Micromechanical measurement of AChBP binding for label-free drug discovery
Koutilya
Buchapudi
a,
Xiaohe
Xu
a,
Yeganeh
Ataian
b,
Hai-Feng
Ji
*a and
Marvin
Schulte
*b
aDepartment of Chemistry, Drexel University, Philadelphia, PA, USA 19104. E-mail: hj56@drexel.edu
bDepartment of Pharmacy, University of Alaska at Fairbanks, Fairbanks, AK, USA 99775. E-mail: mkschulte@alaska.edu
First published on 2nd November 2011
Abstract
A potential binding assay based on binding-driven micromechanical motion is described. Acetylcholine binding protein (AChBP) was used to modify a microcantilever. The modified microcantilever was found to bend on application of the naturally occurring agonist (acetylcholine) or the antagonist (nicotine and d-tubocurarine). Control experiments show that microcantilevers modified without AChBP do not respond to acetylcholine, nicotine, and d-tubocurarine. Kd values obtained for acetylcholine, nicotine, and d-tubocurarine are similar to those obtained from radio-ligand binding assays. These results suggest that the microcantilever system has potential for use in label free, drug screening applications.
Introduction
Ligand gated ion channels (LGICs) are involved in a wide range of physiological processes including nerve conduction, regulation of blood flow, fluid balance and gastric motility. In the central nervous system, these important proteins mediate fast excitatory and inhibitory neurotransmission. The importance of ligand gated ion channels and their endogenous ligands is reflected in the substantial effort that has been applied toward understanding their pharmacology, physiology, and biochemistry. The LGIC superfamily includes 5-HT3, GABA, nicotinic acetylcholine, glycine receptor, and ion-gated and histamine channels (Zn2+).1–7 These are pentameric membrane bound proteins with an amino terminal domain containing the multiple binding sites and a transmembrane region that anchors the protein in the membrane and forms the gated ion channel. Even though LGIC receptors have different channel specificities, they appear to utilize similar mechanisms of ligand specificity, channel opening, ion selectivity and desensitization. Research in the LGIC structure has been greatly facilitated by the discovery of a related acetylcholine binding protein (AChBP).8–10 The AChBP is a naturally occurring, soluble homolog of the amino terminal region of the Nicotinic acetylcholine receptors (nAChR). This protein assembles in a pentameric form and displays structural and functional similarity to the amino terminal domains of the homomeric α7 nAChR and 5-HT3AR. The crystal structure of the AChBP has been determined and provides the framework for developing homology models of 5-HT3AR (as well as other ligand gated ion channels)11 although only a limited amount of information about the specific pharmacology of the AChBP itself has been reported. In its native state this protein can be used in high throughput screening and drug discovery for nAChR ligands.Current high throughput analyses commonly utilize membrane bound receptor proteins or cell lines that express cloned receptor subtypes. These assays typically provide high throughput using either radioligand or fluorescent binding assays in combination with low to moderate throughput functional assays. Functional assays for LGIC receptors often use either automated patch clamp,12 automated Xenopusoocyte two electrode voltage clamp,13 or a system using a fluorescent calcium or voltage sensing dye.14 While these assays provide reasonably high throughput for evaluation of drug candidates, they have significant limitations. Chief among these are the need to prepare isolated membrane preparations and/or maintain cell cultures for each receptor type being evaluated. Receptor proteins are not particularly stable and cannot be easily stored for long periods of time. Cellular assays require continually maintained cell cultures. The assays themselves must be designed so that as many potential cross-reactions with other receptors are identified as possible. While these assays are reasonably high throughput, it is still time consuming to evaluate interactions on large numbers of receptors. In addition, commonly used assays do not identify all interactions with a receptor but only interactions that produce effects observable in the assay system used. As the technology for the rapid production of new chemical entities is extended, so the analytical characterization efforts must increase to support these programs. Such drug screening technologies will need to provide rapid evaluation of drug molecules, multiple receptor analysis, and must be cost efficient. One such technology involves the use of microcantilevers.
