Preparation of highly specific anti-zearalenone antibodies by using the cationic protein conjugate and development of an indirect competitive enzyme-linked immunosorbent assay†
Yuan
Gao‡
ab,
Meihua
Yang‡
a,
Cheng
Peng
b,
Xiaohong
Li
a,
Runlan
Cai
a and
Yun
Qi
*a
aInstitute of Medicinal Plant Development, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, 100193, P.R. China. E-mail: yunqichai@sohu.com; Fax: (+86) 10 62829207; Tel: (+86) 10 62829207
bPharmacy College, Chengdu University of Traditional Chinese Medicine, Chengdu, 610075, P.R. China
First published on 2nd November 2011
Abstract
Although anti-zearalenone (ZEN) antibodies have been widely prepared, these antibodies cross-react with α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), zearalanone (ZAN), α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL). To overcome this problem and improve the specificity of immunoassays, we produced anti-ZEN antibodies based on a ZEN-cationic protein conjugate. In this study, ZEN was coupled with cationic bovine serum albumin (cBSA) via a Mannich reaction. After BALB/c mice were immunized with ZEN-cBSA, an immunological response was rapidly induced. The titers of the polyclonal antisera and monoclonal antibody were 30,000 and 20,000, respectively. Cross-reactivity (CR) values of the anti-ZEN polyclonal antisera and monoclonal antibody with the 5 analogs were <7% and <2%, respectively. An indirect competitive enzyme-linked immunosorbent assay based on the monoclonal anti-ZEN antibody was established. The recovery rates of ZEN in spiked cereal and feed were in the range of 80%–120% with coefficients of variation <15%. The intra-assay variation and inter-assay variation in assay buffer were both <5%. Therefore, the results demonstrated a feasible approach for preparing highly specific, higher titer and more rapidly induced antibodies against ZEN by using a ZEN-cBSA conjugate as the immunogen instead of currently used immunogens.
Introduction
Zearalenone (ZEN) contamination of cereal, feed and the environment is recognized as a worldwide problem. It is a naturally occurring estrogenic mycotoxin produced by numerous species of Fusarium (F.) that grow on grains, especially corn and hay exposed to high moisture levels during storage.1ZEN causes hormone-like effects in warm-blooded animals and serious disturbances to the reproductive system.2 Therefore, in order to minimize the risk to humans and animals, many countries have regulations limiting the acceptable levels of ZEN in cereal and feed.3Immunoassays using specific antibodies, including those targeting ZEN, have been used to detect toxin residues for many years. The key step in the development of an immunoassay is the production of the specific antibody. But the cross-reactivity (CR) of anti-ZEN antibodies with α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), zearalanone (ZAN), α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL) is a serious problem. ZEN and its 5 analogs are structurally identical apart from minor differences at the C7 and C12 positions in the macrocyclic lactone ring (Fig. 1). Therefore it is difficult for an anti-ZEN antibody to distinguish ZEN from its 5 analogs. Anti-ZEN antibodies showed high cross-reactivity (CR) with the 5 analogs (Table 1).
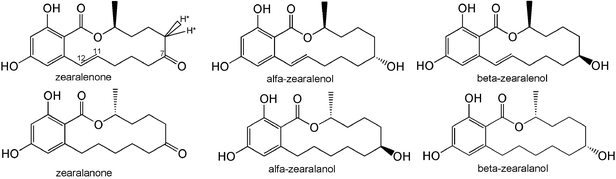 | ||
| Fig. 1 The chemical structures of zearalenone (ZEN), 7α-hydroxy-zearalenol (α-ZOL), 7β-hydroxy-zearalenol (β-ZOL) and their 11–12 reduced analogs: zearalanone (ZAN), 7α-hydroxy-zearalanol (α-ZAL), and 7β-hydroxy-zearalanol (β-ZAL). *The active hydrogen. | ||
| Substance (active derivative) | Coupling reagent (method) | Type of antibody | Time after immunization | Titer of antibody | Cross reactivity (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-ZOL | β-ZOL | ZAN | α-ZAL | β-ZAL | ||||||
| a DCC: Dicyclohexylcarbodiimide. b M: Monoclonal antibody. c —: Not mentioned in literature. d ND: Not detected. e P: Polyclonal antibody. f EDC: 1-ethyl-3(3-dimethylaminopropyl) carbodiimide. | ||||||||||
| ZEN-oxime | DCCa | Mb (IgG2a) | —c | — | 33.7 | 24.5 | NDd | 82.9 | 74 | 19 |
| — | — | M (IgG2a, κ) | — | — | 91 | 21 | 138 | 69 | 6 | 20 |
| — | — | M | — | — | 69 | <1 | 22 | 42 | <1 | 21 |
| ZEN-oxime | DCC | Pe (rabbits) | 11 weeks | 20,480 | 50 | 12 | ND | 5.45 | 2.5 | 17 |
| ZEN-oxime | EDCf | P (pigs) | 14 weeks | 3,500 | 100 | 44 | 100 | 53 | 44 | 22 |
| — | — | — | — | — | 88–120 | 91–95 | 74–81 | 80–101 | 87–116 | 8 |
| ZEN-oxime | EDC | P (rabbits) | — | 1,500 | ND | 18 | 180 | 100 | ND | 23 |
| ZEN | Formaldehyde condensation | P (rabbits) | — | — | 0.15 | <0.02 | 31.7 | 0.12 | ND | 5 |
| ZEN-oxime | DCC | P (rabbits) | — | — | 37.3 | 7.2 | 59.2 | 3.9 | 5.3 | 24 |
| ZEN-oxime | DCC | M (IgG1, κ) | — | — | 121.5 | 65.3 | ND | 21.5 | 18.9 | 25 |
| — | — | M | — | — | 96 | 21 | ND | 24 | 5 | 26 |
| — | — | M (IgE) | — | — | 102 | 71 | 195 | 139 | 20 | 27 |
| — | — | M | 5 weeks | 6,400 | 107 | 29 | ND | 35 | 25 | 28 |
| ZEN-oxime | DCC | M | — | — | 26 | 11 | ND | 8 | 10 | 29 |
| ZEN-oxime | DCC | P (rabbits) | 10 weeks | 1,500 | 280 | 35 | ND | 22 | 10 | 30 |
| 5-NH2-ZEN | glutaraldehyde | M (IgG1, λ) | — | 140,000 | 0.9 | <0.1 | 4.0 | <0.1 | <0.1 | 6 |
