA, TEM, AFM | 38 |
| MWNT-SEBS | CuI/DBU, 60 °C | FT-IR, Raman, XPS, SEM, TEM | 41 |
| MWNT-PMA-PNIPAM | CuSO4/NaAsc, 30 or 55 °C | FT-IR, TGA, SEM, TEM | 42 |
| NT-polyacrylate | CuBr/PMDETA rt | FT-IR, Raman, XPS, TGA, SEM, TEM | 43 |
| MWNT-polyacrylate LbL | CuBr/PMDETA rt | FT-IR, XPS, TGA, SEM, TEM | 44 |
| SWNT-polyurethane | CuI/DBU, 60 °C | FT-IR, Raman, TGA, SEM, TEM | 45 |
| MWNTs-polycaprolactone | CuI/DBU, 60 °C | FT-IR, Raman, TGA, SEM, TEM | 48 |
| SWNT-ZnPc | CuSO4/NaAsc, rt | Raman, Abs/Em, TGA, AFM, SEM | 53 |
| SWNT-dendron porph/phthalo | Cu(MeCN)4PF6 2,6-lutidine, THPTA, rt | Raman, XPS, Abs/Em, TGA, AFM, SEM | 54,55 |
| SWNT-β-CD | CuI/DBU, 70 °C | FT-IR, Raman, Em, TEM | 56 |
| SWNT-aminoacids | CuI, Asc, DIPEA, rt then 80 °C | FT-IR, Raman, TEM | 59 |
| SWNT-AuNPs | CuSO4/NaAsc | Raman, Abs, TEM | 66,67 |
| SWNT-AuNPs | CuSO4/NaAsc, rt | FT-IR, Raman, EDX, TEM | 68 |
| SWNT-Fe2O3 | CuBr/PMDETA rt | Raman, Em, TGA, TEM, Magn | 69 |
| MWNT-MSNs | CuSO4/NaAsc, rt | FT-IR, SEM | 70 |
| NT-polymer-Pd | CuBr/PMDETA rt | FT-IR, XPS, EDX, TGA | 71 |
| MWNT-sulfonate | CuSO4/NaAsc, rt | FT-IR, XPS, TGA | 72 |
Functionalisation of CNTs with polymers
In 2005 the group of Adronov reported for the first time the fabrication of SWNT-polystyrene (PS) hybrids using the CuAAC reaction (Scheme 1).38 To this end, SWNTs were first functionalised with a phenyl diazonium generated in situ from p-aminophenyl propargyl ether and isoamyl nitrite. The reaction was performed using the solvent-free procedure described by Tour and co-workers.39 Independently, several polystyrenes of various molecular weight were synthesised via Atom Transfer Radical Polymerisation (ATRP) and transformed to azido-polymers by reaction with sodium azide. The nanotubes modified with acetylene moieties and the azido-polystyrenes were finally assembled via the CuAAC reaction. Different reaction conditions (i.e. catalytic system and polymer length) were tested to determine the most efficient system for the solubilisation of the composite. It appeared that the CuI/DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) catalytic system allowed the reaction to process at room temperature or under very mild conditions (60 °C) and preserved the grafting of a large amount of polymers on the nanotubes. Note that the length of the polymers influenced the coupling efficiency. For long polymer chains the solubilising effect of the polymer was more important but as a drawback the azide group was less accessible for the reaction.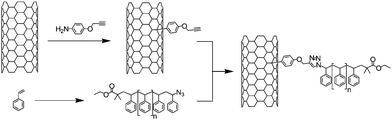 | ||
| Scheme 1 Synthesis of SWNT-polystyrene composite by reaction of 4-propargyloxyphenyl-SWNT and azido-polystyrene via CuAAC. | ||
The best results in terms of solubilisation were obtained with a polystyrene with an average molar mass (Mn) of 4 800 g mol−1. The organo-soluble SWNT-polystyrene composite materials were characterised by infrared (IR) and Raman spectroscopy, thermogravimetry (TGA), transmission electron microscopy (TEM) and atomic force microscopy (AFM). In particular, IR spectra of diazonium-functionalised nanotubes showed the presence of the alkyne stretch at ca 2100 cm−1 and its disappearance after the CuAAC. TGA allowed the estimation of the amount of polymer grafted on the nanotube surfaces.
A few years later, the same group reported the water solubilisation of the SWNT-PS hybrids through direct sulfonation of the grafted polystyrene chains.40 Indeed they used acetyl sulfate in dry chloroform to react with the aromatic part of the polystyrene already linked to SWNTs. The modest degree of functionalisation (from 20 to 34 mol%) was sufficient to induce the water solubility which was the main goal.
In a similar way, Yadav et al.41 functionalised MWNTs with poly(styrene-b-(ethylene-co-butylene)-b-styrene) triblock copolymer (SEBS) using “click chemistry”. The MWNTs were derivatised via the addition reaction with 4-propargyloxybenzene diazonium. Unlike the previous work, here the azide groups were not located at the extremity of the polymers but were introduced chemically on the styrene moieties of the copolymer. The MWNT-SEBS composites were characterised by FT-IR, Raman, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and TEM. The functionalised MWNTs gave good dispersion in the SEBS matrix, and the resulting composites showed a net improvement in the mechanical properties and in the electrical conductivity of the polymer as the amount of nanotubes increased in the mixture.
Liu et al.42 synthesised MWNT composites containing a thermoresponsive diblock poly(N,N-dimethylacrylamide)-poly(N-isopropylacrylamide) (PMA-PNIPAM) copolymer. The copolymer was prepared by Reversible Addition-Fragmentation Transfer (RAFT) polymerisation starting from a thiocarbonylthio-based RAFT agent modified with an azide group. The alkyne moieties were introduced on hydroxylated MWNTs via the sequential reaction of toluene diisocyanate with the hydroxyl of the nanotubes and propargyl alcohol (Scheme 2). Poly(N,N-dimethylacrylamide) and poly(N,N-dimethylacrylamide)-poly(N-isopropylacrylamide) copolymer were attached to the nanotubes thanks to the Huisgen cycloaddition performed in the presence of CuSO4 and sodium ascorbate. The PMA-PNIPAM copolymer was able to form micelles in aqueous solution around MWNTs, facilitating the reaction and then improving the amount of grafted material on the nanotubes. The composites were characterised by the combination of TGA, SEM, TEM and FT-IR. This last technique permitted the monitoring of the introduction and consumption of the alkyne groups on the MWNTs and TGA allowed the evaluation of the amount of polymer grafted on the nanotubes.