Advances in the field of micro-electro-mechanical systems (MEMS) and their uses offer unique opportunities in the design of small-size and cost-effective analytical methods. Microcantilevers are the simplest MEMS device that can be micromachined and mass-produced.15 The unique characteristic of microcantilevers is that they can be made to undergo bending due to molecular adsorption by confining the adsorption to one side of the microcantilever.16–23 Microcantilever sensors hold a position as a cost-effective and highly sensitive sensor platform for medical diagnostics, and environmental and high throughput analysis. Since the microcantilever's deflection is derived from molecular binding, no labeled compound is needed, and this technology could undoubtedly be used to study the interaction of small molecules with drug targets for drug screening. Microcantilevers can also be incorporated into multichannel microcantilever chips that offer improved dynamic response, greatly reduced size, high precision, increased reliability and integration of micromechanical components with on-chip electronic circuitry.19,20 The low cost of the microcantilever technology would be more accessible to smaller laboratories than current high throughput systems, thus providing an added advantage over existing technology.
In our previous work, we have studied interactions of small molecules with the 5-HT3R receptor embedded membrane by using the microcantilever method.24 This membrane immobilized microcantilever and its binding with the naturally occurring agonist serotonin (5-hydroxytryptamine) and the antagonist MDL-72222 were determined. However, although hydrophobic chip surfaces have been created for coupling membrane receptors, these receptors produce much less stable and reliable surfaces than covalently linked proteins. On the other hand, receptor proteins, such as AChBP, could be produced in large quantities and distributed for use in a variety of assay systems, since they are soluble receptor proteins they are relatively easily attached to chip based biosensors and they are stable. In this work, microcantilever bending is used for an AChBP-analytes binding study, which has applications in drug screening and drug development.
Methods
The cDNA sequences for AChBP from Lymnaea stagnalis (GeneBank accession number AF364899.1) were used to synthesize a full length cDNA. A 6X polyHis tag was added to the 3′ end of the cDNA to provide efficient purification of the expressed protein. cDNA synthesis was conducted by GeneArt Inc. (Burlingame, CA). The synthetic AChBP cDNA was inserted into a p3XFLAG-CMV-9 expression vector (Sigma Aldrich). Incorporation of the AChBP sequence into this vector provides an amino terminal Flag tag in addition to the C-terminal 6X His tag. The vector construct was transfected into HEK-293 cells and maintained in 0.4 mg ml−1 G418® (Sigma Aldrich) antibiotic for several weeks. Surviving HEK-293/AChBP cells stably expressed the AChBP protein. The protein was secreted into the extracellular medium and was purified using the Profinia® (Bio-rad) immobilized metal ion chromatography (IMAC) purification system. The purified protein was quantified by UV spectroscopy and further characterized using denaturing and native PAGE to confirm that the expressed protein was pure and present in its pentameric form. The protein was prepared in a 0.01 M phosphate buffer solution (PBS, pH = 7.0) that was used in all subsequent experiments.Purity and assembly of AChBP were determined using native and denaturing PAGE as shown in Fig. 1. The left hand gel shows the results of PAGE (non-denaturing conditions) and the right hand gel shows the result of separation under denaturing conditions (SDS-PAGE). Gels were stained with Coomassie Blue stain (BioRad). The protein sample lane is indicated by S and the BioRad Kaleidoscope standard is indicated by Std. Under non-denaturing conditions, a distinct band is shown to migrate similar to the 150 kDa standard. Under denaturing conditions, the dominant band shifts to slightly less than 37 kDa. The molecular weight of the native protein of about 150 kDa is consistent with the predicted molecular weight of the pentameric AChBP. The 37 kDa single band in the denaturing gel is as expected for the monomeric protein. In the non-denatured protein, a smaller band is evident at around 37 kDa as well suggesting some unassembled monomers are present.