CR is determined by the antibody specificity, which depends on the antigenic determinants exposed to immune cells. This type of CR, where the antigenic determinants do not distinguish between similar binding sites on ZEN and its analogs, has been termed molecular or antigenic mimicry.4 The crucial step to developing a highly specific antibody is to prepare an appropriate immunogen. Because ZEN is a hapten, its antigenicity is only induced by coupling with a carrier protein. Therefore, the preparation of ZEN-carrier protein conjugates is one of the most important steps in antibody production. Different methods of ZEN-protein conjugate preparation will expose different antigenic determinants. In most previous studies, carboxymethyl oxime was introduced to ZEN first, and then the derivatives were coupled with protein by carbodiimide (Table 1). The Mannich reaction was used solely for conjugate preparation.5 Although the antibody specificity was improved somewhat, the problem of CR with ZEN analogs was not solved by using ZEN itself conjugated with carrier protein directly in the Mannich reaction. Teshima and colleagues6,7 introduced a more complex approach in which a hapten mimic (5-NH2-ZEN) was substituted for the purpose of eliciting an antibody to the compound to be assayed, ZEN. The monoclonal antibody obtained showed low CR values with 5 analogs (≤4%), but the CR value with 5-NH2-ZEN itself was not reported yet. In 2007, Erbs et al.,8 who did not manufacture any of the antibodies, tested 3 commercial immunoaffinity columns (IACs) targeting ZEN, and the CR values for the 5 analogs were ≥74% when they were applied either individually or in a mixture. Therefore, it is necessary to establish an effective and simple method to improve the specificity of anti-ZEN antibody.
A cationic protein that served as an efficient carrier was first reported in 1987.9 There are many reports in which conjugates prepared using a cationic protein as a carrier had good immunological properties.10–12 As an antigen, the characteristics of cationic proteins were different from the native proteins.9,13 Cationic proteins had an increased affinity for antigen-presenting cell membranes due to electrostatic interactions with anionic membrane phospholipids.14 Moreover, cationic carrier proteins could minimize cross-linking and generate stronger immune responses compared to their native forms.15 As an efficient carrier, cationic proteins could induce a rapid, specific immunological response against the antigenic determinants of the hapten coupled to the protein.16 In addition, ZEN contains active hydrogen, which is an essential component for conjugating ZEN to a cationic protein. In this study, we describe an efficient and convenient method to construct ZEN-cationic protein conjugates using a Mannich-type reaction. Based on the immunogen synthesized, we prepared polyclonal antisera and a monoclonal antibody. ZEN in cereal and feed samples was analyzed by the established indirect competitive ELISA, including corn, wheat, barley, oat, coix seed and rodent feed. The results confirmed that the monoclonal antibody obtained was highly specific. The goal of this study was to provide an efficient and simple approach to prepare a highly specific anti-ZEN antibody and significantly improve the specificity of immunoassays.
Experimental
Chemicals and materials
ZEN was purchased from Fermentek Ltd. (Jerusalem, Israel). The mouse SP2/0 myeloma cell line was purchased from the Cell Resource Center of Peking Union Medical College (Beijing, P.R. China). Native bovine serum albumin (nBSA), polylysine (PLL; MW 30,000-70,000), ethylenediamine (EDA), 2-(N-morpholino)-ethane sulfonic acid (MES), 50% PEG solution, compete and incomplete Freund's adjuvant, Hypoxanthine aminopterin thymidine (HAT), Hypoxanthine thymidine (HT), α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), zearalanone (ZAN), α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL) were obtained from Sigma-Aldrich (St.Louis, MO, USA). Goat anti-mouse IgG-HRP conjugate was purchased from Jackson ImmunoReasearch Laboratories, Inc. (Lancaster, Pennsylvania, USA). Goat anti-mouse IgM-HRP conjugate was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA). Mouse monoclonal antibody isotyping test kit was purchased from MorphoSys AG Co. (Martinsried, German). The commercial zearalenone ELISA kit was purchased from Abraxis Co. (Warminster, PA, USA). Corn, wheat, barley, oat and coix seed were purchased from supermarkets in China. Other reagents were purchased from Beijing Chemical Reagents Co. (Beijing, P.R. China).Animals
Female BALB/c mice and feed were provided by Vital River Experimental Animal Services (Beijing, P.R. China), and kept in a vivarium under standard conditions of temperature and humidity with a 12 h light/dark cycle. All experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Animals Ethics Committee of the Institute of Medicinal Plant Development of the Chinese Academy of Medical Sciences.Buffers and solutions
Ultrapure deionized water was used for the preparation of the following buffers and solutions: (1) coating buffer: 0.05 mol L−1carbonate buffer, pH 9.6; (2) assay buffer: 0.01 mol L−1phosphate-buffered saline (PBS) pH 7.4, with 8.0 g L−1NaCl; (3) washing buffer (PBST): assay buffer with 0.05% (v/v) Tween 20; (4) blocking solution: 5% goat serum in assay buffer; (5) substrate solution A: 20 mg TMB in 10 mL DMSO and 90 mL water; (6) substrate solution B: 0.1 mol L−1citric acid and 0.2 mol L−1 disodium hydrogen phosphate; (7) working buffer: substrate solution A and B mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v); (8) stop solution: 2 mol L−1H2SO4; and (9) conjugation buffer: 0.1 mol L−1MES pH 4.75.