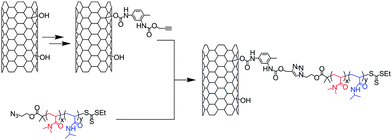 | ||
| Scheme 2 Synthesis of MWNT composites containing poly(N,N-dimethylacrylamide)-poly(N-isopropylacrylamide) diblock copolymer. Hydroxylated MWNT were modified via the sequential reaction of the nanotube with toluene diisocyanate and propargyl alcohol. | ||
Recently Gao and co-workers described the elaboration of carbon nanotube–polymer composites by the combination of two methods: CuAAC and ATRP.43 The first step consisted of introducing the triple bonds onto the carbon nanotubes. It was done using two different strategies depending on the nature of the nanotubes. For MWNTS, acetylenic groups were introduced by esterification of propargyl alcohol on carboxylic functional groups activated by SOCl2; for SWNTs, the nanotubes were functionalised via the 1,3-dipolar cycloaddition of 2-azidoethanol on the sidewall and then a carboxylic acid containing a propargyl group was introduced by esterification. In a second step, a polymethacrylate containing both azide and 2-bromoisobutyryl moieties were attached to the nanotubes using the CuAAC in the presence of CuBr and N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA). The polymer played the role of a clickable macroinitiator on which subsequent reactions could take place. As a proof of concept, poly(n-butyl methacrylate), polystyrene, and poly(ethylene glycol) were grown or attached on CNTs either via the ATRP grafting from approach (for polymethacrylate and polystyrene) or via CuAAC grafting to approach (for poly(ethylene glycol)) (see Fig. 1 for details). It is worth mentioning that the CuBr/PMDETA catalytic system is the same for CuAAC and ATRP. The nanotube derivatives were fully characterised by FT-IR, Raman, XPS spectroscopy, TGA, TEM and SEM. The authors used XPS and TGA to calculate the content of bromine and nitrogen in their composites in order to estimate the amount of polymer grafted on the nanotubes.
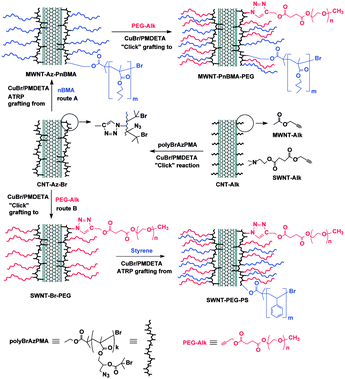 | ||
| Fig. 1 Fabrication of carbon nanotube composites by a combination of “click chemistry” and ATRP. Carbon nanotubes were first modified with acetylene groups and then a polymethacrylate containing both azide and 2-bromoisobutyryl moieties was attached via CuAAC (center of the Figure). The nanotube composites were used as initiators for subsequent grafting to (click chemistry) and grafting from (ATRP) reactions. Reproduced from ref. 43 with permission. | ||
The year after their initial work, the same group reported the construction of MWNT-polymer composites using the Layer by Layer (LbL) technique.44 The carboxylic groups of oxidised MWNTs were esterified with propargyl alcohol and a polymethacrylate containing azide groups was introduced via CuAAC. Then a second layer of polymethacrylate containing pendant acetylene groups was attached on the composite material. Up to three layers of polymer were introduced, giving rise to a clickable platform based on MWNT functional polymers (Scheme 3). The composites were characterised by the combination of TGA, FT-IR, XPS, SEM and TEM. The efficiency of the reaction was confirmed by the TGA: 49.5% and 66.8% weight losses were observed for the composites containing two and three layers of polymer, respectively. To probe the reactivity of their nanotube derivatives, the authors introduced a functional dye (i.e., an alkyne-modified rhodamine B) or monoalkyne-terminated polystyrene at the periphery of their nano-assemblies. The presence of the rhodamine in the MWNT-composite was confirmed by fluorescence and confocal laser scanning microscopy.
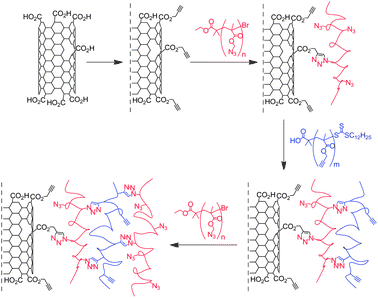 | ||
| Scheme 3 Layer by Layer fabrication of MWNT-polymethacrylate clickable platform. MWNTs were modified with propargyl functional groups and a polymethacrylate containing azide groups (in red) was attached. Then a second layer of polymethacrylate containing pendant acetylene groups (in blue) was introduced on the composite material. Up to three layers of polymer were combined to the nanotubes. | ||
Rana et al.45 reported the synthesis of polyurethane-grafted carbon nanotubes by the coupling of alkyne-functionalised SWNTs with azide-containing polyurethane. The alkyne functional groups were introduced through the addition of 4-propargyloxybenzenediazonium generated in situ. After the [3 + 2]-cycloaddition, the nanotube-polyurethane composites were characterised by FT-IR, Raman spectroscopy, TEM and SEM. The use of CNTs has enormous potential in tissue engineering.46,47 Because of their electrical and mechanical properties, carbon nanotubes can be used for structural reinforcement or as a conductive substrate for neural growth/regeneration. For this kind of application, one has to be sure that the nanotubes are not toxic and do not provoke an immune response. Therefore, the coupling of biocompatible moieties on carbon nanotubes may be of particular interest. To address this particular point, and similarly to their previous work, Rana et al. reported the combination of a biocompatible poly(ε-caprolactone) with MWNTs via CuAAC.48 The nanotubes were functionalised with 4-propargyloxybenzene moieties while the polymer was generated by ring opening co-polymerisation of ε-caprolactone and α-chloro-ε-caprolactone. The azide groups were introduced by nucleophilic substitution of the chlorine atoms along the polymer chain. Finally, the crucial coupling step was performed in the presence of CuI/DBU (Scheme 4). TGA provided an estimation of the amount of polymer (up to 52%) in the composite and electron microscopy (SEM and TEM) demonstrated a homogeneous coating of the nanotubes by the poly(ε-caprolactone). The authors also monitored the disappearance of the azide vibration at around 2100 cm−1 in the polymer by FT-IR.