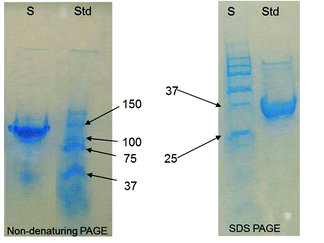 | ||
| Fig. 1 Polyacrylamide gel electrophoresis of AChBP purified by IMAC. | ||
We used commercially available silicon microcantilevers (Veeco Instrument, CA, http://store.veeco.com/) in these experiments. The dimensions of the V-shaped microcantilever are 200 μm in length, 20 μm in width, and 1 μm in thickness. One side of the cantilever had a thin film of chromium (3 nm) followed by a 20 nm layer of gold deposited by e-beam evaporation. The deflection experiments were performed in a flow-through glass cell (Veeco, CA) similar to those used in atomic force microscopy (AFM). For continuous flow-through experiments, initially, the electrolyte solution was driven through the cell using a syringe pump. A constant flow rate at 10 ml h−1 was maintained during each experiment. The cantilever was immersed in the electrolyte solution until a baseline was obtained and the voltage of the position-sensitive detector was set as background corresponding to 0 nm.
Experimental solutions containing different concentrations of analytes were injected directly into the flowing fluid stream via a low-pressure injection port sample loop arrangement with a loop volume of 0.5 ml. This arrangement allows for continuous exposure of the cantilever to the desired solution without disturbing the flow cell or changing the flow rate. Since the volume of the glass cell, including the tubing, was only 0.3 ml, a relatively fast replacement of the liquid in contact with the cantilever was achieved. Deflection measurements were determined using the optical beam deflection method. The bending of the cantilever was measured by monitoring the position of a laser beam reflected from the gold-coated side of the cantilever onto a four-quadrant AFM photodiode. We define bending toward the gold side as “bending up”; “bending down” refers to bending toward the silicon side. In case the adsorption occurs on the gold surface, in general, the downward bending is caused by repulsion or expansion of molecules on the gold surface, which is the so-called compressive stress; vice versa, the upward bending is caused by attraction or contraction of molecules on the gold surface, which is called tensile surface stress. Three cantilevers were prepared for each of the individual experiments to allow statistical comparison of repeatability and efficiency between devices. To eliminate thermomechanical motion of the silicon cantilever caused by temperature fluctuations, we mounted the fluid cell on thermoelectric coolers so that the temperature of the fluid cell could be controlled to 20 ± 0.2 °C.
The Kd, Hill coefficient and Bmax were calculated by fitting the data to the following equation: B/Bmax = 1/(1 + (Kd/[L])n), where B is the microcantilever bending, Bmax is the maximum bending at equilibrium, L is the free ligand (acetylcholine) concentration and n is the Hill coefficient.
Results and discussions
Conjugation chemistry
Significant effort has been focused on microcantilever fabrication and simulation of responses.25 While this is essential to developing inexpensive, practical microcantilever instrumentation, the production of specific biological sensor molecules and optimization of their attachment to the microcantilever surface are equally important. It is often assumed that surface conjugation chemistries utilized by other chip-based microsensors can be transferred to microcantilever devices; however, the mechanism of surface stress induced bending is substantially different from the mechanisms of other sensor platforms such as surface plasmon resonance. Optimization of a biosensor requires surface characteristics such as packing density, be appropriate to the sensor device, and the detection mechanism in use. Surface packing and orientation can be altered by using different conjugation chemistries. It has been observed that microcantilevers modified by different conjugation chemistry responded differently to the analytes.26 The approach using succinic anhydride did not work for microcantilever sensors. The approach using 1-ethyl-3-(3-dimethylaminopropyl) carbodimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) (Scheme 1) provided a relatively more reliable surface modification approach than the succinic anhydride approach. The chemistry of EDC/NHS has been studied in the past.27 In this work, we further investigated the effort of conjugation time on the performance optimization of our microcantilever sensors with improved response characteristics. Improved response characteristics will produce receptors with increased sensitivity.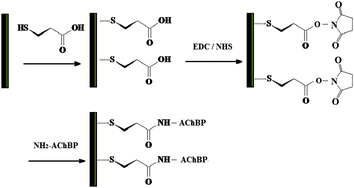 | ||
| Scheme 1 Immobilization of AChBP on the gold surface of microcantilevers by EDC/NHS crosslinker. | ||
In our experiments, clean microcantilevers were stored for 48 hours in a 1 mM ethanolic solution of 11-mercaptoundecanoic acid. These microcantilevers were then thoroughly rinsed with distilled water before being immersed in a solution of 0.5 M NHS and 0.2 M EDC in distilled water for a time period of 12–72 hours. This was followed by thorough rinsing of cantilevers in DI water to remove excess chemicals. The final step was the immobilization of the AChBP on the microcantilever. 5 μl of the AChBP was dissolved in 1 ml of phosphate buffer solution. The microcantilevers were incubated in this solution for a period of 12–48 hours. The microcantilevers were then rinsed to remove any excess AChBP. The chemically modified microcantilevers were stored in buffer solution before use.
