A facile approach for the delivery of inorganic nanoparticles into the brain by passing through the blood–brain barrier (BBB)†
Yeong Shin
Yim
a,
Jin-sil
Choi
b,
Gun Tae
Kim
a,
Chul Hoon
Kim
a,
Tae-Hyun
Shin
b,
Dong Goo
Kim
*a and
Jinwoo
Cheon
*b
aDepartment of Pharmacology, Yonsei University, College of Medicine, Seoul 120-752, Korea. E-mail: dgkimpharm@yuhs.ac
bDepartment of Chemistry, Yonsei University, Seoul 120-749, Korea. E-mail: jcheon@yonsei.ac.kr
First published on 7th November 2011
Abstract
This study provides an easy and simple method to obtain inorganic nanoparticles that can penetrate the blood–brain barrier, the heavily guarded system in the brain, via cross-linked serum albumin surface coatings. Their intact BBB permeability was confirmed in both in vitro and in vivo tests.
Inorganic nanoparticles are becoming key materials for imaging, therapeutic, delivery and actuating capabilities in biomedical research fields.1 Among a variety of challenging applications, they have new potential opportunities in the field of neuroscience including the precise imaging of CNS, drug delivery to treat CNS related diseases and promotion of neuronal differentiation and regeneration.2 However, the blood–brain barrier (BBB) which is known as the protective system of CNS from harmful xenobiotics and endogenous molecules precludes the entrance of drugs and imaging agents into the brain.3 So far, the efforts to achieve organic nanoparticulate systems that can enter into the brain tissue have brought promising results.2,4 For example, organic nanoparticles either made of or conjugated with polysorbate 80, serum albumin or OX antibody are known to be highly effective for drug delivery to the brain.4 Although there have been similar efforts on BBB permeable inorganic nanoparticles with the use of targeting moieties,5 most of the current achievements are either limited to in vitro studies or demonstrated under the BBB breakdown conditions caused by external stimulations (e.g. ultrasound)6 and neural diseases such as cancer or stroke.7Inorganic nanoparticles can possess potentially broad applicability on the imaging and therapeutics of CNS compared to organic nanomaterials due to their excellent multi-modal imaging capabilities such as magnetic resonance imaging (MRI), optical imaging and positron emission tomography (PET) and therapeutic multi-valencies for tagging drugs. Therefore, the development of BBB permeable inorganic nanoparticles is important. In this study, we introduce a simple approach for the preparation of CNS penetrative inorganic nanoparticles, which are composed of a magnetic metal ferrite nanoparticle core and cross-linked serum albumin (SA) surface coatings (Fig. 1a). Accompanying the internalization observations of such nanoparticles in in vitro levels and in vivo mouse brains, we demonstrate that this approach provides an important finding which is the intact BBB permeability of inorganic nanoparticles without any BBB breakdown.
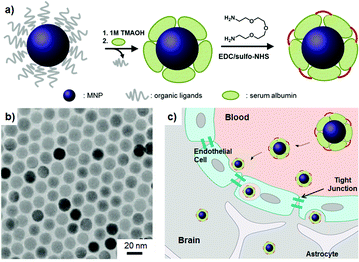 | ||
| Fig. 1 Serum albumin (SA) coated MNPs (SA-MNPs). (a) Surface modification of SA-MNPs. EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, sulfo-NHS: N-hydroxysulfosuccinimide. (b) The transmission electron microscope (TEM) image of MNPs. (c) Schematic of the BBB penetration of nanoparticles. | ||
The magnetic nanoparticles (MNPs, MnFe2O4) are synthesized by thermal decomposition of MnCl2 and iron(III) acetylacetonate in the presence of organic ligands.8 Synthesized MNPs possess a monodisperse size of 15 nm (σ ≈ 5%, Fig. 1b). Their organic ligands are eliminated by the treatment of 1 M trimethylammonium hydroxide (TMAOH) solution and then SAs are introduced onto the MNPs' surface. The stability of SAs on MNPs is ensured by interlinking them with 2,2′-(ethylenedioxy)-bis(ethylamine) (Fig. 1a). Resulting SA-MNPs possess a hydrodynamic size of ca. 30 nm (ESI†). SA-MNPs are labeled with fluorescein (FITC) for their optical recognition in cellular level studies.