1 (v/v); (8) stop solution: 2 mol L−1H2SO4; and (9) conjugation buffer: 0.1 mol L−1MES pH 4.75.
Cationization
The cationization method developed by Muckerheide9 was used with some modifications (Fig. 2(1)–(2)). Briefly, 1 mL of ethylenediamine (EDA) was added to 5 mL of conjugation buffer, and the pH value was adjusted to 5.5 with 1 mol L−1HCl. Then, 5 mL of the mixture was added to 1.68 mL of the same buffer containing 16.8 mg of nBSA and 10 mg EDC and incubated at 37 °C for 2 h with magnetic stirring. The reaction was quenched with 4 mol L−1acetate buffer and dialyzed exhaustively against distilled water for 72 h. The resulting salt-free cationic BSA (cBSA) was lyophilized and stored at −20 °C.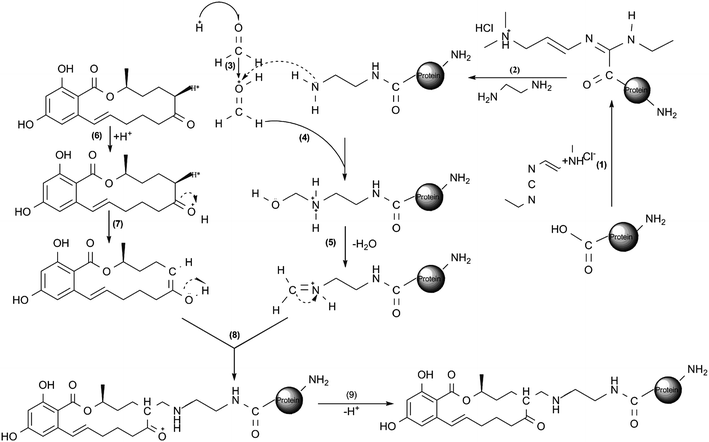 | ||
| Fig. 2 A schematic diagram based on position 6-H for the preparation of ZEN-cationic protein conjugates.16 (1)–(2) cationization of protein; (3)–(5) imidization of N-hydroxymethyl amine groups in the cationic protein; (6)–(7) enolation of ZEN; (8)–(9) conjugation of enolated ZEN with the iminium ionized protein. All the reactions were carried out in MES buffer. This reaction can proceed in other directions: the active hydrogen is also available on the other side of the keton group (position 8), position 3′ and 5′ on the benzene ring and the phenolic OH groups. The ZEN conjugates with cationic protein reaction may be a mixture of conjugates with different conjugation sites. | ||
The preparation of cationic polylysine (cPLL) as another cationic carrier protein was performed according to the procedures mentioned above except that polylysine (PLL) was substituted for nBSA. In addition, PLL can be conjugated with ZEN directly in the next step. It would not affect the assay performance whether PLL was cationized.
Preparation of ZEN-cationic protein conjugates
The preparation of ZEN-cBSA (Fig. 2(3)–(9)) with a Mannich-type reaction was performed as previously described11 with some modifications. Briefly, 600 μg of cBSA and 40 μg of ZEN were dissolved in 600 μL of conjugation buffer and 220 μL of N,N-dimethylformamide (DMF), respectively. After the dropwise addition of the ZEN solution into the protein solution, the mixture was stirred gently; 164 μL of formaldehyde were added to the mixture, and it was immediately stirred gently for 24 h at 37 °C. The conjugate of ZEN-cBSA was dialyzed exhaustively against distilled water for 72 h, and the salt-free ZEN-cBSA was lyophilized and stored at −20 °C. The molar ratio of ZEN to cBSA was determined by measuring the change in the UV absorption (315 nm) of the cBSA after conjugation.The preparation of the coating antigen (ZEN-cPLL) was similar to the procedures mentioned above except for the substitution of cPLL for cBSA.
Immunization
The ZEN-cBSA immunogen was used to immunize mice. The first dose consisted of 60 μg of conjugate, which was intraperitoneally injected as an emulsion of sterilized saline and Freund's complete adjuvant. Subsequent injections emulsified in Freund's incomplete adjuvant were administered intraperitoneally at 1-week intervals. A small volume of blood was collected from the tails of the immunized mice to test the serum titers. When the titers were high, the mice were injected with a final soluble conjugate in sterilized saline without adjuvant.Production of polyclonal antisera and monoclonal antibody (mAb)
3 days after the final booster injection, the mice were anesthetized using ether, and the polyclonal antisera were collected by extirpating the eyeball. The mice were then sacrificed by cervical dislocation. Spleen cells were fused with mouse SP2/0 myeloma cells at the ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of a 50% PEG solution. The hybridomas were cultured in a hypoxanthine aminopterin thymidine (HAT) complete medium containing 20% fetal bovine serum (FBS) in 96-well plates and incubated at 37 °C with 5% CO2. 10 days later, they were expanded in the hypoxanthine thymidine (HT) complete medium. Culture supernatants from each clone were screened for antigen reactivity. Positive hybridomas were subcloned by 2–3 limiting dilutions. The resulting hybridoma clones were propagated and cryopreserved in freezing solution according to the freezing protocol: 30 min at 4 °C, overnight in the gas of a liquid nitrogen jar and then stored in liquid nitrogen.