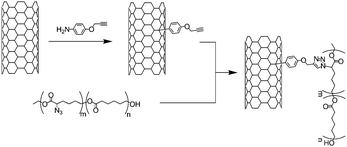 | ||
| Scheme 4 Functionalisation of MWNTs with poly(ε-caprolactone) bearing azide groups. The polymer was synthesised by ring opening co-polymerisation of ε-caprolactone and α-chloro-ε-caprolactone followed by the substitution of the chlorine atoms. | ||
Functionalisation of CNTs with chromophores
Photo-induced processes between SWNTs and photoactive materials have been extensively studied to elaborate optoelectronic devices for sensing or photovoltaic applications. In particular, the combination of dyes with nanotubes allows the fabrication of new functional materials possessing the ability to massively absorb photons to transfer charges and, consequentially, generate photocurrent.7,8 Phthalocyanines (Pc) are synthetic porphyrin analogs, they are attractive molecules for optoelectronic applications and several examples in literature have reported their combination with carbon nanotubes.49–52 However, like many molecules, the fabrication of such chromophores requires several synthetic steps and they are therefore quite expensive to produce. We demonstrated that CuAAC can provide an elegant solution to functionalise SWNTs with such molecules.53 We first synthesised nanotubes bearing phenylacetylene groups by reaction of SWNTs with 4-(2-trimethylsilyl)ethynylaniline in the presence of isoamyl nitrite according a slightly modified literature procedure.39 The diazonium derived from the 4-(2-trimethylsilyl)ethynylaniline was formed in situ and allowed to react with the nanotubes. After the deprotection of the triple bonds with tetrabutylammonium fluoride, a zinc-phthalocyanine bearing an azide group was attached on the nanotubes via the modified Huisgen cycloaddition reaction performed in the presence of CuSO4 and sodium ascorbate. The resulting hybrid materials were characterised by Raman spectroscopy, AFM, SEM and TGA. Raman was used to demonstrate the successful attachment of the phenylacetylene and the presence of the chromophores on the nanotube sidewalls, while TGA permitted the determination of the number of functional groups on the SWNT surface.The photophysical properties of the SWNT-ZnPc conjugates were tested in a series of photophysical measurements. A photoinduced communication between the two photoactive components (i.e., SWNT and ZnPc) was identified. Such beneficial features led us to incorporate the SWNT-ZnPc as photoactive material in an ITO photoanode in a photoelectrochemical cell (Fig. 2). Upon illumination stable and reproducible photocurrents with monochromatic IPCE values as large as 17.3% were obtained.
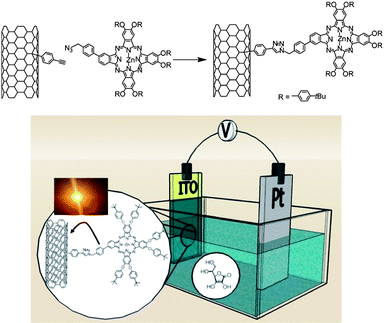 | ||
| Fig. 2 Synthesis of SWNT-ZnPc conjugates (upper part) and schematic representation of the photoelectrochemical cell used for the characterisation of the photovoltaic properties of the nanotube conjugate (lower part). | ||
In order to increase the light harvesting efficiency of the chromophores on the nanotube surfaces, we recently reported on the functionalisation of SWNT with various dendrons containing two zinc-porphyrins (ZnP), two zinc-phthalocyanines (ZnPc), or a dendron containing one ZnP and one ZnPc (Scheme 5).54,55 The dendrons and the nanotube conjugates were synthesised using the CuAAC reaction in the presence of Cu(MeCN)4PF6, 2,6-lutidine and tris-(hydroxypropyltriazolylmethyl)amine (THPTA) as a catalytic system.
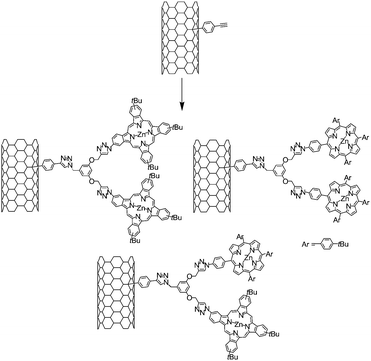 | ||
| Scheme 5 Synthetic pathway for the fabrication of SWNT-ZnPc, SWNT-ZnP and SWNT-ZnP/ZnPc first generation dendrons. | ||
The conjugates were fully characterised and the signals of the ZnP and ZnPc on the nanotubes were detected by absorption, emission, Raman and XPS spectroscopy. As in the previous case, Raman spectroscopy was of great help to prove the covalent functionalisation of the nanotubes and the successful introduction of the chromophores. Moreover, careful examination of high resolution N1S XPS spectra of the conjugates allowed us to identify the signal of the triazole ring, unambiguously confirming the covalent linkage between the nanotubes and the chromophores. Upon illumination, strong electronic interactions between the photo- and electroactive constituents (i.e., SWNT and ZnP/ZnPc) were observed, leading to rapid excited-state deactivation of the chromophores in the presence of the nanotubes.
Functionalisation of CNTs with biomolecules
The CuAAC has been met with large success in the field of bio-conjugation because of its mild reaction conditions, and, more importantly, because of its bio-orthogonality regarding all the chemical functional groups present in biological fluids.34,35 Using CuAAC, Zheng and co-workers reported the conjugation of β-cyclodextrin (β-CD) with carbon nanotubes (Scheme 6).56 Cyclodextrins are cyclic oligosaccharides which are able to encapsulate various organic and biological guests within their hydrophobic cavities in aqueous solution.57 Consequently the combination of β-CD with carbon nanotubes is expected to generate interesting objects for supramolecular chemistry, biomedicine, and nanodevice construction.17 Mono azido β-CD was obtained after mono tosylation followed by nucleophilic substitution by azide of β-CD according to the method described by Muderawan et al.58 while the SWNTs were functionalised with 4-propargyloxybenzene units.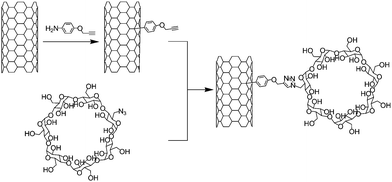 | ||
| Scheme 6 Synthesis of SWNT-β-cyclodextrin conjugates. The nanotubes were functionalised through the addition of 4-propargyloxybenzene diazonium followed by the introduction of mono azido cyclodextrin via the Cu-catalysed Huisgen reaction. | ||
The cyclodextrin and the nanotubes were then coupled together in the presence of the CuI/DBU catalytic system. The SWNT-β-CD hybrids were characterised by Raman, FT-IR spectroscopy and TEM. The hybrids displayed good water solubility and the first test on the inclusion complexation of quinine into the SWNT-β-CD artificial receptors showed promising results for drug delivery.