Table 1 shows the effect of conjugation time of the EDC/NHS methods on the deflection of the AChBP-modified microcantilever surfaces. It shows that the conjugation time of EDC/NHS is more important than that of the following conjugation time of AChBP. It also showed that a protocol of EDC/NHS reaction time 48 hours and the following cross-linking for 48 hours provided the maximum deflection upon exposure to the same concentration of analytes and the standard error was within 7%. We used this protocol in our entire sensor test in this work.
| EDC/NHS reaction time/h | AChBP conjugation time/h | Deflection amplitude/nm |
|---|---|---|
| 12 | 12 | 69.0 ± 20.4 |
| 12 | 24 | 75.2 ± 15.9 |
| 12 | 48 | 75.2 ± 17.7 |
| 24 | 12 | 81.4 ± 17.7 |
| 24 | 24 | 83.2 ± 16.8 |
| 24 | 48 | 83.2 ± 17.8 |
| 48 | 12 | 88.5 ± 9.73 |
| 48 | 24 | 86.7 ± 10.6 |
| 48 | 48 | 90.2 ± 6.20 |
| 72 | 12 | 77.0 ± 13.3 |
| 72 | 24 | 83.2 ± 17.7 |
| 72 | 48 | 85.8 ± 11.5 |
Microcantilever deflection
AChBP coated microcantilever was initially exposed to a constant flow (4 ml h−1) of 0.010 M phosphate buffer solution (pH = 7.0) and the cantilever equilibrated until a stable baseline was obtained (i.e., 0 nm deflection). When a 10−5 M solution of acetylcholine in buffer (pH = 7.0) was switched into the fluid cell to replace the buffer solution at the same flow rate, the microcantilever was deflected until it reached equilibrium (450 seconds). The maximum amplitude of the microcantilever deflection was approximately 90 nm (Fig. 2). After the system reached equilibrium, a phosphate buffer solution was switched into the fluid cell to replace the acetylcholine solution and the cantilever deflection did not return to its original position in the time period we studied in our experiments because of the relatively strong binding of acetylcholine with AChBP. When the concentration of acetylcholine decreases, the deflection of the microcantilever was slower and the bending amplitude was less than that of 10−5 M. Exposure of microcantilevers coated without AChBP to the 10−5 M solution of acetylcholine produced no observable deflection indicating that acetylcholine by itself does not cause the microcantilever to bend. The presence of AChBP receptor is required.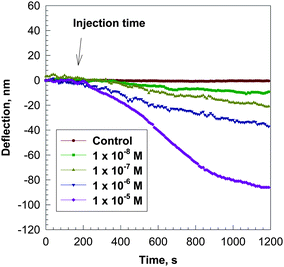 | ||
| Fig. 2 Bending response as a function of time, t, for a silicon microcantilever coated with AChBP on the gold surface after injection of a 10−4 M solution of acetylcholine in 0.01 M phosphate buffer at pH = 7.0. The microcantilever was pre-equilibrated in the 0.01 M phosphate buffer solution before injection of the acetylcholine solution. | ||
The bending amplitudes of the microcantilevers at equilibrium vs. the log of the concentration of acetylcholine are shown in Fig. 3. Each point shown on the curve represents the mean ± SE of three experiments. The standard deviation induced by variation in the cross-linked AChBP on different cantilevers and the position of the focused laser spot at the end of the cantilever was found to be within ±25%. The data in Fig. 3 were used to determine dissociate constants (Kd) of the AChBP to acetylcholine. A Kd value of 1.83 × 10−6 M was calculated from these data using non-linear curve fitting techniques and Graphpad Prism software, which is close to 4.3 × 10−6 M from Smit et al.28 for radioligand binding. A Bmax value of 107 ± 1.7 nm was also determined.