1 in the presence of a 50% PEG solution. The hybridomas were cultured in a hypoxanthine aminopterin thymidine (HAT) complete medium containing 20% fetal bovine serum (FBS) in 96-well plates and incubated at 37 °C with 5% CO2. 10 days later, they were expanded in the hypoxanthine thymidine (HT) complete medium. Culture supernatants from each clone were screened for antigen reactivity. Positive hybridomas were subcloned by 2–3 limiting dilutions. The resulting hybridoma clones were propagated and cryopreserved in freezing solution according to the freezing protocol: 30 min at 4 °C, overnight in the gas of a liquid nitrogen jar and then stored in liquid nitrogen.
In order to gain massive numbers of mAb, mice were intraperitoneally injected with 500 μL of Freund's incomplete adjuvant. 7 days later, 1 × 105 hybridomas were inoculated into the abdominal cavity of each mouse. 1 week later, the ascites were collected and purified via the octanoic acid-saturated ammonium sulfate precipitation method. The ascites were dialyzed exhaustively against distilled water for 48 h, and finally, the salt-free ascites were lyophilized and stored at −20 °C. The isotype classification of the mAb was identified by the mouse monoclonal antibody isotyping test kit.
Development of indirect competitive enzyme-linked immunosorbent assay (icELISA)
The 96-well microtiter plates were coated with 100 μL of ZEN-cPLL in coating buffer and incubated at 4 °C overnight. After 3 washes in washing buffer, the plates were blocked with 200 μL blocking buffer for 1.5 h at 37 °C. After washing, the assay buffer, with or without a competitor (ZEN or other competitors, 50 μL/well), was added, followed by the addition of the anti-ZEN antibody (50 μL/well), and the plate was incubated for 1 h at 37 °C. After washing 5 times, 100 μL of secondary antibody conjugated with HRP in assay buffer was added, and the plates were incubated at 37 °C for 1 h. The plates were washed 5 times, and the colorimetric reaction was developed using 100 μL of working buffer for 10 min at 37 °C. The reaction was terminated by 50 μL of stop solution, and the optical density (OD) was read at 450 nm.Evaluation of antibody specificity
The specificity of each antibody was investigated by measuring its CR value with 5 ZEN analogs. The icELISA was performed by adding various free competitors at different concentrations to estimate their respective IC50 value. After reading the plate, the result was expressed as the percentage inhibition as follows: % inhibition = % B/B0, where B was the absorbance at each concentration of analyte and B0 was the absorbance in the absence of analyte. The IC50 value was determined by using the concentration of the inhibitor that leads to a 50% decrease in the maximum signal. The CR (%) was calculated as follows: CR (%) = (IC50, ZEN/IC50, analyte) × 100. An inhibition standard curve was generated by plotting absorbance in 450 nm versus concentration of ZEN.Preparation of cereal and feed samples
The method developed by Peseka17 was used with some modifications. Briefly, the corn, wheat, barley, oat, coix seed and rodent feed (containing ground shell corn and soybean meal) samples were refrigerated at 4 °C prior to grinding and analysis. For analysis, each finely ground sample (5.0 g) was weighed into a 100 mL screw-cap bottle and extracted with 25 mL of methanol-water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) by shaking for 3 min on a rotary shaker. The extracts were filtered, and the filtrate was diluted at least 3.5 times in assay buffer to give a final methanol concentration ≤ 20% (v/v) for analysis.
30, v/v) by shaking for 3 min on a rotary shaker. The extracts were filtered, and the filtrate was diluted at least 3.5 times in assay buffer to give a final methanol concentration ≤ 20% (v/v) for analysis.
Sample determination based on the anti-ZEN mAb
The spiked samples were artificially spiked with ZEN, α-ZOL, β-ZOL, ZAN, α-ZAL and β-ZAL standard solution in assay buffer. Each sample contained 5 analogs (each at final concentration 25 μg L−1) and also ZEN (at different final concentration 12.5, 25 and 50 μg L−1) was analyzed to determine the recovery rate of ZEN. The recovery rate was calculated as follows.| Recovery (%) = (amount of ZEN in spiked sample − amount of ZEN in unspiked sample)/added amount of ZEN × 100 |
In order to validate the specificity of the anti-ZEN mAb prepared in our laboratory, we purchased the ZEN competitive ELISA (cELISA) kit from Abraxis Co. which served as a control. The cereal and feed samples were analyzed by icELISA based on the anti-ZEN mAb and Abraxis zearalenone plate kit (AZPK) simultaneously. The procedure of the AZPK was carried out according to its directions. The accuracies of these two methods were ascertained by analysis of the recovery in ZEN standard solution. The assay buffer was fortified by ZEN standard solution at the final concentration of 12.5, 25 and 50 μg L−1.The recovery results were calculated as the ratio of measured level to spiked level (n = 4).
