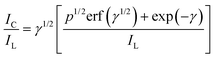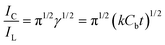DOI:
10.1039/C1AN15746A
(Paper)
Analyst, 2012,
137, 209-215
Electrochemical sensor for neurotransmitters at physiological pH using a heterocyclic conducting polymer modified electrode†‡
Received
17th August 2011
, Accepted 27th September 2011
First published on 3rd November 2011
Abstract
We report the simultaneous determination of two neurotransmitters, norepinephrine (NEP) and serotonin (5-HT), at physiological pH using the electropolymerized film of 3-amino-5-mercapto-1,2,4-triazole modified glassy carbon (p-AMTa) electrode. A bare glassy carbon (GC) electrode fails to resolve the voltammetric signals of NEP and 5-HT due to the surface fouling caused by the oxidized products of them. However, the p-AMTa electrode not only separates the voltammetric signals of NEP and 5-HT with a potential difference of 150 mV between NEP and 5-HT but also shows higher oxidation currents for them. The simultaneous determination of NEP and 5-HT was successfully achieved at p-AMTa electrode using differential pulse voltammetry method. The amperometric current response increased linearly with increasing NEP and 5-HT concentration in the range of 1.0 × 10−8 to 1.0 × 10−4 M and 1.0 × 10−8 to 5.0 × 10−5 M, respectively, and the detection limit was found to be 1.65 × 10−11 for NEP and 1.32 × 10−11 M for 5-HT (S/N = 3). The p-AMTa electrode shows better recovery results for spiked NEP and 5-HT in human blood plasma samples.
Introduction
Norepinephrine (NEP) is a catecholamine and it acts as a biochemical messenger in mammalian central nervous systems. A 3.8-fold excess of NEP in neuronal cell body causes neuronal death in Alzheimer's disease.1 Increased plasma NEP is associated with coronary heart disease2 and muscle sympathetic nerve traffic in human obesity.3 The loss of NEP in brain neuronal cells is associated with Parkinson's disease.4 Higher over-commitment to work was associated with lower NEP secretion and leads to psychosocial stress in humans.5 Increased 24-hour urinary NEP excretion is a symptom of depression and anxiety for middle-aged women.6Serotonin or 5-hydroxytryptamine (5-HT) is another important neurotransmitter and it is involved in signal transduction pathways in the human body.7 Increased levels of brain 5-HT is related to Huntington's disease8 and reduced levels of brain 5-HT induces Down's syndrome, as in Alzheimer's disease.9 The ratio of blood plasma to whole-blood 5-HT has been reported as a novel marker of atherosclerotic cardiovascular disease.10 A reduced level of thrombocyte 5-HT is connected to the suicidal behaviour of psychiatric patients.11DNA damage was induced by 5-HT in the presence of copper ions.12 Thus, accurate determination of both NEP and 5-HT is very important from a clinical point of view.
Several methods have been employed for the determination of NEP individually, which include microdialysis,13 end-column chemiluminescene,14HPLC capillary electrophoresis coupled with amperometric detection,15ion chromatography with direct conductivity detection,16 micellar electrokinetic capillary chromatography with UV absorbance detection17 and voltammetry.18–22 Similar methods have been used for the determination of 5-HT individually.23NEP and 5-HT coexist in the spinal cord,24 brain cells,25–28 and plasma.10,29 Thus, simultaneous determination of them is essential to investigate the above mentioned diseases. To date, only a limited number of methods have been reported for the simultaneous determination of NEP and 5-HT, which include microdialysis,24HPLC,25,26,30 and LC.27,31 However, these methods are very tedious, time consuming processes and more expensive. On the other hand, an electrochemical method of determination has several advantages, such as being less expensive, more convenient and highly selective and sensitive. Since the oxidation potentials of NEP and 5-HT are very close, simultaneous determination of them is a difficult task for the researchers by electrochemical method. Only one report was available in the literature for the simultaneous determination of these two neurotransmitters by electrochemical method using eriochrome cyanine R film modified glassy carbon (GC) electrode.32 However, stable determination of 5-HT was not achieved at this electrode due to the formation of new intermediate products. Further, a poor detection limit was achieved for both NEP and 5-HT.32 In the present study, we wish to report a highly stable determination of NEP and 5-HT simultaneously using the electropolymerized film of 3-amino-5-mercapto-1,2,4-triazole on GC electrode (p-AMTa) at physiological pH. Further, we have achieved detection limit of 1.65 × 10−11 for NEP and 1.32 × 10−11 M for 5-HT (S/N = 3) by amperometry method. The practical application of the present modified electrode was demonstrated by determining NEP and 5-HT in human blood plasma samples.
Experimental
Chemicals
3-Amino-5-mercapto-1,2,4-triazole (AMTa), norepinephrine (NEP) and serotonin (5-HT) were purchased from Aldrich and were used as received. All other chemicals used in this investigation were of analytical grade. pH 7.2 phosphate buffer (PB) solution was prepared using Na2HPO4 and NaH2PO4. Double distilled water was used to prepare the solutions used in this investigation.