In the same way, the group of Cho described the functionalisation of SWNTs with azides derived from various amino acids (alanine, leucine, phenylalanine and valine).59 The CuAAC was performed in the presence of CuI, ascorbic acid and N,N-diisopropylethylamine. The functional nanotubes were characterised mainly by Raman and FT-IR spectroscopy. The authors observed an increase of the D-band, in Raman spectra, after the functionalisation of the SWNTs with 4-propargyloxybenzene moieties and the presence of the stretching bands of the triple bonds in IR spectra; the two IR signals (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and
C and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H) tended to disappear after the CuAAC coupling.
C–H) tended to disappear after the CuAAC coupling.
Functionalisation of CNTs with nanoparticles
Nanoparticles made with gold, palladium or iron oxide were found to be very promising materials for biomedical applications (diagnostic and therapy),60,61 in catalysis62,63 and for their plasmonic properties.64,65 The combination of nanoparticles with carbon nanotubes allows the formation of new materials combining the properties of the nanotubes with those of the nanocrystals. In 2007 and 2008 Rao and co-workers published the first examples of gold nanoparticle-SWNT conjugates made by CuAAC.66,67 The gold nanoparticles were capped with hex-5-yn-1-thiol while the carboxylic functional groups of oxidised SWNTs were coupled to 4-azidobutylamine (Scheme 7). The nanotube–nanoparticle hybrids were characterised by TEM.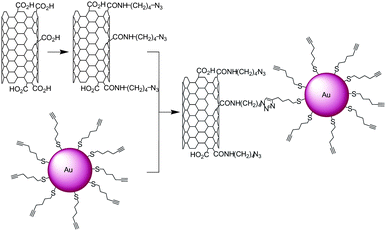 | ||
| Scheme 7 Synthesis of SWNT-Au nanoparticle conjugates. Azide groups were introduced on the nanotubes while the nanoparticles were modified with triple bonds. The two nano-objects were reacted together in the presence of CuSO4 and sodium ascorbate. | ||
Similar results were obtained by Rana et al.68 in 2009. The main difference concerned the way the gold colloids and the SWNTs were functionalised. Indeed the nanoparticles were modified with 11-azido-1-undecanethiol while the nanotubes were functionalised through diazonium addition starting from p-aminophenylpropargyl ether. The nanotubes and nanoparticles were assembled together through CuAAC performed in the presence of CuSO4 and sodium ascorbate. The SWNT derivatives were characterised by Raman, FT-IR, TEM and EDX (energy-dispersive X-ray spectroscopy). The infrared signal of the azide groups of the nanoparticles permitted the reaction progress to be followed and EDX revealed the qualitative composition of the nano-assemblies.
Iron oxide nanoparticles were “attached” by Gao and co-workers on their previously described “clickable” nanotube platforms.44 Fe3O4 magnetic nanoparticles coated with water soluble poly(acrylic acid) were modified either with azide or alkyne functional groups and then attached on MWNTs functionalised either with azide- or alkyne-containing polymers (Scheme 8).69 In both cases the Cu-catalysed Huisgen reaction was performed with CuBr/PMDETA catalytic system. The nanohybrids were characterised by TGA, Raman, and TEM. In addition, the magnetic properties of the MWNT-Fe3O4 as well as those of the iron oxide nanoparticles precursor were investigated; the nanohybrids exhibited superparamagnetic behaviour with a lower saturation magnetisation compared with that of pure magnetic nanoparticles.
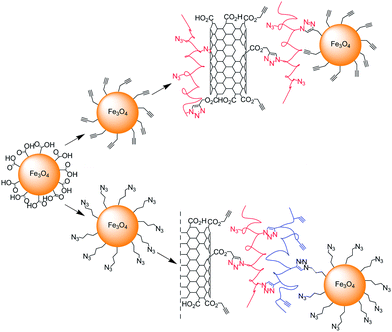 | ||
| Scheme 8 Grafting via CuAAC of magnetic nanoparticles on the “clickable” nanotube platforms described in Scheme 3. The iron oxide nanoparticles containing either alkyne or azide functionalities were attached on the azide or alkyne MWNT composites. | ||
Interestingly, the authors observed that the direct functionalisation of alkyne-modified MWNTs with azide-containing nanoparticles was not feasible. Therefore the use of MWNT-functional polymer composites is expected to open avenues for the facile fabrication of multifunctional hybrid compounds with tailor-made structures and properties.
Song et al.70 fabricated a copper ion detector using magnetic silica nanoparticles (MSNs) clicked onto MWNTs (Fig. 3). The sensing effect was based on the high peroxidase-like activity of MWNT-MSNs. Oxidised MWNT bearing acid functional groups were activated using N,N′-dicyclohexylcarbodiimide (DCC) and subsequently coupled to propargyl amine. MSN-N3 were obtained in two steps by the grafting of the (3-chloropropyl)trimethoxysilane on SiO2 nanoparticles followed by the substitution of the chlorine atoms by azido groups in DMF. In the presence of copper(II) and sodium ascorbate, the MWNTs and MSNs properly functionalised with alkyne and azide reacted together to give a MWNT-MSN hybrid possessing intrinsic peroxidase-like activity. After formation, the hybrid was removed from the reaction mixture thanks to the magnetic properties of the silica nanoparticles. In the absence of copper only MSNs were collected. After addition of H2O2 and tetramethylbenzidine (TMB) to the collected fractions, a fast coloured reaction occurred in the solution in the presence of the MWNT-MSN hybrid. The change could be monitored by the absorbance change at 652 nm, indirectly demonstrating the initial presence of Cu(II) in the solution.