Validation of the icELISA
The repeatability of icELISA based on the anti-ZEN mAb was measured. For intra-assay (within-plate) repeatability, 6 replicates of each dilution were tested in the same plate on a single day. For inter-assay (between-run) repeatability, triplicates of each dilution were run on 5 different days. The detection limit (LOD) was calculated from the ZEN standard solution. The mean OD value, standard deviation (SD) and coefficient of variation (CV) were calculated for each test.Results and discussion
Synthesis and characterization of hapten
ZEN is a small antigen with a molecular mass of 318.15. Because ZEN alone is not antigenic, its conjugation to a carrier protein was the most critical step of the project. Common protocols used for the preparation of complete antigens could not be applied in this case due to the absence of a known coupling group such as NH, COOH and SH on ZEN. Instead, the hydrogen group of ZEN was used to construct a hapten-carrier protein complexvia a Mannich-type reaction. This reaction is a common amino-alkylated reaction, in which compounds containing active hydrogen, such as ketones, esters, acetylenes, α-picolines, etc., can be linked to an amine or amide group by formaldehyde in weakly acidic conditions.16BSA is one of the most commonly used carrier proteins, and it usually yields satisfactory results. A two-step strategy was used to link ZEN to BSA. The first step corresponded to a modification of the BSA carboxylic groups to generate a cationic protein with a high isoelectric point. The carboxylic groups were modified to form aminoethylamide side chains. In the second step, the abundance of primary amines on cBSA made it very receptive in an amino-alkylation reaction, during which amine moieties of cBSA were linked to ZEN through active hydrogen.
The UV absorption spectra of nBSA and cBSA are shown in Fig. 3. The maximum absorbing wavelength in the cBSA UV spectra, which are characteristic of the protein, was slightly blue-shifted from 278 to 276 nm compared with nBSA. This might be caused by the increase in cBSA polarity that is due to the induction of excessive cationic groups and amino-ethylamine groups on nBSA.16 Native-polyacrylamide gel electrophoresis was run, and the protein was not degenerated before electrophoresis indicating that the positive charge of the cationic protein could be maintained in this condition. When the electrode was not reversed, cBSA did not migrate, and nBSA showed a series of bands (data not shown). These results clearly indicated that the cationization was successful.12
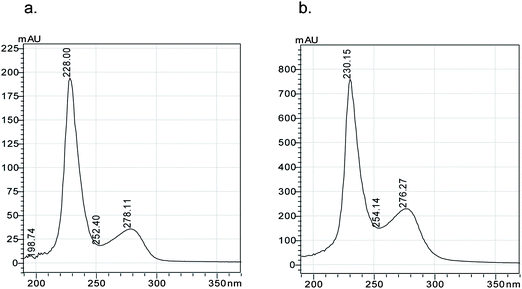 | ||
| Fig. 3 UV spectra of: (a) 1.0 g L−1nBSA and (b) 4.0 g L−1cBSA. | ||
Production of polyclonal antisera and monoclonal antibody (mAb)
ZEN-cBSA with a molar ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was injected into mice. 40 days later, titers of the polyclonal antiserum from individual animals injected with the same immunogen were high. Pre-immune serum controls showed negligible absorbance, whereas the pooled antisera had a titer of 30,000.
1 was injected into mice. 40 days later, titers of the polyclonal antiserum from individual animals injected with the same immunogen were high. Pre-immune serum controls showed negligible absorbance, whereas the pooled antisera had a titer of 30,000.
2 weeks after cell fusion, culture supernatants from each clone were subjected to screening. Positive hybridomas were subcloned 3 times by limiting dilution. The mAb produced from the hybridoma cells was IgM with a κ-type light chain. The titer of the mAb was 20,000. This result indicated that the antibody obtained had a higher affinity when using the ZEN-cBSA as the immunogen. It also suggested that a cationic protein had good antigenicity, as the carrier protein could not only induce a more rapid immune response but also a higher titer of antibody [22,28,30 in Table 1].
Optimization of the assay conditions
To establish a specific icELISA method, the optimum assay conditions were determined by the adjustment of several parameters, such as the concentration of the coating antigen and the dilution of the anti-ZEN antibody and secondary antibody.18 In this study, the optimal concentration of the coating antigen, ZEN-cPLL, was found to be 10 μg mL−1. The best dilution for the polyclonal antisera and mAb were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,000 and 1
2,000 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,500, respectively. Suitable dilutions of the goat anti-mouse IgG-HRP and goat anti-mouse IgM-HRP were 1
1,500, respectively. Suitable dilutions of the goat anti-mouse IgG-HRP and goat anti-mouse IgM-HRP were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,000 and 1
20,000 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, respectively. Using the standard curve shown in Fig. 4 (a, c), ZEN could be determined over the range from 6.25 to 400 μg L−1 (polyclonal antisera) and 1.56 to 100 μg L−1 (mAb).
500, respectively. Using the standard curve shown in Fig. 4 (a, c), ZEN could be determined over the range from 6.25 to 400 μg L−1 (polyclonal antisera) and 1.56 to 100 μg L−1 (mAb).
