The preparation of a novel organic–inorganic hybrid monolithic column with sonication-assist and its application
Junjie
Ma
,
Gengliang
Yang
*,
Cuihong
Yan
,
Yanzhao
Gu
,
Ligai
Bai
,
Yanhui
Duan
and
Jia
Li
College of Pharmacy, Hebei University, Hebei Province Key Laboratory of Pharmaceutical Quality Control, Baoding, Hebei 071002, China. E-mail: ygl@hbu.edu.cn; Fax: +86-312 5971107; Tel: +86-312 5971108
First published on 23rd November 2011
Abstract
A novel hydroxyl functionalized organic–inorganic hybrid monolithic column for high performance liquid chromatography (HPLC) was synthesized viafree radical copolymerization with sonocation-assist, which could shorten the time of the sol–gel process. Vinyltrimethoxysilane (VTMS) was used as the monomer and vinyl ester resin was used as both the monomer and crosslinker. The conditions of preparation were investigated and the characteristics of the hybrid column were studied by SEM and Fourier transform infrared spectroscopy. The obtained column showed high permeability and low backpressure. The column was used to separate lysozyme from egg white in a short time (6 min) by HPLC, and benzene and its homologs were separated by the hybrid column. In addition, influences on the elution of lysozyme, such as the pH value and the buffer concentration, were studied. Additionally, the lysozyme separated by the hybrid column showed high biological activity, which was assayed by the method of turbidimetry.
1 Introduction
Monolithic columns can be described as integrated continuous porous separation media for separation science. In the past decade, monolithic column have drawn our attention and applied widely in chromatographic separation. There are many advantages that monolithic columns possess, including easy preparation, versatility in surface modification, great permeability, and good peak capacity. These unique merits have made monolithic columns widely used in analytical separation science.1,2Basically, monolithic materials are divided into two groups: organic polymer and inorganic polymer. Organic polymer-based monolithic columns possess many advantages, such as good stability to pH. But there are also disadvantages, such as poor mechanical stability, which results in a short lifetime, and undesirable retention reproducibility in some cases.3,4 Additionally, lack of commercially available polar monomers and the limited solubility of very polar monomers have influenced the development of hydrophilic polymer-monolithic columns. As a result, reports on hydrophilic polymer-based monolithic columns are still very limited. In contrast, the inorganic silica-based monolithic columns demonstrate good solvent resistance and high mechanical stability. Nevertheless, silica-based columns suffer from the disadvantage of poor pH resistance and are time consuming to prepare. This is because certain steps (e.g. the drying and cladding of the rods) are difficult to overcome in academic laboratories and it took us several days to fabricate silica-based columns.5–8
In order to overcome the disadvantages of inorganic and organic monolithic columns, the organic–inorganic hybrid monolithic columns have been developed via a sol–gel process and have attracted great attention since the organic functional moieties can be incorporated into the inorganic silica monolithic matrixes to combine the merits of inorganic and organic monoliths, such as good mechanical stability and solvent resistance, and overcome the disadvantages of both inorganic and organic monolithic columns.7–11 Yan et al. have prepared an octyl-functionalized hybrid silica monolithic column using tetraethoxysilane (TEOS) and n-octyltriethoxysilane (C8-TEOS) as the monomers and the octyl-functionalized hybrid silica monolithic column was successfully used in electrochromatography, where polycyclic aromatic hydrocarbons (PAHs) and phenols were successfully separated with high column efficiency of up to 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plates m−1.12
000 plates m−1.12