Instrumentation
Electrochemical measurements were performed in a conventional two compartment, three electrode cell with a mirror-polished 3 mm GC electrode as a working electrode, Pt wire as a counter electrode and a NaCl saturated Ag/AgCl reference electrode. All of the electrochemical measurements were carried out on a CHI model 634B (Austin, TX, USA) Electrochemical Workstation. For differential pulse voltammetry measurements, pulse width of 0.06 s, amplitude of 0.05 V, sample period of 0.02 s and pulse period of 0.20 s were used. For chronoamperometric measurements, pulse width of 0.25 s and potential step of one were used. All of the electrochemical measurements were carried out under a nitrogen atmosphere at 27 °C. The tapping mode AFM images were recorded using a Nanoscope (IV) instrument (Vecco). The Field Emission Scanning Electron Microscopy (FE-SEM) images were recorded using a JEOL-model JSM 6701F. For AFM and FE-SEM measurements, indium tin oxide (ITO) purchased from Asahi Beer Optical Ltd., Japan was used as a substrate.
Fabrication of p-AMTa modified GC electrode
The GC electrode was polished with 0.05 μm alumina slurry and then rinsed thoroughly with water. Then the electrode was sonicated in water for 5 min to remove any adsorbed alumina particles. The electropolymerization of AMTa on GC electrode was carried out by 15 successive potential sweeps between −0.20 V to +1.70 V at a scan rate of 50 mV s−1 in 1 mM AMTa containing 0.1 M H2SO4 (Fig. ES1 in the ESI†).33 After the electropolymerization, the polymer modified electrode was removed from the solution, washed with water and then used for electrochemical measurements. We have also obtained identical electropolymerization behavior on an ITO surface.
Results and discussion
AFM studies of p-AMTa film on ITO electrode
The size and the morphology of the p-AMTa film were investigated by AFM. Fig. 1 shows the tapping mode AFM image (5 μm × 5 μm) of the p-AMTa film. The AFM image shows the formation of homogeneous film on an ITO surface. The inset of Fig.1 shows that the deposited p-AMTa film has a spherical-like structure. The diameter of each spherical-like structure was 20–30 nm and the thickness of the p-AMTa film was found to be ∼30 nm.
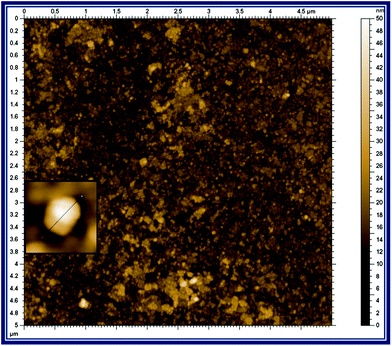 |
| | Fig. 1
Tapping mode AFM image of the p-AMTa film deposited on an ITO electrode. Inset: Magnified AFM image of single spherical particles of p-AMTa. | |
The morphology of the polymer film was also characterized by FE-SEM. Fig. ES2 in the ESI† shows the FE-SEM image of the p-AMTa film. The image represents the uniform polymer film formation on the ITO surface. It also indicates the formation of spherical-like structures. The size of each spherical-like structure was found to be ∼20 nm.
Electrochemical behavior of NEP and 5-HT at bare and p-AMTa modified electrodes
We have optimized the electrocatalytic activity of the p-AMTa electrode towards NEP and 5-HT with respect to deposition cycles and pH. We found that the p-AMTa film deposited by 15 cycles on the GC electrode showed higher electrocatalytic activity towards NEP and 5-HT when compared to films deposited by more than 15 cycles. Further, we have performed the oxidation of NEP and 5-HT at different pH using the p-AMTa electrode. We obtained a higher oxidation current with less positive potentials for NEP and 5-HT at pH 7.2 (Fig. ES3 in the ESI†). Thus, p-AMTa film deposited by 15 cycles and 0.2 M PB solution (pH 7.2) were chosen for the determination of NEP and 5-HT. Fig. 2A shows the cyclic voltammograms (CVs) obtained for 0.5 mM NEP at bare GC and p-AMTa electrodes in 0.2 M PB solution at pH 7.2. The bare GC electrode shows a broad irreversible oxidation peak at 0.23 V for NEP in the first cycle (curve a). In the subsequent cycles, the oxidation peak current was decreased (curve b). This is due to the surface fouling effect caused by the oxidation products of NEP at the bare GC electrode. On the other hand, the p-AMTa electrode shows a sharp oxidation peak at 0.20 V with an enhanced current for NEP, which is 30 mV less positive potential than at the bare GC electrode and a reduction peak at 0.15 V in the first cycle (curve c). The peak separation was found to be 50 mV. In the subsequent cycles, the redox peak of NEP remains very stable and after 8 cycles only a very slight decrease in peak current was observed (curve d). This indicates that p-AMTa electrode was effectively catalyzing the NEP oxidation without any fouling effect.