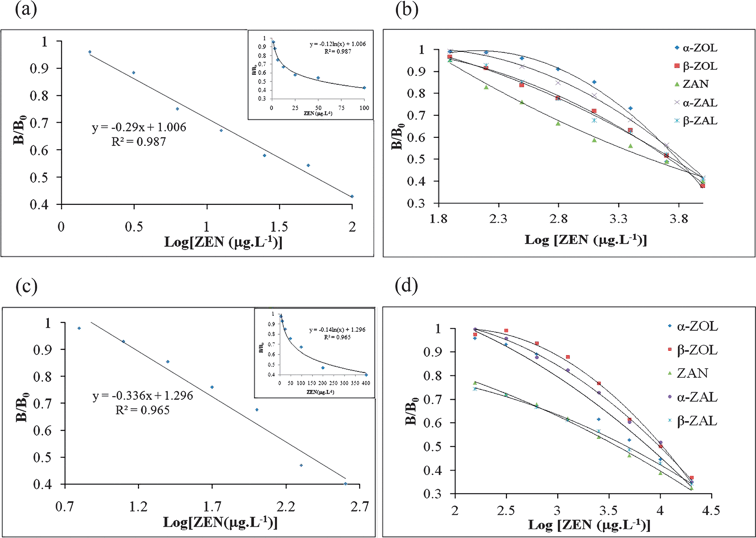 | ||
| Fig. 4 icELISA competition curves. a. icELISA competition curve for the binding of ZEN to anti-ZEN mAb. b. icELISA competition curves for the binding of 5 ZEN analogs to anti-ZEN mAb. c. icELISA competition curve for the binding of ZEN to anti-ZEN polyclonal antisera. d. icELISA competition curves for the binding of 5 ZEN analogs to anti-ZEN polyclonal antisera. | ||
Cross-reactions of polyclonal antisera and monoclonal antibody
The icELISA was used to characterize the sensitivity (IC50) and specificity (CR) of the obtained antibodies. The specificity of the antibodies was estimated by measuring inhibition curves using 5 structurally related analogs. The IC50 and CR values for each analog are given in Table 2, and the competition curves for the binding of the antibodies to ZEN and its 5 analogs are shown in Fig. 4. From Table 2, it can be seen that the CR values of the anti-ZEN polyclonal antisera and monoclonal antibody with the 5 analogs are <7% and <2%, respectively. These low CR values suggested that, on the one hand, the C7 carbonyl and C12 double bond in the macrocyclic lactone ring might be exposed more efficiently on ZEN-cBSA. It might be a result of the application of both cBSA and the Mannich reaction. On the other hand, more highly specific antibodies, which included polyclonal antisera and a monoclonal antibody, were obtained by using ZEN-cBSA as an immunogen (Table 1).| Compound | Polyclonal antisera | Monoclonal antibody | ||
|---|---|---|---|---|
| IC50 (μg L−1) | Cross-reactivitya (%) | IC50 (μg L−1) | Cross-reactivitya (%) | |
| a The percentage of cross-reactivity is defined as the ratio of IC50 value for ZEN to that for competitors. | ||||
| ZEN | 233.35 | 100 | 55.72 | 100 |
| α-ZOL | 7430.19 | 3.14 | 8542.80 | 0.65 |
| β-ZOL | 11912.42 | 1.96 | 5956.62 | 0.94 |
| ZAN | 3435.58 | 6.79 | 3767.04 | 1.48 |
| α-ZAL | 10375.28 | 2.25 | 8830.80 | 0.63 |
| β-ZAL | 4130.48 | 5.65 | 6063.17 | 0.92 |
icELISA validation
The icELISA based on the anti-ZEN mAb and AZPK was used to determine the ZEN in corn, wheat, barley, oat, coix seed and rodent feed simultaneously. According to the direction, the antibody utilized in the AZPK is specific for ZEN and closely related structures. The CR values of this kit with β-ZOL, ZAN, α-ZAL and β-ZAL were 13%, 81%, 30% and 8%, respectively. As is shown in Table 3, for all spiked level, the average recovery rates of icELISA were all in the range of 80%–120%, and the CV values were all less than 15%. But all of the average recovery rates of AZPK were out of the acceptable range. When we tested the ZEN standard solution in assay buffer in the absence of 5 analogs, the average recovery rates of these two methods were all in the range of 80%–120%, and the CV values were all less than 5% (Table 4). It is suggested that both of these two methods could accurately test ZEN content, but the AZPK could not effectively distinguish ZEN and its 5 analogs. These results demonstrated that the satisfactory precision of the immunoassay applied in the real cereal and feed system was obtained. It also suggested that the icELISA based on the mAb in our study showed higher specific and more accurate than the commercial ELISA kit.| Sample | Spiked ZEN (μg L−1) | Indirect competitive ELISA | AZPK | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detected ZEN (μg L−1) | Mean detected ZEN (μg L−1) | Recoveryb (%) | CV (%) | Detected ZEN (μg L−1) | Mean detected ZEN (μg L−1) | Recoveryb (%) | CV (%) | ||||||
| a The unspiked sample which did not contain ZEN and its 5 analogues. b All unspiked samples contained some background level of ZEN and this had to be subtracted from the level measured for spiked samples in order to calculate the recovery. | |||||||||||||
| Corn | 0a | 19.73 | 19.73 | 20.67 | 20.04 | - | 2.71 | 33.78 | 37.35 | 34.76 | 35.30 | — | 5.22 |
| 12.5 | 29.54 | 30.94 | 30.47 | 30.32 | 82.20 | 2.36 | 73.31 | 78.76 | 84.62 | 78.90 | 348.76 | 7.17 | |
| 25 | 41.56 | 54.96 | 48.54 | 48.36 | 113.26 | 13.86 | 116.03 | 97.67 | 106.46 | 106.72 | 285.68 | 8.60 | |
| 50 | 66.22 | 79.78 | 70.46 | 72.15 | 104.23 | 9.61 | 194.49 | 186.30 | 231.04 | 203.94 | 337.28 | 11.68 | |
| Wheat | 0 | 6.65 | 6.45 | 6.35 | 6.48 | — | 2.38 | 2.28 | 2.52 | 2.74 | 2.51 | — | 9.29 |
| 12.5 | 19.12 | 18.25 | 17.97 | 18.45 | 95.67 | 3.25 | 48.35 | 49.76 | 48.35 | 48.82 | 370.50 | 1.66 | |
| 25 | 34.50 | 26.09 | 28.19 | 29.59 | 92.41 | 14.79 | 40.13 | 40.13 | 40.70 | 40.32 | 151.23 | 0.83 | |
| 50 | 65.20 | 58.48 | 56.70 | 60.13 | 107.27 | 7.46 | 188.99 | 203.05 | 188.99 | 193.68 | 382.33 | 4.19 | |