![Maximum deflection of a silicon cantilever coated with AChBP on the gold surface as a function of the concentration of acetylcholine in 0.01 M phosphate buffer at pH = 7.0. The Kd and Bmax were calculated by fitting the data to the following equation: B/Bmax = 1/(1 + (Kd/[L])n), where B is the microcantilever bending, Bmax is the maximum bending at equilibrium, L is the free ligand (acetylcholine) concentration and n is the Hill coefficient.](/image/article/2012/AN/c1an15734e/c1an15734e-f3.gif) | ||
| Fig. 3 Maximum deflection of a silicon cantilever coated with AChBP on the gold surface as a function of the concentration of acetylcholine in 0.01 M phosphate buffer at pH = 7.0. The Kd and Bmax were calculated by fitting the data to the following equation: B/Bmax = 1/(1 + (Kd/[L])n), where B is the microcantilever bending, Bmax is the maximum bending at equilibrium, L is the free ligand (acetylcholine) concentration and n is the Hill coefficient. | ||
Competitive antagonists were also tested to verify the specificity of microcantilever bending resulting from binding of ligands to the AChBP receptor and to identify any variation in binding kinetics for agonistsversusantagonists. Nicotine and d-tubocurarine were chosen due to their availability and they were well studied for ready comparison.
Similar to application of acetylcholine, 10−6 M nicotine or d-tubocurarine produced a deflection of the microcantilever coated with the AChBP (Fig. 4). The time to plateau is much faster for d-tubocurarine and nicotine compared to that for acetylcholine, indicating a high binding constant between AChBP with nicotine or d-tubocurarine. In the absence of AChBP no bending was observed on application of 10−6 M nicotine or d-tubocurarine indicating that nicotine or d-tubocurarine by itself does not produce a deflection of the microcantilever. The deflection amplitude at equilibrium of the microcantilever vs. the concentration of nicotine in the buffer solutions is shown in Fig. 5. Kd values of 1.92 × 10−8 M and 7.1 × 10−9 M−1 for nicotine and d-tubocurarine, respectively, were calculated from these data as described above. The Bmax values are 13 ± 0.47 nm and 32 ± 0.67 nm for nicotine and d-tubocurarine, respectively.
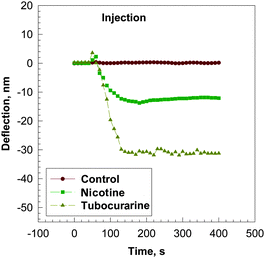 | ||
| Fig. 4 Bending response as a function of time, t, for a silicon microcantilever coated with AChBP after injection of a 10−6 M solution of nicotine (■) and d-tubocurarine (◆) in 0.01 M phosphate buffer at pH = 7.0. The microcantilever was pre-equilibrated in the 0.01 M phosphate buffer solution before injection of the analyte solution. Control experiments were done by introducing 10−6 M solution of nicotine (●) and d-tubocurarine (line not shown) to a microcantilever without AChBP in 0.01 M phosphate buffer at pH = 7.0. | ||
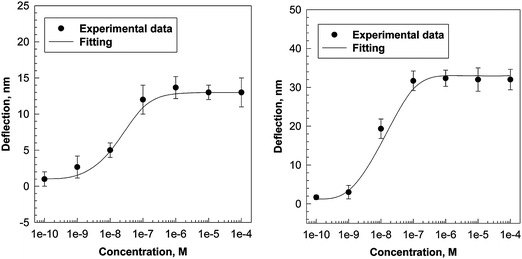 | ||
| Fig. 5 Maximum deflection of a silicon cantilever coated with AChBP as a function of the concentration of nicotine (left) and d-tubocurarine (right) in 0.01 M phosphate buffer at pH = 7.0. The Kd and Bmax were calculated as described in Fig. 2 for acetylcholine. | ||
The data presented here demonstrate that the deflections we observed are the result of binding of acetylcholine, nicotine, and d-tubocurarine with AChBP. From a molecular point of view, binding can result in electrostatic repulsion, attraction, steric effects, intermolecular interactions or a combination of effects that alter the surface stresses on the cantilever.29 The mechanism responsible for producing deflections of the AChBP-modified microcantilever in response to ligand binding may be due to a slight conformational change of the AChBP on the microcantilever derived from the binding of the ligand to AChBP. The conformational change of the AChBP upon binding to agonists and antagonists has been demonstrated by using a fluorescent probe.30 The surface