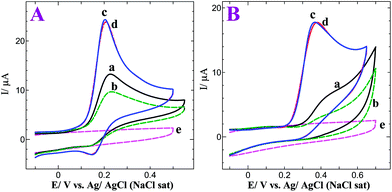 |
| | Fig. 2
(A)
CVs obtained for 0.5 mM NEP at a bare GC electrode (a) 1st cycle and (b) after 6 cycles and a p-AMTa electrode (c) 1st cycle and (d) after 8 cycles in 0.2 M PB solution (pH 7.2) at a scan rate of 50 mV s−1. CV obtained in the absence of NEP (e) at a p-AMTa electrode in PB solution at pH 7.2 at a scan rate of 50 mV s−1. (B)CVs obtained for 0.5 mM 5-HT at a bare GC electrode (a) 1st cycle and (b) after 6 cycles and a p-AMTa modified GC electrode (c) 1st cycle and (d) after 8 cycles in 0.2 M PB solution (pH 7.2) at a scan rate of 50 mV s−1. CV obtained in the absence of 5-HT (e) at a p-AMTa electrode in PB solution at pH 7.2 at a scan rate of 50 mV s−1. | |
Fig. 2B shows the CVs obtained for 0.5 mM 5-HT at bare GC and p-AMTa electrodes in 0.2 M PB solution at pH 7.2. The bare GC electrode shows an ill-defined oxidation wave around 0.44 V for 5-HT in the first cycle (curve a). In the subsequent cycles, the oxidation wave was shifted to more positive potential with decreased current. After 6 cycles, the obtained ill-defined oxidation wave had almost disappeared (curve b). The adsorption of oxidized products of 5-HT on the GC electrode surface was the possible reason for the decreased oxidation current with more positive potential shift at the bare GC electrode. Thus, the bare GC electrode was not suitable for the stable determination of 5-HT. On the other hand, a well-defined oxidation peak at 0.37 V with enhanced current was observed for 5-HT at the p-AMTa electrode (curve c), which is 70 mV less positive potential than at the bare GC electrode. After 8 cycles, a slight shift in the oxidation potential was observed (curve d). This indicates that p-AMTa electrode effectively prevents the fouling caused by the oxidation products of 5-HT. The above results show that the p-AMTa electrode can be used for the stable determination of NEP and 5-HT. The p-AMTa electrode does not show any signal in the absence of analytes in 0.2 M PB solution at pH 7.2 (curve e).
Further, we have calculated the catalytic rate constant (k) of NEP oxidation at bare GC and p-AMTa electrodes. Since NEP oxidation is a reversible process, we have used the method of Galus (eqn (1))22,34 to calculate the k value of the catalytic process of NEP.
| | 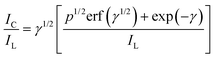 | (1) |
where
IC is the catalytic current of NEP at p-AMTa
electrode,
IL is the limiting current in the absence of NEP (
IC and
IL obtained from
chronoamperometry) and
γ =
kCbt (
Cb is the bulk concentration of NEP and
t is the time elapsed) and
γ is the argument of the error function. In the cases where
γ exceeds 2, the error function is almost equal to
eqn (1) and therefore the above equation can be reduced to
eqn (2),
| | 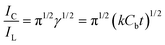 | (2) |
The eqn (2) can be used to calculate the k value. Based on the slope of the IC/ILversus t1/2 plot; k can be obtained for a given NEP concentration. From the values of the slopes, an average value of k was found to be 2.82 and 6.21 × 104 M−1 s−1 for bare GC and p-AMTa electrodes, respectively. The obtained higher value of k at p-AMTa electrode indicates that the catalyzed oxidation process of NEP was higher at the p-AMTa electrode. Further, the heterogeneous rate constant (ks) for the oxidation of NEP at p-AMTa electrode was calculated as 3.76 × 10−2 cm s−1 (Γ = 6.05 × 10−10 mol cm−2).33 5-HT oxidation is two electrons involved irreversible process35,36 and hence we have used Velasco eqn (3)37 to calculate ks at bare GC and p-AMTa electrodes.
| | | kS = 1.11D01/2(Ep − Ep/2)−1/2v1/2 | (3) |
where
Do is apparent diffusion coefficient;
Ep is
oxidation peak potential;
Ep/2 is half-wave
oxidation peak potential and
ν is scan rate. The
D0 value was determined based on cottrell slope obtained from a single potential
chronoamperometry technique. The estimated
ks values for the oxidation of 5-HT at bare
GC and p-AMTa electrodes were found to be 5.4 × 10
−4 and 3.0 × 10
−3 cm s
−1, respectively. The higher obtained
ks value for 5-HT at the p-AMTa electrode indicated that the oxidation of 5-HT was faster at the p-AMTa electrode than at the bare GC
electrode.
The effect of scan rate on the oxidation of NEP and 5-HT were studied. The CVs obtained for NEP and 5-HT at the p-AMTa electrode at scan rates from 50 to 1000 mV s−1 in PB solution at pH 7.2 are shown in Fig. ES4A and Fig. ES4B in the ESI.† A good linearity was obtained while plotting the current against the square root of the scan rate with correlation coefficients of 0.9949 (inset of Fig. ES4A†), and 0.9967 (inset of Fig. ES4B†) for NEP and 5-HT oxidations, respectively, indicating that the oxidation of NEP and 5-HT at p-AMTa electrode was a diffusion-controlled process.