Other approaches have been attempted to prepare the hybrid columns and the performance of the hybrid columns have been investigated.13 Zou et al. reported an approach (one pot process) in which the hydrolyzed alkoxysilanes of tetramethoxysilane (TMOS) and vinyltrimethoxysilane (VTMS) as precursors were copolymerized with the organic monomer of allyldimethyldodecylammonium bromide (ADDAB) for synthesis of a hybrid monolith. The column was also applied in the analysis of tryptic digests of bovine serum albumin (BSA) and mouse liver extract by micro-liquid chromatography tandem mass spectrometry (μLC-MS/MS), demonstrating its potential in proteome analysis.8 There are also other kinds of hybrid monolithic columns. For example, metals can be used as the inorganic moiety, as reported in other articles.14 Bai et al. used vinyl ester resin as the monomer, sodium bisulfite both as organic adjunct and coadunate initiator to prepare a strong cation hybrid column, which was used to separate lysozyme (Lys) from egg white.15
Hou et al. have prepared an organic–inorganic hybrid silica monolith based immobilized titanium ion affinity chromatography column. Using such a hybrid silica monolithic Ti4+–IMAC column, phosphopeptides were effectively isolated from the digest mixture of α-casein and BSA with a molar ratio as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, illustrating its superior selectivity.16 Xu et al. used glycidyl methacrylate (GMA), ethylene dimethacrylate (EDMA) and 2-mercaptoethanol to fabricate a thiol-modified monolithic column, then in situreduction of chloroauric acid within the column was used to form gold nanoparticles attached to the surface of the pores of the monolith. The monolithic column provided a means to selectively retain cysteine-containing peptides. Application of the gold-modified hybrid monolith in tandem with a packed C18 capillary column was demonstrated with baseline separation of six peptides (three cysteine-containing peptides).17 However, the column was not directly used in analysis and the effect of column was enriching peptides. Additionally the preparation process of the column was complicated and would take us at least two days to fabricate the columns. Yang et al. used the method of radical polymerization of the continuous phase of oil in water high-internal-phase emulsions to fabricate porous poly(vinyl ester) resin monolithic supports, and it could be used to separate immunoglobulin from human plasma and chicken egg yolk.18 However, the preparation method of the column was complicated and it was difficult to control the formation of column.
200, illustrating its superior selectivity.16 Xu et al. used glycidyl methacrylate (GMA), ethylene dimethacrylate (EDMA) and 2-mercaptoethanol to fabricate a thiol-modified monolithic column, then in situreduction of chloroauric acid within the column was used to form gold nanoparticles attached to the surface of the pores of the monolith. The monolithic column provided a means to selectively retain cysteine-containing peptides. Application of the gold-modified hybrid monolith in tandem with a packed C18 capillary column was demonstrated with baseline separation of six peptides (three cysteine-containing peptides).17 However, the column was not directly used in analysis and the effect of column was enriching peptides. Additionally the preparation process of the column was complicated and would take us at least two days to fabricate the columns. Yang et al. used the method of radical polymerization of the continuous phase of oil in water high-internal-phase emulsions to fabricate porous poly(vinyl ester) resin monolithic supports, and it could be used to separate immunoglobulin from human plasma and chicken egg yolk.18 However, the preparation method of the column was complicated and it was difficult to control the formation of column.
In our work, an organic–inorganic hybrid monolithic column with hydroxyl functional group was developed with sonication-assist and it could be used to separate lysozyme from egg white, and benzene from its homologs. With the use of sonication, the time of sol–gel process was shortened from 12 h to 20 min. The characteristics of the hybrid column and factors influencing elution were investigated. The results showed that the column exhibited good mechanical strength and pH resistance. Moreover, the lysozyme separated from egg white by the hybrid column possessed high biological activity after being assayed by the method of turbidimetry.