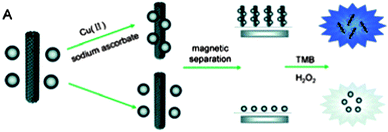 | ||
| Fig. 3 Schematic representation of the indirect detection of CuII based on the CuAAC between MWNTs and magnetic silica nanoparticles. Reproduced from ref. 70 with permission. | ||
For catalytic purposes, palladium nanoparticles were synthesised in MWNTs-polymer conjugates assembled by “click chemistry”.71 In this work, poly(glycidyl methacrylates) modified with azide groups were attached on nanotubes, functionalised with phenylacetylene moieties, via the CuAAC in the presence CuBr/PMDETA. The nanotube hybrids were used for in situ growth of 3 nm palladium nanoparticles and the resulting system was tested as catalyst for Suzuki coupling reactions. The CNT-polymer-Pd exhibited a good stability and a very satisfactory catalytic activity. The nanotube derivatives were characterised by the combination of FT-IR, XPS, EDX spectroscopy and TGA. Infrared spectroscopy demonstrated the presence of the azides and triple bonds in the two components (i.e. polymers and nanotubes, respectively) and their effective coupling during the reaction. Elemental analysis showed the presence of palladium in the hybrids.
A last example of nanotube functionalisation via CuAAC was reported recently by the group of Swager.72 SWNTs and MWNTs were first functionalised with propargyl groups using their own methology73 based on the 1,3-dipolar cycloaddition of an acetylenedicarboxylate derivative in the presence of N,N-dimethylaminopyridine and an alcohol (for this reaction dipropargyl acetylenedecarboxylate and propargyl alcohol were used). The alkyne-modified nanotubes and sodium 3-azidopropane-1-sulfonate were reacted together in the presence of CuSO4 and sodium ascorbate. The resulting water soluble materials were characterised by FT-IR, XPS and TGA. The interest in the work came from the fact that the sulfonate groups on the nanotubes could act as metal binding sites for Pd(II) and Cu(II). The MWNT hybrid materials were tested for catalytic Wacker-type olefin oxidation. In the catalytic process, the palladium(II) catalyst is first reduced to palladium(0) and then regenerated by oxidative electron transfer to copper(II).
From Table 1, one can draw some conclusions: i) in general Cu(I) catalysts are preferred to Cu(II). The two catalytic systems give positive results and the choice of the catalyst can depend on the solvent used for the reaction; for example in water CuSO4/NaAsc can be used very easily while in organic solvents Cu(I) in the presence of a nitrogen-containing ligand is preferred. It is worth to mention that some works have demonstrated that ATRP catalytic system (i.e. CuBr/PMDETA) is perfectly suitable for CuAAC. ii) the characterisation of the assemblies is mainly based on the combination of spectroscopic techniques like infrared and Raman; on one hand Raman spectroscopy, with the G-, D- and eventually RBM bands, gives important information on the nanotubes (structure, defects, metallic/semiconducting character, etc…) and on the other hand infrared spectroscopy allows the identification of the alkyne and azide functional groups (specific peaks at ca 2100 cm−1 for N3 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and at ca 3300 cm−1 for
C and at ca 3300 cm−1 for ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H) and their disappearance after the formation of the triazole rings. Other spectroscopic techniques, like EDX or XPS, permits the qualitative or quantitative analysis of the nanotube materials while microscopy (AFM, SEM; TEM) allows the morphological characterisation the nanotube assemblies.
C–H) and their disappearance after the formation of the triazole rings. Other spectroscopic techniques, like EDX or XPS, permits the qualitative or quantitative analysis of the nanotube materials while microscopy (AFM, SEM; TEM) allows the morphological characterisation the nanotube assemblies.
Functionalisation of CNTs using other reactions as a key step
In this section, we wanted to give an overview of the other “click chemistry” reactions which have been used to functionalise the nanotubes. Indeed, among the reaction listed as “click chemistry”, to the best of our knowledge, only the thiol-maleimide reaction (1,4-Michael addition)21 has been applied to nanotubes to fabricate functional materials.The first example of thiol-maleimide coupling on nanotubes was described in 2002 with the introduction of single stranded DNA on modified SWNTs.74 The strategy employed by the authors was to generate amino groups on CNTs thanks to chemical oxidation followed by reaction of the carboxylic groups with ethylene diamine. Then the maleimide moiety was introduced on the nanotubes using a reagent commercially available: the succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC). Then a 32-base single-stranded DNA containing a thiol in 5′ position was introduced on the maleimide (Scheme 9). The presence of DNA on the nanotubes was proven by hybridisation experiments using two different 16-mer oligonucleotides (fully complementary or with mismatches) containing a fluorophore.
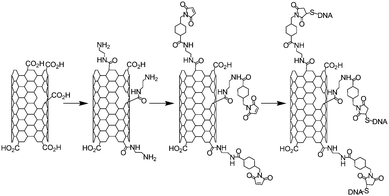 | ||
| Scheme 9 Synthetic reaction pathway for the elaboration of SWNT-DNA hybrids via the 1,4-Michael addition of thiol on maleimide. | ||
Holleitner and co-workers described the combination and the properties of SWNTs with photosystem I (PS I) proteins.75–77 PS I is a chlorophyll protein complex located in the membranes of chloroplasts and cyanobacteria. It has a cylindrical shape with a diameter of about 15 nm and a height of 9 nm. It is involved in electron transfer processes during photosynthesis. In the systems described by Holleitner, a cysteine mutation was introduced on the protein to enable the reaction with the maleimide-modified nanotubes. The maleimide groups were introduced by reaction of ethylene diamine-modified nanotubes with several commercial heterobifunctional linkers (sulfo-SMCC, sulfo-MBS) containing the maleimide unit at one end and an activated carboxylic functional group at the other end. Fig. 4 represents a biohydrid device obtained by on-chip functionalisation of a maleimide-modified nanotube with the PS I protein.77 The interest in thiol-maleimide coupling is due to the fact that the reaction occurs spontaneously without addition of catalyst or reagent. The resulting nanotube–protein biohybrids were characterised by AFM and their optoelectronic properties were tested. The maleimide-functionalised SWNTs deposited on the Si/SiO2 surface and connected to gold electrodes were reacted with PS I via thiol-maleimide coupling. Upon illumination, the nanohybrid-based devices exhibited a photoconductive gain effect due to the presence of the PS I. Indeed, in the presence of light, charge separations occurred in the protein. The electrons are transferred along the electron transfer chain of the reaction center leaving a hole at the oxidising side of the PSI which is coupled to the CNT surface.76,77
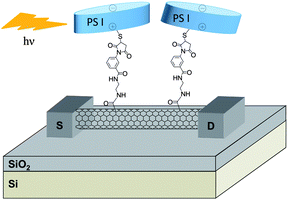 | ||
| Fig. 4 Schematic representation of the SWNT-PS I optoelectronic device. Upon illumination, an increase in the electrical response of the device corresponding to the absorption of the protein was observed. | ||
In 2003, the group of Prato combined maleimide-modified SWNTs with one or two peptides derived from the viral envelope protein VP1 of the foot-and-mouth disease virus (FMDV).78,79 The SWNTs were functionalised with primary amine via the 1,3-dipolar cycloaddition80 and then N-succinimidyl-3-maleimidopropionate was introduced either on the primary amine of the nanotubes or on lysine-functionalised nanotubes (Fig. 5).