Electrochemical behavior of NEP and 5-HT in a mixture at bare GC and p-AMTa electrodes
Since NEP and 5-HT co-exist in spinal cord,24 brain25–27 and plasma fluids,10,29 it is essential to determine them from their mixture. Fig. 3 exhibits the differential pulse voltammograms (DPVs) obtained for a mixture of 0.5 mM each of NEP and 5-HT at bare GC and p-AMTa electrodes in 0.2 M PB solution (pH 7.2). The bare GC electrode shows an oxidation wave at 0.31 V for 5-HT with a shoulder wave for NEP oxidation in the first cycle (curve a). In the subsequent cycles, 5-HT oxidation wave was drastically decreased with a slight shift to more a positive potential and the shoulder wave due to NEP completely disappeared (curve b). The observed results indicate that the bare GC electrode was not suitable for the determination of NEP and 5-HT in a mixture. On the other hand, the p-AMTa electrode not only resolved the voltammetric signals of NEP and 5-HT with a potential difference of 150 mV but also enhanced their oxidation currents (curve c). The oxidation potentials of NEP and 5-HT were observed at 0.14 and 0.29 V, respectively. Even after 6 cycles, stable oxidation peaks were observed for NEP and 5-HT at the p-AMTa electrode (curve d). The obtained potential difference of 150 mV is enough for the simultaneous determination of NEP and 5-HT in a mixture at p-AMTa electrode.
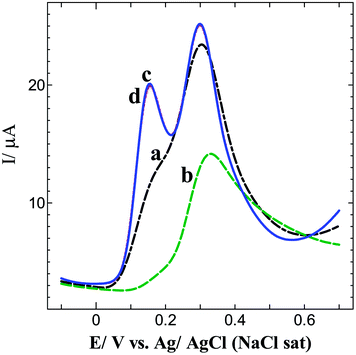 |
| | Fig. 3
DPVs obtained for 0.5 mM each of NEP and 5-HT at bare GC and p-AMTa electrodes; 1st cycle (a and c) and after 6 cycles (b and d) in 0.2 M PB solution (pH 7.2). | |
The p-AMTa film contains an –NH– group in the heterocyclic ring. Thus, interactions of NEP and 5-HT with the –NH– group of the p-AMTa film are possible viahydrogen bonding (Scheme ES1†).38–40 Further, hydrophobic interactions between the p-AMTa electrode and NEP and 5-HT molecules are also possible.41,42 These are the plausible reasons for the enhanced oxidation peak currents of NEP and 5-HT at the p-AMTa electrode when compared to the bare GC electrode.
Simultaneous determination of NEP and 5-HT using p-AMTa electrode
The main objective of the present work is to simultaneously determine NEP and 5-HT using a p-AMTa electrode. The obtained large peak separation between NEP and 5-HT as well as their stability encouraged us to determine them simultaneously. Fig. 4 shows the DPVs obtained for the simultaneous determination of NEP and 5-HT in PB solution (pH 7.2) at a p-AMTa electrode. A well-defined signal was observed for 8 μM NEP and 7 μM 5-HT at 0.15 and 0.30 V, respectively (curve a). When the concentration of NEP was increased from 8 μM to 56 μM and 5-HT was increased from 7 μM to 49 μM (curves a–g), the peak currents of the respective analytes increased linearly with a correlation coefficient of 0.9903 for NEP (inset of Fig. 4A) and 0.9965 for 5-HT (inset of Fig. 4B). The oxidation peak potentials of NEP and 5-HT remain the same for further increases in concentration.