2 Experiments
2.1 Chemicals
Vinyl ester resin was synthesized from bisphenol A diglycidyl ether (BADE). Lysozyme was obtained from Sigma Chemical Co. (St Louis, MO, USA). Dodecyl alcohol was obtained from China Medicament Co. Ltd. (Beijing, China). Polyethylene glycol (PEG, MW = 10 000) was purchased from Sigma Chemical Co. (St Louis, MO, USA). 2, 2′-Azobisisobutyronitrile (AIBN) was purchased from Shanghai Chemical Plant (Shanghai, China). Vinyltrimethoxysilane (VTMS) was purchased from Guibao Co. Ltd. (Hangzhou, China). HPLC-grade methanol was used for the preparation of mobile phases. All of these chemicals were analytical reagent grade. Triplex distilled water was used for all experiments.A 1100 system from Agilent Technologies (Shanghai, China) was applied to chromatographic studies. The HPLC system consisted of a quaternary pump with an online vacuum degasser, an autosampler with variable injection capacity from 0.1 to 100 μL and a UV detector. Chromatographic separation of Lys was carried out on the polymeric monolithic column (50 mm × 4.6 mm i.d.). All sample solutions injected into the chromatographic system were filtered through a Millipore membrane (0.45 μm) to remove particles. Scanning electron microscopy was purchased by Hitachi High Technologies, (S-4300, Japan).
2.2 Preparation of the organic–inorganic hybrid monolith
 | ||
| Fig. 1 The synthesis process of vinyl ester resin. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW, 0.01 g) and acetic acid (0.05 M, 0.1 mL) were mixed in a polytetrafluoroethylene tube, and then sonicated to form a homogeneous solution at 40 °C for 20 min. After that, 1 mL of dodecyl alcohol, 0.8 mL of vinyl ester resin and 2 mg of AIBN were added into the homogeneous solution with 10 min sonication. The solution was then injected into the stainless-steel tube of a 50 mm × 4.6mm i.d. chromatographic column, both ends sealed with rubber and the column was submerged in a water bath at 60 °C for 12 h for polymerization. After that, the column was rinsed by methanol to flush out the residual material for further use.
000 MW, 0.01 g) and acetic acid (0.05 M, 0.1 mL) were mixed in a polytetrafluoroethylene tube, and then sonicated to form a homogeneous solution at 40 °C for 20 min. After that, 1 mL of dodecyl alcohol, 0.8 mL of vinyl ester resin and 2 mg of AIBN were added into the homogeneous solution with 10 min sonication. The solution was then injected into the stainless-steel tube of a 50 mm × 4.6mm i.d. chromatographic column, both ends sealed with rubber and the column was submerged in a water bath at 60 °C for 12 h for polymerization. After that, the column was rinsed by methanol to flush out the residual material for further use.
2.3 Chromatographic characters of the monolith
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 4 °C for 15 min. The supernatant fluid was used as the Lys source. The chromatographic separation was performed using a gradient which was: water for the first 2.5 min and 0.02 mol L−1Na2HPO4 (pH = 4.0) for the next 10 min. The chromatography of separation is shown in Fig. 6.
000 rpm) at 4 °C for 15 min. The supernatant fluid was used as the Lys source. The chromatographic separation was performed using a gradient which was: water for the first 2.5 min and 0.02 mol L−1Na2HPO4 (pH = 4.0) for the next 10 min. The chromatography of separation is shown in Fig. 6.
2.4 Assay binding capacity of hybrid column
The method of frontal analysis was carried out to determine the dynamic binding capacity of hybrid monolithic column for Lys, with 3 mg mL−1 Lys in the mobile phase of 0.02 mol L−1Na2HPO4 aqueous solution (pH = 4).2.5 The determination of Lys activity
The biological activity of lysozyme separated on the hybrid column was measured by a turbidimetric assay. The main procedures were followed from the article reported previously.20,21 Firstly, the lysozyme separated by the hybrid column was lyophilized, then dissolved in 0.05 mol L−1phosphate (pH = 6.2). Secondly, a Micrococcus lysodeikticus suspension (0.05 mol L−1phosphate, pH = 6.2) was poured into Lys solution and mixed to form a homogeneous solution. Thirdly, the homogeneous solution was placed on a rocking bed (25 °C, 50 rpm, 1 min), and the decline of the absorbance measured at 450 nm every 15 s was detected. All samples were assayed in triplicate.3 Results and discussions