| Barley | 0 | 6.25 | 6.16 | 5.97 | 6.13 | — | 2.36 | 4.66 | 4.66 | 4.73 | 4.69 | — | 0.83 |
| 12.5 | 16.63 | 16.89 | 20.03 | 17.85 | 93.76 | 10.62 | 387.28 | 381.76 | 387.28 | 385.44 | 3045.99 | 0.83 | |
| 25 | 30.47 | 29.08 | 29.54 | 29.69 | 94.26 | 2.38 | 345.28 | 335.51 | 340.36 | 340.38 | 1342.77 | 1.43 | |
| 50 | 54.96 | 64.19 | 57.58 | 58.91 | 105.57 | 8.08 | 244.69 | 255.45 | 251.81 | 250.65 | 491.92 | 2.18 | |
| Oat | 0 | 13.80 | 14.24 | 12.97 | 13.67 | — | 4.71 | 16.72 | 18.23 | 17.46 | 17.47 | — | 4.30 |
| 12.5 | 25.68 | 26.09 | 27.33 | 26.37 | 101.57 | 3.25 | 79.90 | 77.64 | 79.90 | 79.14 | 493.40 | 1.65 | |
| 25 | 42.21 | 44.92 | 43.54 | 43.56 | 119.55 | 3.10 | 156.83 | 159.10 | 161.40 | 159.11 | 566.55 | 1.43 | |
| 50 | 57.58 | 62.23 | 61.27 | 60.36 | 93.39 | 4.07 | 251.81 | 278.42 | 255.45 | 261.89 | 488.84 | 5.51 | |
| Coix seed | 0 | 365.36 | 327.73 | 312.80 | 335.30 | — | 8.07 | 340.36 | 335.51 | 335.51 | 337.13 | — | 0.83 |
| 12.5 | 354.19 | 343.36 | 348.70 | 348.76 | 107.65 | 1.55 | 466.70 | 447.03 | 466.71 | 460.14 | 984.10 | 2.47 | |
| 25 | 382.78 | 338.07 | 371.10 | 363.98 | 114.69 | 6.37 | 546.49 | 570.53 | 587.14 | 568.05 | 923.70 | 3.60 | |
| 50 | 420.15 | 371.08 | 388.80 | 393.33 | 116.06 | 6.32 | 707.54 | 738.67 | 697.47 | 714.56 | 754.86 | 3.00 | |
| Rodent feed | 0 | 232.90 | 222.30 | 215.50 | 223.57 | — | 3.92 | 290.66 | 299.12 | 278.42 | 289.40 | — | 3.60 |
| 12.5 | 247.82 | 225.78 | 232.90 | 235.50 | 95.46 | 4.78 | 570.53 | 554.39 | 595.63 | 573.52 | 2272.92 | 3.62 | |
| 25 | 276.28 | 247.83 | 229.30 | 251.14 | 110.27 | 9.42 | 494.27 | 578.78 | 554.39 | 542.48 | 1012.31 | 8.02 | |
| 50 | 298.58 | 272.02 | 276.30 | 282.29 | 117.45 | 5.05 | 793.61 | 760.17 | 760.17 | 771.32 | 963.84 | 2.50 | |
| Level (μg L−1) | Indirect competitive ELISA | AZPK | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Measured amounta (μg L−1) | CVb (%) | R c (%) | n | Measured amounta (μg L−1) | CVb (%) | R c (%) | |
| a Mean ± SD. b CV: coefficient of variation= SD/mean *100. c R: recovery. | ||||||||
| 12.5 | 4 | 10.75 ± 0.010 | 2.17 | 85.96 | 4 | 14.19 ± 0.004 | 1.08 | 113.50 |
| 25 | 4 | 24.12 ± 0.005 | 1.20 | 96.49 | 4 | 23.23 ± 0.005 | 1.54 | 92.93 |
| 50 | 4 | 52.40 ± 0.004 | 1.06 | 104.80 | 4 | 52.61 ± 0.002 | 0.60 | 105.21 |
In the optimum conditions, the LOD of icELISA based on anti-ZEN mAb was 1.56 μg L−1. The repeatability test (Table 5) was performed by comparing the OD ratios of 6 replicates in the same plate (intra-assay repeatability) or triplicates at different days (inter-assay repeatability). The intra-assay CV of 6 tests ranged from 1.60% to 3.63%, with a median value of 2.42%. The inter-assay CV of 3 tests was between 4.20% and 4.90%, with a median value of 4.46%. These data showed that the assay was repeatable and yielded a low and acceptable variation.
| Level (μg L−1) | Intra-assaya | Inter-assayb | ||||
|---|---|---|---|---|---|---|
| n | OD value c | CVd (%) | n | OD valuec | CVd (%) | |
| a Intra-assay variation was calculated from 6 replicates on a single day. b Inter-assay variation was calculated from triplicates on 5 different days. c Mean ± SD. d CV: coefficient of variation. | ||||||
| 25 | 6 | 0.277 ± 0.006 | 2.13 | 3 | 0.283 ± 0.012 | 4.20 |
| 50 | 6 | 0.262 ± 0.004 | 1.50 | 3 | 0.267 ± 0.011 | 4.27 |
| 100 | 6 | 0.204 ± 0.007 | 3.63 | 3 | 0.211 ± 0.010 | 4.90 |
Conclusion
In our research, a novel conjugate of ZEN-cBSA was prepared and used to prepare anti-ZEN antibodies. The resulting antibodies not only induced a fast immune response but also had a high titer and high specificity as expected. The 5 ZEN analogs affected the antibody interaction much less and showed lower CR with the anti-ZEN antibodies. Under the optimum ELISA conditions, the icELISA based on the mAb was used to detect ZEN in cereal and feed samples from markets in China and showed highly specific and satisfactory precision. In summary, the problem of anti-ZEN antibody specificity towards the 5 ZEN analogs was overcome in this study. The icELISA method developed in this study is ideally suited to be used as a specific detection method for ZEN contaminants in cereal and feed.Acknowledgements
This work was supported by the Important New Drug Research Project of the Ministry of Science and Technology of China (2009ZX09502-025), the Scientific Research Project of Traditional Chinese Medicine Vocation (200807042), and the Open Research Fund of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources (Chengdu, P.R. China).References
- J. W. Bennett and M. Klich, Clin. Microbiol. Rev., 2003, 16, 497 CrossRef CAS.