stress resulting from protein conformation change has been a recent focus of MCL research.31 Conformational changes are capable of altering immobilization of molecules on the surface, distances between molecules, relative orientations and surface interactions; thus it is reasonable that conformational changes will alter MCL bending. The Bmax for nicotine and d-tubocurarine is much less than that for acetylcholine, which reflects a difference in conformational change of the AChBP on the surface. Partial agonists and antagonists, such as nicotine and d-tubocurarine, would likely produce smaller conformational changes compared to a full agonist like acetylcholine.
Conclusion
In summary, we demonstrated that AChBP-modified microcantilevers can be used to study the interactions of small molecules with AChBP. The microcantilever technology provides new opportunities for drug identification in an increasing number of therapeutic areas. This approach has the advantage that it might be applicable to a large number of proteins. A state-of-the-art screening microchip capable of developing and running biochemical assays such as enzyme inhibition as well as binding assays could be established.The bending approach based on adsorption-induced surface stress is of particular interest in the study of conformational change in proteins. Surface stress changes due to protein conformational change on interaction with analytes could act as transducers of chemical information. The AChBP is an example of a protein that undergoes conformational change on ligand binding. Microcantilevers responding to these stresses would be ideal for high sensitivity detection of the small dimensional changes expected. These microcantilevers could also be used to investigate conformational changes that do not involve analyte interactions.
While the use of the AChBP in high throughput applications may be limited, the similarity of this protein to the LGIC family makes it likely that other similar proteins might be developed with similar functionality on microcantilevers. Kostelidou et al. have reported the expression of multiple nicotinic subunits in a soluble form similar to the AChBP. Further advance in this direction could provide a wide array of human LGICs for use in microcantilever arrays.32 The development of microchip arrays makes it possible to evaluate a single drug candidate on a wide variety of receptors simultaneously. In addition to the development of large arrays containing multiple receptor subtypes, it would also be possible to develop arrays designed to identify the specific binding site on a receptor at which a drug candidate binds. This array would be composed of fully functional receptor proteins along with receptors mutated at different known binding sites on the protein. An inkjet printing technique can be used to modify the cantilever array, each cantilever modified by specific AChBP mutants or references.
Acknowledgements
This work is supported by National Institutes of Health (NIH) Grant Number 1R01NS057366.References
- A. Karlin and M. H. Akabas, Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins, Neuron, 1995, 15, 1231–1244 CrossRef CAS.
- A. Devillers-Thiery, J. L. Galzi, J. L. Eisele, S. Bertrand, D. Bertrand and J. P. Changeux, Functional architecture of the nicotinic acetylcholine receptor: a prototype of ligand-gated ion channels, J. Membr. Biol., 1993, 136, 97–112 CrossRef CAS.
- G. Grenningloh, A. Rienitz, B. Schmitt, C. Methfessel, M. Zensen, K. Beyreuther, E. D. Gundelfinger and H. Betz, The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors, Nature, 1987, 328, 215–220 CrossRef CAS.
- P. R. Schofield, M. G. Darlison, N. Fujita, D. R. Burt, F. A. Stephenson, H. Rodriguez, L. M. Rhee, J. Ramachandran, V. Reale and T. A. Glencorse, Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family, Nature, 1987, 328, 221–227 CrossRef CAS.