3.1 Characterizations of the hybrid monolithic column
| No. | PEG (g) | 0.05 M HAc (mL) | Vinyl ester resin (mL) | Dodecyl alcohol (mL) | VTMS (mL) | AIBN (g) | Polycondensation temperature (°C) | Copolymerization temperature (°C) |
|---|---|---|---|---|---|---|---|---|
| C1 | 0.01 | 0.1 | 0.9 | 1 | 0.6 | 0.01 | 40 | 60 |
| C2 | 0.01 | 0.1 | 0.8 | 1 | 0.6 | 0.01 | 40 | 60 |
| C3 | 0.01 | 0.1 | 0.7 | 1 | 0.6 | 0.01 | 40 | 60 |
| C4 | 0.01 | 0.1 | 0.8 | 0.9 | 0.6 | 0.01 | 40 | 60 |
| C5 | 0.01 | 0.1 | 0.8 | 0.8 | 0.6 | 0.01 | 40 | 60 |
| C6 | 0.01 | 0.1 | 0.8 | 1 | 0.6 | 0.01 | 50 | 60 |
| C7 | 0.01 | 0.1 | 0.8 | 1 | 0.6 | 0.01 | 30 | 60 |
| C8 | 0.02 | 0.1 | 0.8 | 1 | 0.6 | 0.01 | 40 | 60 |
| C9 | 0.005 | 0.1 | 0.8 | 1 | 0.6 | 0.01 | 40 | 60 |
| C10 | 0.02 | 0.1 | 0.8 | 1 | 0.7 | 0.01 | 40 | 60 |
| C11 | 0.02 | 0.1 | 0.8 | 1 | 0.5 | 0.01 | 40 | 60 |
From Table 1, we learned that if the ratio of vinyl ester resin was increased, the permeability of the column would be decreased and the backpressure would increase because the vinyl ester resin was not only the monomer but also the cross linking agent. Dodecyl alcohol was the porogenic solvent, and a low content of it could result in a drop in permeability; that is, the backpressure of the column would increase. HAc aqueous solution was used as the catalyzer of polycondensation in our studies. Generally, the polycondensation reaction comprises of two stepwise reactions: hydrolysis and condensation of metal alkoxide. Si–O–C bonds of VTMS were hydrolyzed and formed Si–OH in the presence of HAc aqueous solution, then Si–OH condensed and formed the polycondensation product Si–O–Si in the presence of PEG, which induced the separation of phases. The polycondensation of silicone oxide is usually performed at 40 °C for 12 h.8,12 The copolymerization of monomers is performed at 60 °C for 12 or 24 h. With the assistance of sonication, the process of sol–gel accelerates, the time of the sol–gel process will decrease and the total time of preparation of the hybrid column will be shortened. Compared with the ordinary method of sol–gel process at 40 °C for 12 h without sonication-assist, there is no obvious change in the hybrid monolithic column prepared with sonication-assist. As a result, the use of sonication decreases the total time of preparation of the hybrid column.
In our work, the polycondensation was performed at a relatively low temperature (30, 40 or 50 °C), and the copolymerization was performed at 60 °C, as usually adopted for the preparation of organic monolithic columns with AIBN as the initiator. The results showed that the temperature of 30 °C was not appropriate for the polycondensation of VTMS, because the column of C7 had a high permeability. When the temperature is low, the reaction of polycondensation is not complete. However, when the temperature was increased to 50 °C, the column of C6 showed bad permeability and high backpressure, so 40 °C was chosen as the polycondensation temperature. The content of PEG and VTMS also affected the permeability of the hybrid monolithic column. With an increase of PEG, column C8 showed low permeability and high backpressure. With a decrease of PEG, column C9 showed low backpressure with high permeability. That was because PEG induced the separation of phases in the sol–gel process. The phenomenon of PEG in the formation of hybrid column was similar to the article reported previously.22 In our research, the content of VTMS was also considered. The polycondensation product of VTMS was not only the monomer but also the cross linker in the formation of the hybrid column. Therefore, an increase in the VTMS in the composition led to high backpressure and low permeability (C10). The backpressure became low with a decrease in VTMS in the composition of hybrid column (C11). As a result, the conditions of C2 gave the best preparation conditions for the column.