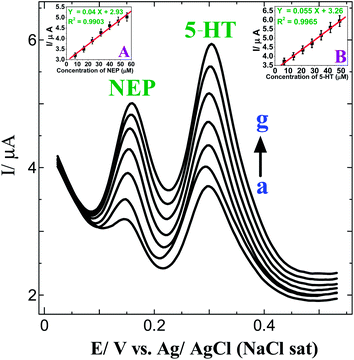 |
| | Fig. 4
DPVs obtained for the addition of 8–56 μM NEP and 7–49 μM 5-HT (curves a–g) at a p-AMTa electrode in 0.2 M PB solution (pH 7.2). Insets: (A) Plot of concentration of NEPvs. current. (B) Plot of concentration of 5-HT vs. current. | |
Selective determination of NEP and 5-HT
It is known that NEP and 5-HT co-exist in human fluids and their concentrations vary depending upon the particular disease. For example, a 3.8-fold excess of NEP in neuronal cell bodies causes the neuronal death in Alzheimer's disease1 and the ratio of blood plasma to whole-blood 5-HT can be used as a novel marker of atherosclerotic cardiovascular disease.10 Hence, from a clinical point of view, the selective determination of NEP and 5-HT in the presence of a high concentration of the other analyte is important. Fig. ES5A† shows the DPV obtained for 15 μM NEP in the presence of 0.6 mM 5-HT in 0.2 M PB solution (pH 7.2) at a p-AMTa electrode. A clear voltammetric signal was observed for 15 μM NEP in the presence of 0.6 mM 5-HT, which revealed that detection of a low concentration of NEP is possible even in the presence of 40-fold 5-HT. Fig. ES5B† shows the DPV obtained for 5 μM 5-HT in the presence of 0.2 mM NEP in 0.2 M PB solution (pH 7.2) at p-AMTa electrode. A clear voltammetric signal was observed for 5 μM 5-HT in the presence of 0.2 mM NEP, indicating that detection of a low concentration of 5-HT is possible even in the presence of 40-fold NEP. These results revealed that the p-AMTa electrode is selective towards NEP and 5-HT in the presence of high concentrations of the other analyte. Further, it is known that ascorbic acid (AA), uric acid (UA) and tyrosine (Tyr) co-exist with NEP and 5-HT in human fluids6,10,29 and hence the simultaneous determination of NEP and 5-HT in the presence of high concentrations of AA, UA and Tyr is important. Fig. ES6† shows the DPV obtained for 5 μM each of NEP and 5-HT in the presence of 0.5 mM each of AA, UA and Tyr in 0.2 M PB solution (pH 7.2) at the p-AMTa electrode. Clear voltammetric signals were observed for 5 μM each of NEP and 5-HT in the presence of 0.5 mM each of AA, UA and Tyr. This suggests that detection of low concentrations of NEP and 5-HT are possible even in the presence of 100-fold AA, UA and Tyr.
Amperometric determination of NEP and 5-HT
The amperometric method was used to examine the sensitivity of the p-AMTa electrode towards the detection of NEP and 5-HT individually. Fig. 5A shows the amperometric i–t curve for NEP at a p-AMTa electrode in a homogeneously stirred 0.2 M PB solution (pH 7.2) by applying a potential of +0.4 V. The p-AMTa electrode shows the current response for each increase of 10 nM NEP. The current response increases and the steady state current response was attained within 3 s for further increases of 10 nM NEP in each step with a sample interval of 50 s. The dependence of the response current with respect to concentration of NEP was linear from 10 nM to 80 nM at the p-AMTa electrode with a correlation coefficient of 0.9909 (inset of Fig. 5A). The amperometric i–t curve for each increase of 10 nM NEP showed linear current increase without noise. Fig. 5B shows that the amperometric current response increased linearly with increasing NEP concentration in the range of 1.0 × 10−8–1 × 10−4 M with a correlation coefficient of 0.9914 (inset of Fig. 5B) and the detection limit was found to be 1.65 × 10−11 M (S/N = 3) for NEP.
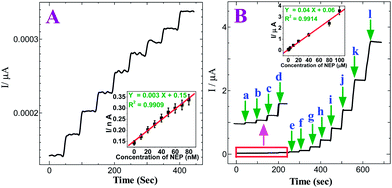 |
| | Fig. 5
(A) Amperometric i–t curve for the determination of NEP at p-AMTa electrode in 0.2 M PB solution (pH 7.2). Each addition increases the concentration of NEP by 10 nM at a regular interval of 50 s. Eapp = +0.4 V. Inset: Plot of concentration of NEPvs. current. (B) Amperometric i–t curve for the increment of (a) 0.01 (b) 0.1 (c) 0.2 (d) 0.4 (e) 0.8 (f) 2 (g) 4 (h) 10 (i) 20 (j) 40 (k) 80 and (l) 100 μM NEP at p-AMTa electrode in 0.2 M PB solution (pH 7.2) at a regular interval of 50 s. Eapp = +0.4 V. Inset: Plot of concentration of NEPvs. current. | |
Fig. 6A shows the amperometric i–t curve for 5-HT at the p-AMTa electrode in a homogeneously stirred 0.2 M PB solution (pH 7.2) by applying a potential of +0.6 V. The p-AMTa electrode shows the current response for each increase of 10 nM 5-HT. The current response increases for further increase of 10 nM 5-HT in each step with a sample interval of 50 s. The dependence of the response current with respect to concentration of 5-HT was linear from 10 nM to 60 nM at p-AMTa electrode with a correlation coefficient of 0.9989 (inset of Fig. 6A). Fig. 6B shows that the amperometric current response increased linearly with increasing 5-HT concentration in the range of 1.0 × 10−8–5 × 10−5 M with a correlation coefficient of 0.9902 (inset of Fig. 6B) and the detection limit was 1.32 × 10−11 M (S/N = 3) for 5-HT.