- P. A. Davies, W. Wang, T. G. Hales and E. F. Kirkness, A novel class of ligand-gated ion channel is activated by Zn2+, J. Biol. Chem., 2003, 278, 712–717 CrossRef CAS.
- Y. B. Zheng, B. Hirschberg, J. Yuan, A. P. Wang, D. C. Hunt, S. W. Ludmerer, D. M. Schmatz and D. F. Cully, Identification of two novel drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye, J. Biol. Chem., 2002, 277, 2000–2005 CrossRef CAS.
- I. Witte, H. J. Kreienkamp, M. Gewecke and T. Roeder, Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion, J. Neurochem., 2002, 83, 504–514 CrossRef CAS.
- K. Brejc, W. J. van Dijk, R. V. Klaassen, M. Schuurmans, J. van Der Oost, A. B. Smit and T. K. Sixma, Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors, Nature, 2001, 411, 269–276 CrossRef CAS.
- G. Maksay, Z. Bikadi and M. Simonyi, Binding interactions of antagonists with 5-hydroxytryptamine3A receptor models, J. Recept. Signal Transduct. Res., 2003, 23, 255–270 CrossRef CAS.
- D. C. Reeves, M. F. Sayed, P. L. Chau, K. L. Price and S. C. Lummis, Prediction of 5-HT3 receptor agonist-binding residues using homology modeling, Biophys. J., 2003, 84(4), 2338–2344 CrossRef CAS.
- S. B. Hansen, T. T. Talley, Z. Radic and P. Taylor, Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica, J. Biol. Chem., 2004, 279, 24197–24202 CrossRef CAS.
- S. Friis, C. Mathes, M. Sunesen, M. R. Bowlby and J. Dunlop, Characterization of compounds on nicotinic acetylcholine receptor alpha7 channels using higher throughput electrophysiology, J. Neurosci. Methods, 2009, 177, 142–148 CrossRef CAS.
- U. Pehl, C. Leisgen, K. Gampe and E. Guenther, Automated higher-throughput compound screening on ion channel targets based on the Xenopus laevis oocyte expression system, Assay Drug Dev. Technol., 2004, 2, 515–524 CAS.
- C. Joesch, E. Guevarra, S. P. Parel, A. Bergner, P. Zbinden, D. Konrad and H. Albrecht, Use of FLIPR membrane potential dyes for validation of high-throughput screening with the FLIPR and microARCS technologies: identification of ion channel modulators acting on the GABA(A) receptor, J. Biomol. Screening, 2008, 13, 218–228 CrossRef CAS.
- Y. Tang, F. Fang, X. Yan and H.-F. Ji, Fabrication and characterization of SiO2 microcantilever for microsensor application, Sens. Actuators, B, 2004, 97, 109–113 CrossRef.
- J. Fritz, M. K. Baller, H. P. Lang, H. Rothuizen, P. Vettiger, E. Meyer, H. J. Guntherodt, C. Gerber and J. K. Gimzewski, Translating biomolecular recognition into nanomechanics, Science, 2000, 288, 316–318 CrossRef CAS.
- G. Wu, R. Datar, K. M. Hansen, T. Thundat, R. J. Cote and A. Majumdar, Bioassay of prostate specific antigen (PSA) using microcantilevers, Nat. Biotechnol., 2001, 19, 856–860 CrossRef CAS.
- H. F. Ji, T. Thundat, R. Dabestani, G. M. Brown, P. F. Britt and P. Bonnesson, Ultrasensitive detection of trace CrO42− using a microcantilever sensor, Anal. Chem., 2001, 73, 1572–1576 CrossRef CAS.
- D. Lange, C. Hagleitner, A. Hierlemann, O. Brand and H. Baltes, Complementary metal oxide semiconductor cantilever arrays on a single chip: mass-sensitive detection of volatile organic compounds, Anal. Chem., 2002, 74, 3084–3095 CrossRef CAS.