 | ||
| Fig. 2 The IR spectrum of the hybrid column. | ||
 | ||
| Fig. 3 Scanning electron microscopy of the hybrid column. | ||
 | ||
| Fig. 4 The relationship of flow rate and backpressure (chromatographic conditions: the prepared hybrid monolith C2, 50 mm × 4.6 mm i.d.; mobile phase: water.). | ||
The pore diameter distribution of C2 was characterized by mercury intrusion porosimetry and is shown in Fig. 5. From the results, it was determined that the general pore volume, average pore diameter and interval porosity were 1.327 mL g−1, 0.74 μm and 65.38%, respectively.
 | ||
| Fig. 5 Pore size distribution profiles for the monolith by mercury intrusion porosimetry. | ||
3.2 Separation of Lys from egg white
Fig. 6 shows the chromatogram and two peaks were observed. Lys was separated from egg white within 6 min was found to be the second peak. In previous studies, there were many methods which were used for the separation of Lys. Bai15 used the –SO3H hybrid column to separate Lys from egg white in 10 min. But the hybrid column we prepared had an advantage: the time of separation of Lys was shorter than that reported previously; it only took us 6 min to separate the Lys from egg white. What is more, the hybrid column had a good stability in the experiment. The RSD of the retention peak area of the Lys in different times and column-to-column were 0.81% (n = 5) and 2.51% (n = 5), respectively. | ||
| Fig. 6 The figure of separation of Lys from egg white. HPLC conditions: the prepared hybrid monolith C2, 50 mm × 4.6 mm i.d; a) mobile phase: water for the first 2.5 min and 0.02 mol L−1NaH2PO4 (pH = 4.0) for 10 min; b) mobile phase: water. | ||
3.3 Effect of pH and buffer concentration on the elution of Lys
The effects of pH and the concentration of the mobile phase were studied in our research, and Fig. 7 and 8 show the results. When the mobile phase was water, Lys was not eluted from the hybrid column (Fig. 6), so we chose aqueous phosphate solution as the elution agent. As the salt concentration increased, the protein bound to the column desorbed.23Fig. 7 showed that the concentration of buffer salt had an effect on the elution of Lys. The elution effect increased with the increase of salt concentration and the greatest elution effect on Lys was at 0.02 mol L−1 aqueous phosphate solution. It is not difficult to explain the phenomenon. The stability of a protein is related to the size of mass point, electric charge and hydration layer, and electrolyte destroys the structure of electric double layer. So the stability of Lys solution was degraded and the Lys precipitated. As a result, the Lys was eluted by aqueous phosphate solution and 0.02 mol L−1NaH2PO4 which possessed the greatest ability of elution was chosen as the mobile phase. Fig. 8 revealed the effect of pH on the elution of Lys and it showed that the ability of elution decreased with an increase in pH value; that is, the Lys was retained on the hybrid monolith. The phenomenon can be explained as follows: because the isoelectric point (pI) of Lys is close to 11.0, when pH < pI, Lys is positively charged. When the value of pH increases in the range of 4 to 10, the positive charge on Lys decreases. There are lots of hydroxyl group on the stationary phase, which leads to positively charged stationary phase. So the repulsion of positive charges which exists between the monolith with hydroxyl functional groups and the Lys will reduce with the increase of pH in the range of 4 to 7. In contrast, when pH value of the mobile phase is higher than 7, the hydroxyl group on the monolith will be hydrolyzed, and the stationary phase is negative charged. Therefore, there will be an electrostatic attraction between the stationary phase and Lys, and the electrostatic attraction will be enhanced with the increase of pH in the range of 7 to 10 and the ability of elute will decrease. As a result, 0.02 mol L−1NaH2PO4 (pH = 4) was selected as the elution mobile phase. | ||