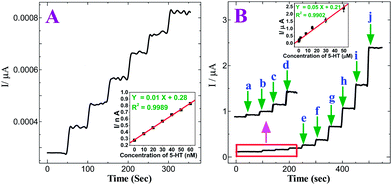 |
| | Fig. 6
(A) Amperometric i–t curve for the determination of 5-HT at p-AMTa electrode in 0.2 M PB solution (pH 7.2). Each addition increases the concentration of 5-HT by 10 nM at a regular interval of 50 s. Eapp = +0.6 V. Inset: Plot of concentration of 5-HT vs. current. (B) Amperometric i–t curve for the increment of (a) 0.01 (b) 0.1 (c) 0.2 (d) 0.4 (e) 0.8 (f) 2 (g) 8 (h) 15 (i) 30 and (j) 50 μM 5-HT at p-AMTa electrode in 0.2 M PB solution (pH 7.2) at a regular interval of 50 s. Eapp = +0.6 V. Inset: Plot of concentration of 5-HT vs. current. | |
The observed linear range and the lowest detection limit of NEP at the p-AMTa electrode were compared with the reported modified electrodes18–22,32 and are given in Table 1. As can be seen from Table 1, the present modified electrode showed the lowest detection limit (1.65 × 10−10 M) and a wide range for NEP (1.0 × 10−8–1 × 10−4 M) compared to the reported modified electrodes. We have also compared the linear range and the lowest detection limit of 5-HT at the p-AMTa electrode with the reported modified electrodes32,35,36,43–45 and the values are given in Table 2. As can be seen from Table 2, the present modified electrode showed the lowest detection limit (1.32 × 10−10 M) and a wide range for 5-HT (1.0 × 10−8–5 × 10−5 M) compared to the reported modified electrodes.
Table 1 Comparison of different modified electrodes for the determination of NEP with a p-AMTa electrode
Table 2 Comparison of different modified electrodes for the determination of 5-HT with a p-AMTa electrode
|
Electrodes
|
pH |
Linear range (M) |
Detection limit (M) |
Reference |
|
Gold nanocluster and polypyrrole composite modified GC electrode |
7.0 |
7.0 × 10−9–2.2 × 10−6 |
1.0 × 10−9 |
35
|
| Chitosan coated carbon fiber microelectrode |
7.4 |
2.0 × 10−9–1.0 × 10−7 |
1.6 × 10−9 |
44
|
|
Gold nanoparticles modified indium tin oxide electrode |
7.2 |
1.0 × 10−8–2.5 × 10−4 |
3.0 × 10−9 |
43
|
|
Nafion
membrane coated colloidal gold screen-printed electrode |
7.4 |
2.0 × 10−8–5.0 × 10−4 |
5.0 × 10−9 |
36
|
| Poly(phenosafranine) modified GC electrode |
7.1 |
1.0 × 10−5–2.0 × 10−7 |
2.0 × 10−8 |
45
|
|
Erichrome cyanine R electrodeposited GC electrode |
7.0 |
5.0 × 10−8–5.0 × 10−6 |
5.0 × 10−8 |
32
|
| Poly(3-amino-5-mercapto-1,2,4-triazole) modified GC electrode |
7.2 |
1.0 × 10−8–5.0 × 10−5 |
1.32 × 10−11 |
This work |
Anti-interference ability and stability of the p-AMTa electrode
The detection of NEP and 5-HT at p-AMTa electrode was carried out in the presence of various common ions such as Na+, K+, NH4+, Mg2+, Ca2+, Cl−, F−, CO32− and SO42− and some physiological interferents such as glucose, urea and oxalate by amperometric method. No change in the amperometric current response was observed for 10 nM each of NEP and 5-HT in the presence of 10 μM of MgSO4, CaCl2, NaCl, K2CO3, NaF, NH4Cl, urea, glucose and oxalate, indicating that the present p-AMTa electrode is highly selective towards the determination of NEP and 5-HT individually in the presence of 1000-fold excess of these interferents.
In order to investigate the stability of the p-AMTa electrode, the CVs were recorded for 0.5 mM each of NEP and 5-HT individually in 0.2 M PB solution at 5 min intervals. It was found that oxidation peak currents of NEP and 5-HT remained same with a relative standard deviation of 1.45% and 1.97%, respectively, for 20 repetitive measurements, indicating that this electrode has a good reproducibility. The NEP oxidation current response decreased by about 1.25% in 1 week and 3.08% in 2 weeks when the electrode was kept in pH 7.2 PB solution. The 5-HT oxidation current response decreased by about 2.03% in 1 week and 4.15% in 2 weeks when the electrode was kept in pH 7.2 PB solution. To ascertain the reproducibility of the results further, three different GC electrodes were modified with the p-AMTa electrode and their response towards the oxidation of 0.5 mM each of NEP and 5-HT in a mixture was recorded by 15 repeated measurements. The separation between the voltammetric peaks of NEP and 5-HT was same at all three electrodes. The peak current obtained in the 15 repeated measurements of three independent electrodes showed a relative standard deviation of 2.93%, confirming that the results are reproducible. The above results showed that the present p-AMTa electrode was very stable and reproducible towards these analytes.