- C. L. Britton, Jr, R. J. Warmack, S. F. Smith, P. I. Oden, R. L. Jones, T. Thundat, G. M. Brown, W. L. Bryan, J. C. Depriest, M. N. Ericson, M. S. Emery, M. R. Moore, G. W. Turner, A. L. Wintenberg, T. D. Threatt, Z. Hu, L. G. Clonts and J. M. Rochelle, Multiple-input micorcantilever sensor with capacitive readout, in Symposium on Advanced Research in VLSI, 1999 Search PubMed.
- H. F. Ji, E. Finot, R. Dabestani, T. Thundat, G. M. Brown and P. F. Britt, A noval self-assembled monolayer coated microcantilever for low level cesium detection, Chem. Commun., 2000, 457–458 RSC.
- G. Y. Chen, T. Thundat, E. A. Wachter and R. J. Warmack, Absorption-induced surface stress and its effect on resonance frequency of microcantilevers, J. Appl. Phys., 1995, 77, 3618–3622 CrossRef CAS.
- H. P. Lang, M. K. Baller, R. Berger, Ch. Gerber, J. K. Gimzewaki, F. M. Battiston, P. Fornaro, J. P. Ramseyer, E. Meyer and H. J. Guntherodt, An artificial nose based on a micromechanical cantilever array, Anal. Chim. Acta, 1999, 393, 59–65 CrossRef CAS.
- Y. Zhang, S. P. Venkatachalan, H. Xu, X. Xu, P. Joshi, H.-F. Ji and M. Schulte, Micromechanical measurement of membrane receptor binding for label-free drug discovery, Biosens. Bioelectron., 2004, 19, 1473–1478 CrossRef CAS.
- M. S. K. Mutyala, D. Bandhanadham, L. Pan, V. R. Pendyala and H. F. Ji, Mechanical and electronic approaches to improve the sensitivity of microcantilever sensors, Acta Mech. Sin., 2009, 25, 1–12 CrossRef CAS.
- S. Velanki, H.-F. Ji, T. Thundat and D. A. Blake, Detection of Cd2+ using antibody based microcantilevers, Ultramicroscopy, 2007, 107, 1123–1128 CrossRef CAS.
- S. Sam, L. Touahir, J. S. Andresa, P. Allongue, J. N. Chazalviel, A. C. Gouget-Laemmel, C. H. de Villeneuve, A. Moraillon, F. Ozanam, N. Gabouze and S. Djebbar, Semiquantitative study of the EDC/NHS Activation of acid terminal groups at modified porous silicon surfaces, Langmuir, 2010, 26, 809–814 CrossRef CAS.
- A. B. Smit, N. I. Syed, D. Schaap, J. van Minnen, J. Klumperman, K. S. Kits, H. Lodder, R. C. vander Schors, R. van Elk, B. Sorgedrager, K. Brejc, T. K. Sixma and W. P. Geraerts, Aglia-derived acetylcholine-binding protein that modulates synaptic transmission, Nature, 2001, 411, 261–268 CrossRef CAS.
- R. Berger, E. Delamarche, H. P. Lang, Ch. Gerber, J. K. Gimewski, E. Meyer and H.-J. Guntherodt, Surface stress in the self-assembly of alkanethiols on gold probed by a force microscopy technique, Appl. Phys. A: Mater. Sci. Process., 1998, 66, S55–S59 CrossRef CAS.
- R. E. Hibbs, T. T. Talley and P. Taylor, Acrylodan-conjugated cystein side chains reveal conformational state and ligand site locations of the acetylcholine-binding proteins, J. Biol. Chem., 2004, 279, 28483–28491 CrossRef CAS.
- H.-F. Ji, H. Gao, K. R. Buchapudi, X. Yang, X. Xu and M. K. Schulte, Microcantilever biosensors based on conformational change of proteins, Analyst, 2008, 132, 434–443 RSC.
- K. Kostelidou, N. Trakas, M. Zouridakis, K. Bitzopoulou, A. Sotiriadis, I. Gavra and S. J. Tzartos, Expression and characterization of soluble forms of the extracellular domains of the beta, gamma and epsilon subunits of the human muscle acetylcholine receptor, FEBS J., 2006, 273, 3557–3566 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