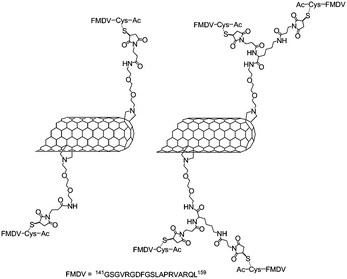 | ||
| Fig. 5 Representation of SWNTs functionalised with the peptide derived from the viral envelope protein VP1 of the FMDV. The nanotubes were first functionalised with maleimide groups and then the FMDV peptide containing a cysteine residue was introduced. | ||
The antigenicity of the FMDV peptide-SWNTs was proven by surface plasmon resonance analysis and enzyme-linked immunosorbent assay (ELISA).78 The results from both experiments suggested that the peptide bound to the SWNT support adopted the correct secondary conformation necessary for recognition by specific antibodies. Moreover, an in vivo study showed that the FMDV peptide-SWNTs were also immunogenic. High antibody titers were obtained after immunisation of mice, and the antibodies were able to neutralise the virus.79 This result highlighted the potential use of peptide-functionalised carbon nanotubes in vaccine delivery. In order to determine the cell penetration of functionalised nanotubes and their potential use for drug delivery, a fluorescent 13-mer peptide derived from the GS protein was linked to the SWNT-maleimide derivative.81 It was demonstrated that the functionalised SWNTs were able to cross the cell membrane and could accumulate in cells with concentration as high as 10 μM without significant cytotoxicity.
Baldacchini et al.82 reported the covalent linkage between SWNTs and yeast cytochrome C (YCC) redox proteins. The cytochrome was attached to maleimide-terminated nanotubes78 through the thiol group of a cysteine located in the region opposite to the lysine-rich protein docking site. The nanotube–maleimide derivatives were characterised by TGA and Raman spectroscopy. After coupling with the cytochrome, the biohybrids were characterised by tapping mode AFM and current sensing scanning probe techniques: i.e. conductive AFM (C-AFM) and scanning tunneling microscopy (STM). The current sensing near field experiments provided stable and high resolution single-molecule current images of protein-coated metallic SWNTs.
Finally, two nanotube polyfunctional hybrids, the first one containing a thiol-modified RGD ligands and an oligonucleotide and the second containing a (111In) radiometal chelate and the complementary oligonucleotide, were fabricated for radiotherapy purposes.83 Cyclic RGD peptide (arginine-glycine-aspartate) found in integrin proteins is responsible for cell adhesion with extracellular matrix (ECM); it was introduced on nanotubes using the maleimide/thiol Michael addition. To this end, the nanotubes were modified with pyrrolidine groups using the methodology described by Prato and co-workers80 and the maleimide was incorporated by the reaction of the functionalised nanotubes with succinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxy-[6-amidocaproate]. Then, the thiol-modified RGD ligands were introduced. The activity of the separated components (i.e. SWNT-RGD-oligonucletotide and SWNT-111In-oligonucletotide) were tested in vivo, and both demonstrated the expected activity.
Conclusion
Fabrication of innovative materials based on carbon nanotubes is very challenging. Since the covalent functionalisation of nanotubes often requires a large excess of reagents, it is unrealistic to perform the direct functionalisation with expensive and complex molecules. Thus, the introduction of small and inexpensive anchor molecules possessing reactive functional groups on CNTs and the realisation of the key coupling reaction that follows under mild conditions is a valuable strategy. The CuAAC reaction has been extensively used for the past 10 years in many fields of chemistry, from material science to bio-organic chemistry. Applied to carbon nanotubes, this reaction demonstrated its potential and versatility. The 1,4-Michael addition was also successfully used to functionalise carbon nanotubes, but the sensitivity of the reactive functional groups (thiol and maleimide groups are subject to dimerisation/oxidation and nucleophilic attack, respectively) makes this reaction less convenient than the CuAAC.Other carbon-based nanomaterials like carbon onions,84 graphite and carbon electrodes,85 carbon nanofibers86 and also graphene87 or oxidised graphene88–91 have been functionalised using CuAAC. Due to the recent and considerable development of graphene in literature, we can assume that CuAAC will also play a major role in the functionalisation of this material.
Acknowledgements
GC acknowledges the ANR (Agence National de la Recherche) project f-DNA n°ANR-09-NANO-005-01 for postdoctoral funding. This work has been funded with the partial support of the ANR (project f-DNA n°ANR-09-NANO-005-01 and TRANCHANT n°ANR 2010 BLAN 1009 4). We are particularly grateful to E. Anquillare for careful proof-reading.Notes and references
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun'ko, Carbon, 2006, 44, 1624 CrossRef CAS.
- L. Bokobza, Polymer, 2007, 48, 4907 CrossRef CAS.
- P. Avouris, Z. Chen and V. Perebeinos, Nat. Nanotechnol., 2007, 2, 605 CrossRef CAS.