- T. Kuiper-Goodman, P. M. Scott and H. Watanabe, Regul. Toxicol. Pharmacol., 1987, 7, 253 CrossRef CAS.
- H. P. V. Egmond, M. A. Jonker, Worldwide regulations for mycotoxins in food and feed in 2003., Food and Agriculture Organization of the United Nations, Rome, 2004, p. 165 Search PubMed.
- L. C. James and D. S. Tawfik, Protein Sci., 2003, 12, 2183 CrossRef CAS.
- A. A. Burkin, G. P. Kononenko and N. A. Soboleva, Prikl. Biokhim. Mikrobiol., 2002, 38, 305 CAS.
- R. Teshima, M. Kawase, T. Tanaka, K. Hirai, M. Sato, J. Sawada, H. Ikebuchi, M. Ichinoe and T. Terao, J. Agric. Food Chem., 1990, 38, 1618 CrossRef CAS.
- T. Tanaka, P. Teshima, H. Ikebuchi, J. Sawada and M. Ichinoe, J. Agric. Food Chem., 1995, 43, 946 CrossRef CAS.
- M. Erbs, N. Hartmann and T. D. Bucheli, J. Aoac. Int., 2007, 90, 1197 CAS.
- A. Muckerheide, R. J. Apple, A. J. Pesce and J. G. Michael, J. Immunol., 1987, 138, 833 CAS.
- F. S. Chu, H. P. Lau, T. S. Fan and G. S. Zhang, J. Immunol. Methods, 1982, 55, 73 CrossRef CAS.
- J. Jean, C. Turcotte, R. E. Simard and I. Fliss, J. Immunol. Methods, 1999, 223, 155 CrossRef CAS.
- W. J. Fischer, I. Garthwaite, C. O. Miles, K. M. Ross, J. B. Aggen, A. R. Chamberlin, N. R. Towers and D. R. Dietrich, Environ. Sci. Technol., 2001, 35, 4849 CrossRef CAS.
- A. Muckerheide, P. L. Domen and J. G. Michael, J. Immunol., 1987, 138, 2800 CAS.
- R. J. Apple, P. L. Domen, A. Muckerheide and J. G. Michael, J. Immunol., 1988, 140, 3290 CAS.
- G. T. Hermanson, Bioconjugate techniques, Academic Press, San Diego, 1996, p. 785 Search PubMed.
- Y. Zhou, J. Wu, W. Yu, Y. Xu, P. Wang, B. Xie and F. Chen, J. Immunol. Methods, 2007, 328, 79 CrossRef CAS.
- M. T. Liu, B. P. Ram, L. P. Hart and J. J. Pestka, Appl. Environ. Microbiol., 1985, 50, 332 CAS.
- I. S. Kim, S. J. Setford and S. Saini, Anal. Chim. Acta, 2000, 422, 167 CrossRef CAS.
- R. Dietrich, E. Schneider, E. Usleber and E. Märtlbauer, Nat. Toxins, 1995, 3, 288 CrossRef CAS.
- A. Y. Kolosova, S. De Saeger, L. Sibanda, R. Verheijen and C. Van Peteghem, Anal. Bioanal. Chem., 2007, 389, 2103 CrossRef CAS.
- E. Y. Basova, I. Y. Goryacheva, T. Y. Rusanova, N. A. Burmistrova, R. Dietrich, E. Märtlbauer, C. Detavernier, C. Van Peteghem and S. De Saeger, Anal. Bioanal. Chem., 2010, 397, 55 CrossRef CAS.
- D. Thouvenot and R. F. Morfin, Appl. Environ. Microbiol., 1983, 45, 16 CAS.
- A. A. Burkin, G. P. Kononenko and N. A. Soboleva, Prikl. Biokhim. Mikrobiol., 2002, 38, 194 CAS.
- E. Usleber, V. Renz, E. Märtlbauer and G. Terplan, Zentralbl. Veterinarmed B., 1992, 39, 617 CrossRef CAS.
- T. Thongrussamee, N. S. Kuzmina, W. B. Shim, T. Jiratpong, S. A. Eremin, J. Intrasook and D. H. Chung, Food. Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk. Assess., 2008, 25, 997 CAS.
- J. L. Urraca, E. Benito-Pena, C. Perez-Conde, M. C. Moreno-Bondi and J. J. Pestka, J. Agric. Food Chem., 2005, 53, 3338 CrossRef CAS.
- C. M. Maragos and E. K. Kim, J. Food. Prot., 2004, 67, 1039 CAS.
- G. A. Bennett, T. C. Nelsen and B. M. Miller, J. Aoac. Int., 1994, 77, 1500 CAS.
- Q. Yuan, J. R. Clarke, H. R. Zhou, J. E. Linz, J. J. Pestka and L. P. Hart, Appl. Environ. Microbiol., 1997, 63, 263 CAS.
- R. Warner, B. P. Ram, L. P. Hart and J. J. Pestka, J. Agric. Food Chem., 1986, 34, 714 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1an15487g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