| Fig. 7 Effect of buffer salt concentration on the elution of Lys. HPLC conditions: the prepared hybrid monolith C2, 50 mm × 4.6 mm i.d.; sample: Lys, 1 mg mL−1; volume: 1.0 μL. Mobile phases (NaH2PO4 aqueous solution): a, 0.005 mol L−1; b, 0.01 mol L−1; c, 0.02 mol L−1; d, 0.05 mol L−1; e, 0.1 mol L−1; f, 0.2 mol L−1. | ||
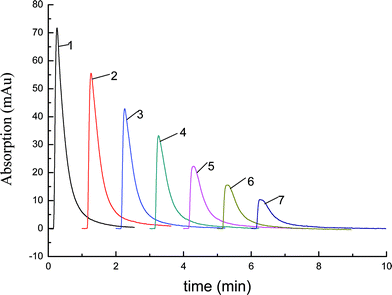 | ||
| Fig. 8 The pH effect on the elution of Lys. HPLC conditions: the prepared hybrid monolith C2, 50 mm × 4.6 mm i.d.; sample: Lys, 1 mg mL−1; volume: 5.0 μL. Mobile phase: 0.02 mol L−1buffer phosphate with the pH as follows: 1. pH = 4.0; 2. pH = 5.0; 3. pH = 6.0; 4. pH = 7.0; 5. pH = 8.0; 6. pH = 9.0; 7. pH = 10.0. | ||
3.4 Separation of benzene and its homologs from a mixture
The monolith was also used to separate benzene and its homologs from a mixture with the mobile phase methanol/water (80/20, v/v). The chromatogram was shown in Fig. 9. The peaks were benzene, toluene and ethyl benzene, in that order. The order of the peaks can be explained by the theory of hydrophobic interaction. The stationary phase of the column is composed of two parts, one is the inorganic moiety and the other is the resin moiety, which provides sufficient hydrophobic properties (phenyl groups), allowing the hybrid column to be used in the separation of organic small molecules.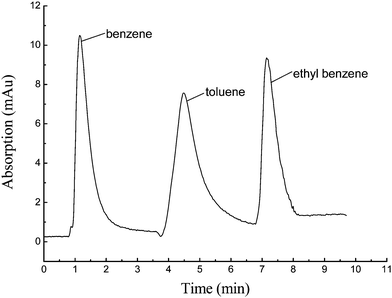 | ||
| Fig. 9 Chromatogram of benzene and its homologs. HPLC conditions: the prepared hybrid monolith C2, 50 mm × 4.6 mm i.d.; samples: benzene, toluene and ethyl benzene, 1μg mL−1; volume: 5.0 μL. Mobile phase: methanol/water (80/20, v/v). | ||
3.5 The dynamic binding capacity of the hybrid monolith for Lys
Using the method described in section 2.4, the dynamic binding capacity of the hybrid monolith for Lys is 0.18 mg g−1.3.6 Biological activity assay of Lys
One unit of enzyme activity is defined as the reduction of the absorbance at 450 nm by 0.001 min−1 under the conditions (25 °C, pH = 6.2) employed. Specific activity is defined in terms of units of activity per milligram of protein (U mg−1). The results of the biological activity of Lys are shown in Table 2 and the specific activity of Lys was 14572 ± 140 U mg−1. The biological activity we assayed is constant with the result reported previously,23 indicating that the Lys separated by the hybrid column was not deactivated and possessed high biological activity, meaning it can be used in food and medicine industries.| Concentration (μg mL−1) | 5 | 6.25 | 7.5 | 10.0 | 12.5 |
|---|---|---|---|---|---|
| ΔA | 0.0760 | 0.09686 | 0.1110 | 0.1521 | 0.1856 |
| Specific activity (U mg−1) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 125 200 ± 125 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 150 500 ± 150 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 140 800 ± 140 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 ± 120 210 ± 120 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 ± 165 850 ± 165 |
| Average specific activity (U mg−1) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 572 ± 140 572 ± 140 |
4 Conclusions
A novel organic–inorganic hybrid column with hydroxyl functional groups was prepared from VTMS and vinyl ester resin. The column showed high permeability, low backpressure, strong pH resistance and strong mechanical stability. The results demonstrated the hybrid column could be used as the solid phase of HPLC and used to separate lysozyme from egg white in a short time; benzene and its homologs were also successfully separated. Additionally, the Lys separated by the hybrid column possesses high biological activity. Therefore, such hybrid columns with hydroxyl functional groups could be used in the separation of proteins and organic molecules by HPLC.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21175031), Hebei Province Programs for Science and Technology Development (No. 11966411D) and the Education Department of Hebei Province (No. ZD2010234).References
- N. Tanaka, H. Kobayashi and N. Ishizuka, J. Chromatogr. A, 2002, 96, 535–49 Search PubMed.