Recovery test of NEP and 5-HT in human plasma samples
The practical application of the present method was tested by measuring the concentrations of NEP and 5-HT in human blood plasma samples. The human plasma samples were collected from a clinical laboratory and diluted to 20 times with 0.2 M PB solution (pH 7.2) without any treatment. The recovery experiments were carried out by a standard addition method in blood plasma samples. When blood plasma samples were spiked with 5 μM NEP or 5-HT, the oxidation peaks of NEP and 5-HT were appeared in DPV. The recovery results were obtained and the results are summarized in Table 3 and Table 4. The proposed method showed a good recovery of spiked NEP and 5-HT in human samples, indicating that the present modified electrode could be applied to determine NEP and 5-HT in different human fluids.
Table 3 Determination and recovery test of NEP in human blood plasma
| Blood plasma |
Spiked NEP (μM) |
Found (μM) |
Recoveries (%) |
| Sample 1 |
5 |
4.94 |
98.80 |
| 10 |
9.80 |
98.00 |
| 15 |
14.72 |
98.13 |
| Sample 2 |
5 |
4.92 |
98.40 |
| 10 |
9.82 |
98.20 |
| 15 |
14.71 |
98.06 |
Table 4 Determination and recovery test of 5-HT in human blood plasma
| Blood plasma |
Spiked 5-HT (μM) |
Found (μM) |
Recoveries (%) |
| Sample 1 |
5 |
4.89 |
97.80 |
| 10 |
9.76 |
97.60 |
| 15 |
14.71 |
98.06 |
| Sample 2 |
5 |
4.88 |
97.60 |
| 10 |
9.82 |
98.20 |
| 15 |
14.69 |
97.93 |
Conclusions
In this paper, we reported the simultaneous determination of NEP and 5-HT using a p-AMTa electrode in 0.2 M PB solution at physiological pH. The bare GC electrode failed to resolve the voltammetric signals of NEP and 5-HT in a mixture while the p-AMTa electrode successfully resolved them. Unlike the bare GC electrode, the p-AMTa electrode effectively prevented the surface fouling effect and thus a stable determination of NEP and 5-HT with a potential difference of 150 mV was achieved. The oxidation currents of NEP and 5-HT were enhanced at the p-AMTa electrode when compared to the bare GC electrode due to possible hydrogen bonding interactions between heterocyclic –NH– group of the p-AMTa film and NEP and 5-HT. The detection of 10 nM each NEP and 5-HT were achieved at the p-AMTa electrode using the amperometric method. The amperometric current response increased linearly with increasing NEP concentration in the linear range of 1.0 × 10−8–1 × 10−4 M and the detection limit was found to be 1.65 × 10−11 M (S/N = 3), and in the range of 1.0 × 10−8–5 × 10−5 M for 5-HT and the detection limit was found to be 1.32 × 10−11 M. From a clinical point of view, the p-AMTa electrode showed good recoveries for spiked NEP and 5-HT in human plasma samples.
References
- W. J. Burke, S. W. Li, C. A. Schmitt, P. Xia, H. D. Chung and K. N. Gillespie, Brain Res., 1999, 816, 633 CrossRef CAS.
- R. M. Carney, K. E. Freedland, R. C. Veith, P. E. Cryer, J. A. Skala, T. Lynch and A. S. Jaffe, Biol. Psychiatry, 1999, 45, 458 CrossRef CAS.
- G. Grassi, G. Seravalle, R. Dell'Ore, F. Arenare, R. Facchetti and G. Mancia, Nutr., Metab. Cardiovasc. Dis., 2009, 19, 469 CrossRef CAS.
- K. S. Rommelfanger and D. Weinshenker, Biochem. Pharmacol., 2007, 74, 177 CrossRef CAS.
- P. H. Wirtz, J. Siegrist, U. Rimmele and U. Ehlert, Psychoneuroendocrinology, 2008, 33, 92 CrossRef CAS.
- J. W. Hughes, L. Watkins, J. A. Blumenthal, C. Kuhn and A. Sherwood, J. Psychosom. Res., 2004, 57, 353 Search PubMed.
- M. B. Hansen and E. Skadhauge, Comp. Biochem. Physiol., 1997, 118A, 283 CrossRef CAS.
- G. P. Reynolds and S. J. Pearson, Neurosci. Lett., 1987, 22, 233 CrossRef.
- C. M. Yates, J. Simpson and A. Gordon, Neurosci. Lett., 1986, 65, 189 CrossRef CAS.
- K. Hara, Y. Hirowatari, M. Yoshika, Y. Komiyama, Y. Tsuka and H. Takahashi, J. Lab. Clin. Med., 2004, 144, 31 CrossRef CAS.
- N. Ruljancic, M. Mihanovic and I. Cepelak, Progress Neuro-Psychopharmacol. Biol. Psychiatry, 2011, 35, 1261 CrossRef CAS.
- N. Hadi, S. Singh, A. Ahmad and R. Zaidi, Neurosci. Lett., 2001, 308, 83 CrossRef CAS.
- C. W. Berridge and E. D. Abercrombie, Neuroscience, 1999, 93, 1263 CrossRef CAS.
- Z. Lin, X. Wu, X. Lin and Z. Xie, J. Chromatogr., A, 2007, 1170, 118 CrossRef CAS.