- P. Avouris, M. Freitag and V. Perebeinos, Nat. Photonics, 2008, 2, 341 CrossRef CAS.
- B. L. Allen, P. D. Kichambare and A. Star, Adv. Mater., 2007, 19, 1439 CrossRef CAS.
- H. Ago, K. Petritsch, M. S. P. Shaffer, A. H. Windle and R. H. Friend, Adv. Mater., 1999, 11, 1281 CrossRef CAS.
- D. M. Guldi, G. M. A. Rahman, F. Zerbetto and M. Prato, Acc. Chem. Res., 2005, 38, 871 CrossRef CAS.
- V. Sgobba and D. M. Guldi, Chem. Soc. Rev., 2009, 38, 165 RSC.
- T. Umeyama and H. Imahori, Energy Environ. Sci., 2008, 1, 120 CAS.
- A. C. Dillon, Chem. Rev., 2010, 110, 6856 CrossRef CAS.
- E. Katz and I. Willner, ChemPhysChem, 2004, 5, 1084 CrossRef CAS.
- K. Kostaleros, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108 CrossRef.
- N. A. Kotov, J. O. Winter, I. P. Clements, E. Jan, B. P. Timko, S. Campidelli, S. Pathak, A. Mazzatenta, C. M. Lieber, M. Prato, R. V. Bellamkonda, G. A. Silva, N. Wong Shi Kam, F. Patolsky and L. Ballerini, Adv. Mater., 2009, 21, 3970 CrossRef CAS.
- P. Singh, S. Campidelli, S. Giordani, D. Bonifazi, A. Bianco and M. Prato, Chem. Soc. Rev., 2009, 38, 2214 RSC.
- C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen and C. Mioskowski, Science, 2003, 300, 775 CrossRef CAS.
- N. Nakashima, Y. Tomonari and H. Murakami, Chem. Lett., 2002, 638 CrossRef CAS.
- Y.-L. Zhao and J. F. Stoddart, Acc. Chem. Res., 2009, 42, 1161 CrossRef CAS.
- D. A. Britz and A. N. Khlobystov, Chem. Soc. Rev., 2006, 35, 637 RSC.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- M. A. Gauthier and H.-A. Klok, Chem. Commun., 2008, 2591 RSC.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355 RSC.
- U. Mansfeld, C. Pietsch, R. Hoogenboom, C. R. Becer and U. S. Schubert, Polym. Chem., 2010, 1, 1560 RSC.
- N. K. Devaraj, R. Weissleder and S. A. Hilderbrand, Bioconjugate Chem., 2008, 19, 2297 CrossRef CAS.
- M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518 CrossRef CAS.
- W. Song, Y. Wang, J. Qu and Q. Lin, J. Am. Chem. Soc., 2008, 130, 9654 CrossRef CAS.
- W. Song, Y. Wang, J. Qu, M. M. Madden and Q. Lin, Angew. Chem., Int. Ed., 2008, 47, 2832 CrossRef CAS.
- J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302 RSC.
- J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272 RSC.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- R. Huisgen, Angew. Chem., Int. Ed. Engl., 1968, 7, 321 CrossRef CAS.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS.
- S. I. Presolski, V. Hong, S.-H. Cho and M. G. Finn, J. Am. Chem. Soc., 2010, 132, 14570 CrossRef CAS.
- M. D. Best, Biochemistry, 2009, 48, 6571 CrossRef CAS.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974 CrossRef CAS.
- W. S. Horne, C. D. Stout and M. R. Ghadiri, J. Am. Chem. Soc., 2003, 125, 9372 CrossRef CAS.
- W. S. Horne, M. K. Yadav, C. D. Stout and M. R. Ghadiri, J. Am. Chem. Soc., 2004, 126, 15366 CrossRef CAS.
- H. Li, F. Cheng, A. M. Duft and A. Adronov, J. Am. Chem. Soc., 2005, 127, 14518 CrossRef CAS.
- J. Bahr and J. M. Tour, Chem. Mater., 2001, 13, 3823 CrossRef CAS.
- H. Li and A. Adronov, Carbon, 2007, 45, 984 CrossRef CAS.
- S. K. Yadav, S. S. Mahapatra, J. W. Cho and J. Y. Lee, J. Phys. Chem. C, 2010, 114, 11395 CAS.
- J. Liu, Z. Nie, Y. Gao, A. Adronov and H. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7187 CrossRef CAS.
- Y. Zhang, H. He and C. Gao, Macromolecules, 2008, 41, 9581 CrossRef CAS.
- Y. Zhang, H. He, C. Gao and J. Wu, Langmuir, 2009, 25, 5814 CrossRef CAS.
- S. Rana, J. W. Cho and I. Kumar, J. Nanosci. Nanotechnol., 2010, 10, 5700 CrossRef CAS.
- B. S. Harrinson and A. Atala, Biomaterials, 2007, 28, 344 CrossRef.
- C. M. Voge and J. P. Stegemann, J. Neural Eng., 2011, 8, 011001 CrossRef.
- S. Rana, H. J. Yoo, J. W. Cho, B. C. Chun and J. S. Park, J. Appl. Polym. Sci., 2011, 119, 31 CrossRef CAS.
- G. de la Torre, W. Blau and T. Torres, Nanotechnology, 2003, 14, 765 CrossRef CAS.
- B. Ballesteros, S. Campidelli, G. de la Torre, C. Ehli, D. M. Guldi, M. Prato and T. Torres, Chem. Commun., 2007, 2950 RSC.
- B. Ballesteros, G. de la Torre, C. Ehli, G. M. A. Rahman, F. Agulló-Rueda, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2007, 129, 5061 CrossRef CAS.
- R. Chitta, A. D. Sandanayaka, A. L. Schumacher, L. D'Souza, Y. Araki, O. Ito and F. D'Souza, J. Phys. Chem. C, 2007, 111, 6947 CAS.
- S. Campidelli, B. Ballesteros, A. Filoramo, D. Díaz-Díaz, G. de la Torre, T. Torres, G. M. A. Rahman, C. Ehli, D. Kiessling, F. Werner, V. Sgobba, D. M. Guldi, C. Cioffi, M. Prato and J.-P. Bourgoin, J. Am. Chem. Soc., 2008, 130, 11503 CrossRef CAS.