- E. G. Vlakh and T. B. Tennikova, J. Sep. Sci., 2007, 30, 2802–2813 Search PubMed.
- M. M. Zheng, M. Y. Zhang, G. Y. Peng and Y. Q. Feng, Anal. Chim. Acta, 2008, 625, 35–49 Search PubMed.
- M. M. Zheng, G. D. Ruan and Y. Q. Feng, J. Chromatogr., A, 2009, 1216, 7739–7746 CrossRef CAS.
- Y. F. Cheng, T. H. Walter, Z. Lu, P. Iraneta, B. A. Alden, C. Gendreau, U. D. Neue, J. M. Grassi, J. L. Carmody, J. E. O’Gara and R. Fisk, LC-GC, 2000, 18, 1162–1172 CAS.
- J. F. Ma, Z. Liang and X. Q. Qiao, Anal. Chem., 2008, 80, 2949–2956 CrossRef CAS.
- M. M. Zheng, G. D. Ruan and Y. Q. Feng, J. Chromatogr., A, 2009, 1216, 7739–7746 CrossRef CAS.
- M. H. Wu, R. A. Wu, F. J. Wang, L. B. Ren, J. Dong, Z. Liu and H. F. Zou, Anal. Chem., 2009, 81, 3529–3536 CrossRef CAS.
- M. H. Wu, R. A. Wu and R. B. Li, Anal. Chem., 2010, 82, 5447–5454 CrossRef.
- L. Xu and H. K. Lee, J. Chromatogr., A, 2008, 1195, 78–84 CrossRef CAS.
- L. Angiolini, D. Caretti and R. D. Vito, et al. , J. Inorg. Organomet. Polym., 1997, 7, 151–162 CrossRef CAS.
- L. J. Yan, Q. H. Zhang and Y. Q. Feng, J. Chromatogr., A, 2006, 1121, 92–98 Search PubMed.
- T. Hara and S. Makino, J. Chromatogr., A, 2010, 1217, 89–98 Search PubMed.
- P. Jandera, J. Chromatogr., A, 2010, 1217, 22–33 Search PubMed.
- L. G. Bai, H. Y. Liu and Y. K. Liu, J. Chromatogr., A, 2011, 1218, 100–106 Search PubMed.
- C. Y. Hou, J. F. Ma and D. Y. Tao, J. Proteome Res., 2010, 9, 4093–4101 CrossRef CAS.
- Y. Xu, Q. Cao and F. Svec, Anal. Chem., 2010, 82, 3352–3358 CrossRef CAS.
- J. Yang, G. L. Yang and H. Y. Liu, J. Appl. Polym. Sci., 2011, 119, 412–418 Search PubMed.
- J. G. Gao, H. L. Du and H. N. Sun, Chin. J. Catal., 1989, 10, 422–428 Search PubMed.
- Y. H. Liao, M. B. Brown and G. P. Martin, J. Pharm. Pharmacol., 2001, 53, 549–554 Search PubMed.
- S. C. Goheen, J. Chromatogr., A, 1998, 816, 89–96 CrossRef CAS.
- O. Núňez, K. Nakanishi and N. Tanaka, J. Chromatogr., A, 2008, 1191, 231–252 CrossRef CAS.
- F. T. Meng and G. H. Ma, J. Control. Release, 2003, 91, 407–416 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