- M. Novotny, V. Quaiserova-Mocko, E. A. Wehrwein, D. L. Kreulen and G. M. Swain, J. Neurosci. Methods, 2007, 163, 52 CrossRef CAS.
- C. L. Guan, J. Ouyang, Q. L. Li, B. H. Liu and W. R. G. Baeyens, Talanta, 2000, 50, 1197 CrossRef CAS.
- G. Liu, J. Chen and Y. Ma, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 805, 281 CrossRef CAS.
- R. N. Goyal and S. Bishnoi, Talanta, 2010, 84, 78 CrossRef.
- P. Kalimuthu and S. A. John, Electrochim. Acta, 2011, 56, 2428 CrossRef CAS.
- L.-P. Lu, S.-Q. Wang and X.-Q. Lin, Anal. Chim. Acta, 2004, 519, 161 CrossRef CAS.
- M. Mazloum-Ardakani, H. Beitollahi, M. K. Amini, F. Mirkhalaf and M. Abdollahi-Alibeik, Sens. Actuators, B, 2010, 151, 243 CrossRef.
- M. Mazloum-Ardakani, H. Beitollahi, M. A. Sheikh-Mohseni, H. Naemi and N. Taghavinia, Appl. Catal., A, 2010, 378, 195 CrossRef CAS.
- Y. Hirowatari, K. Hara, H. Kamihata, T. Iwasaka and H. Takahashi, Clin. Biochem., 2004, 37, 191 CrossRef CAS.
- T. L. Lisi, K. N. Westlund and K. A. Sluka, J. Neurosci. Methods, 2003, 126, 187 CrossRef CAS.
- M. E. P. Hows, L. Lacroix, C. Heidbreder, A. J. Organ and A. J. Shah, J. Neurosci. Methods, 2004, 138, 123 CrossRef CAS.
- M. K. Lakshmana and T. R. Raju, Anal. Biochem., 1997, 246, 166 CrossRef CAS.
- T. Yoshitake, K. Fujino, J. Kehr, J. Ishida, H. Nohta and M. Yamaguchi, Anal. Biochem., 2003, 312, 125 CrossRef CAS.
- T. Yoshitake, J. Kehr, S. Yoshitake, K. Fujino, H. Nohta and M. Yamaguchi, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 807, 177 CrossRef CAS.
- E. R. Peskind, D. Wingerson, M. Pascualy, L. Thal, R. C. Veith, D. M. Dorsa, S. Bodenheimer and M. A. Raskind, Biol. Psychiatry, 1995, 38, 532 CrossRef CAS.
- V. Carrera, E. Sabater, E. Vilanova and M. A. Sogorb, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 847, 88 CrossRef CAS.
- S. Rinne, A. Holm, E. Lundanes and T. Greibrokk, J. Chromatogr., A, 2006, 1119, 285 CrossRef CAS.
- H. Yao, S. Li, Y. Tang, Y. Chen, Y. Chen and X. Lin, Electrochim. Acta, 2009, 54, 4607 CrossRef CAS.
- S. B. Revin and S. A. John, Electrochim. Acta, 2011, 56, 8934 CrossRef CAS.
-
Fundamentals of Electrochemical Analysis, ed. Z. Galus, 1976, Ellis Horwood, New York Search PubMed.
- J. Li and X. Lin, Sens. Actuators, B, 2007, 124, 486 CrossRef.
- M. Liu, J. Xiang, J. Zhou and H. Ding, J. Electroanal. Chem., 2010, 640, 1–7 CrossRef CAS.
- J. G. Velasco, Electroanalysis, 1997, 9, 880 CrossRef.
- P.-Y. Chen, R. Vittal, P.-C. Nien, G.-S. Liou and K.-C. Ho, Talanta, 2010, 80, 1145 CrossRef CAS.
- L. Özcan and Y. Sahin, Sens. Actuators, B, 2007, 127, 362 CrossRef.
- E. Pardieu, H. Cheap, C. Vedrine, M. Lazerges, Y. Lattach, F. Garnier, S. Remita and C. Pernelle, Anal. Chim. Acta, 2009, 649, 236 CrossRef CAS.
- P. R. Roy, T. Okajima and T. Ohsaka, Bioelectrochemistry, 2003, 59, 11 CrossRef CAS.
- V. S. Vasantha and S.-M. Chen, J. Electroanal. Chem., 2006, 592, 77 CrossRef CAS.
- R. N. Goyal, V. K. Gupta, M. Oyama and N. Bachheti, Talanta, 2007, 72, 976 CrossRef CAS.
- R. E. Ozel, K. N. Wallace and S. Andreescu, Anal. Chim. Acta, 2011, 695, 89 CrossRef CAS.
- T. Selvaraju and R. Ramaraj, Electrochem. Commun., 2003, 5, 667 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1an15746a |
| ‡ This article is part of a web theme in Analyst and Analytical Methods on Future Electroanalytical Developments, highlighting important developments and novel applications. Also in this theme is work presented at the Eirelec 2011 meeting, dedicated to Professor Malcolm Smyth on the occasion of his 60th birthday. |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here.