- T. Palacin, H. Le Khanh, B. Jousselme, P. Jégou, A. Filoramo, C. Ehli, D. M. Guldi and S. Campidelli, J. Am. Chem. Soc., 2009, 131, 15394 CrossRef CAS.
- K. H. Le Ho, L. Rivier, B. Jousselme, P. Jégou, A. Filoramo and S. Campidelli, Chem. Commun., 2010, 46, 8731 RSC.
- Z. Guo, L. Liang, J.-J. Liang, Y.-F. Ma, X.-Y. Yang, D.-M. Ren, Y.-S. Chen and J.-Y. Zheng, J. Nanopart. Res., 2007, 10, 1077 CrossRef.
- G. Gattuso, S. A. Nepogodiev and J. F. Stoddart, Chem. Rev., 1998, 98, 1919 CrossRef CAS.
- I. W. Muderawan, T. T. Ong, T. C. Lee, D. J. Young, C. B. Ching and S. C. Ng, Tetrahedron Lett., 2005, 46, 7905 CrossRef CAS.
- I. Kumar, S. Rana, C. V. Rode and J. W. Cho, J. Nanosci. Nanotechnol., 2008, 8, 3351 CrossRef CAS.
- H.-T. Song, J.-S. Choi, Y.-M. Huh, S. Kim, Y.-W. Jun, J.-S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 9992 CrossRef CAS.
- J. Xie, S. Lee and X. Chen, Adv. Drug Delivery Rev., 2010, 62, 1064 CrossRef CAS.
- R. W. J. Scott, O. M. Wilson and R. M. Crooks, J. Phys. Chem. B, 2005, 109, 692 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS.
- H. Wang, D. W. Brandl, P. Nordlander and N. J. Halas, Acc. Chem. Res., 2007, 40, 53 CrossRef CAS.
- C. Girard, E. Dujardin, G. Baffou and R. Quidant, New J. Phys., 2008, 10, 105016 CrossRef.
- R. Voggu, P. Suguna, S. Chandrasekaran and C. N. R. Rao, Chem. Phys. Lett., 2007, 443, 118 CrossRef CAS.
- R. Voggu, S. Pal, S. K. Pati and C. N. R. Rao, J. Phys.: Condens. Matter, 2008, 20, 215211 CrossRef.
- S. Rana, I. Kumar, H. J. Yoo and J. W. Cho, J. Nanosci. Nanotechnol., 2009, 9, 3261 CrossRef CAS.
- H. He, Y. Zhang, C. Gao and J. Wu, Chem. Commun., 2009, 1655 RSC.
- Y. Song, K. Qu, C. Xu, J. Ren and X. Qu, Chem. Commun., 2010, 46, 6572 RSC.
- S. Mahouche Chergui, A. Ledebt, F. Mammeri, F. Herbst, B. Carbonnier, H. Ben Romdhane, M. Delamar and M. M. Chehimi, Langmuir, 2010, 26, 16115 CrossRef CAS.
- J. M. Schnorr and T. M. Swager, J. Mater. Chem., 2011, 21, 4768 RSC.
- W. Zhang and T. M. Swager, J. Am. Chem. Soc., 2007, 129, 7714 CrossRef CAS.
- S. E. Baker, W. Cai, T. L. Lasseter, K. P. Weidkamp and R. J. Hamers, Nano Lett., 2002, 2, 1413 CrossRef CAS.
- I. Carmeli, M. Mangold, L. Frolov, B. Zebli, C. Carmeli, S. Richter and A. W. Holleitner, Adv. Mater., 2007, 19, 3901 CrossRef CAS.
- S. M. Kaniber, F. C. Simmel, A. W. Holleitner and I. Carmeli, Nanotechnology, 2009, 20, 345701 CrossRef.
- S. M. Kaniber, M. Brandstetter, F. C. Simmel, I. Carmeli and A. W. Holleitner, J. Am. Chem. Soc., 2010, 132, 2872 CrossRef CAS.
- D. Pantarotto, C. D. Partidos, R. Graff, J. Hoebeke, J.-P. Briand, M. Prato and A. Bianco, J. Am. Chem. Soc., 2003, 125, 6160 CrossRef CAS.
- D. Pantarotto, C. D. Partidos, J. Hoebeke, F. Brown, E. Kramer, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Chem. Biol., 2003, 10, 961 CrossRef CAS.
- V. Georgakilas, N. Tagmatarchis, D. Pantarotto, A. Bianco, J.-P. Briand and M. Prato, Chem. Commun., 2002, 3050 RSC.
- D. Pantarotto, J.-P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16 RSC.
- C. Baldacchini, M. A. Herrero, M. Prato and S. Cannistaro, Adv. Funct. Mater., 2011, 21, 153 CrossRef CAS.
- C. H. Villa, M. R. McDevitt, F. E. Escorcia, D. A. Rey, M. Bergkvist, C. A. Batt and D. A. Scheinberg, Nano Lett., 2008, 8, 4221 CrossRef CAS.
- K. Flavin, M. N. Chaur, L. Echegoyen and S. Giordani, Org. Lett., 2010, 12, 840 CrossRef CAS.
- A. Devadoss and C. E. D. Chidsey, J. Am. Chem. Soc., 2007, 129, 5370 CrossRef CAS.
- E. C. Landis and R. J. Hamers, Chem. Mater., 2009, 21, 724 CrossRef CAS.
- H.-X. Wang, K.-G. Zhou, Y.-L. Xie, J. Zeng, N.-N. Chai, J. Li and H.-L. Zhang, Chem. Commun., 2011, 47, 5747 RSC.
- R. Salvio, S. Krabbenborg, W. J. M. Naber, A. H. Velders, D. N. Reinhoudt and W. G. van der Weil, Chem.–Eur. J., 2009, 15, 8235 CrossRef CAS.
- S. Sun, Y. Cao, J. Feng and P. Wu, J. Mater. Chem., 2010, 20, 5605 RSC.
- Y. Pan, H. Bao, N. G. Sahoo, T. Wu and L. Li, Adv. Funct. Mater., 2011 DOI:10.1002/adfm.201100078.
- Y. Cao, Z. Lai, J. Feng and P. Wu, J. Mater. Chem., 2011, 21, 9271 RSC.
| This journal is © The Royal Society of Chemistry 2011 |