Prior to pursuing the in vivoBBB penetration studies, primary cells associated with the BBB and CNS are examined to confirm the uptake of SA-MNPs (Fig. 1c and 2a). SA-MNPs (100 μg ml−1) are incubated for 1 h at 37 °C with three different types of cells including primary cultured brain microvascular endothelial cells (Fig. 2b), neurons (Fig. 2c) and astrocytes (one of the neuroglia cells, Fig. 2d). All of these cells maintain their original characteristics unlike carcinoma cells.10 Each type of cell is identified by its characteristic indicator; endothelial cells with an actin recognizer called phalloidin, neurons with anti-NeuN (neuronal specific nuclear protein) and astrocytes with anti-GFAP (glial fibrillary acidic protein). We utilize confocal laser scanning microscopy (Carl Zeiss, USA) where optical images are at the same focal plane to ensure the presence of SA-MNPs inside the cells. In Fig. 2b, multiple green spots of fluorescence from SA-MNPs are observed inside the endothelial cells. In addition, in the merged image, SA-MNPs are found inside the cytosol, but not in the nucleus (DAPI, blue). Similarly, in neurons and astrocytes, green fluorescence from SA-MNPs is observed in cytosol not in nuclei of cells (Fig. 2c and d). To confirm the SA-MNPs presence inside cells, the sectioned image of SA-MNPs treated neurons is taken with TEM. As seen in the red circles of Fig. 2e, SA-MNPs are consistently observed in the cytosol as dark spots.
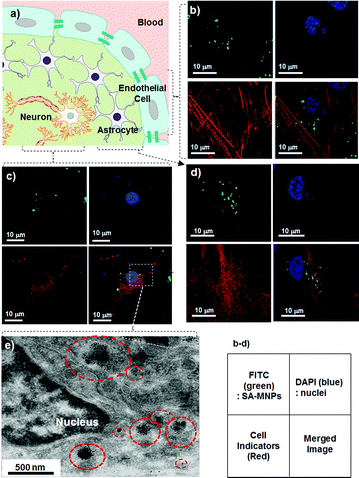 | ||
| Fig. 2 In vitro internalization of SA-MNPs into primary cells. (a) BBB is composed of endothelial cells and is surrounded by astrocytes.9 The confocal images of (b) endothelial cells, (c) neurons and (d) astrocytes which are treated with SA-MNPs (100 μg ml−1). The green FITC labeled SA-MNPs are observed in the cells. The nuclei of cells are stained with blue DAPI. Each of the endothelial cells, neurons and astrocytes is shown in red after being counter-stained with phalloidin (action marker), anti-NeuN (neuron marker) and anti-GFAP (neuroglia marker), respectively. The small box is the legend for the optical images. (e) TEM image of SA-MNPs treated neuron. Red circles indicate SA-MNPs. | ||
Based on the successful in vitro experiments on the individual cells, the internalization of SA-MNPs into the brain is examined by measuring the changes of MRI signals. SA-MNPs are very useful to serve as an MR imaging agent with high MRI contrast enhancement effects.8 SA-MNPs are injected into BALB/c mice through the tail vein at a dose of 20 mg kg−1 and then the MRI signal changes are monitored at the brain region. In the color-coded MR image where the red color represents low signal intensity and the blue color is high signal intensity, the MRI signal changes from red to blue at 20 min after the nanoparticle injection (Fig. 3a). Then, it is changed into green/orange in 50 min. The graph of T2 relaxivity change (ΔR2/R2pre-injection, R2 = 1/T2) vs. time shows the changes of MRI signals in the brain more clearly (Fig. 3b). The MRI signal in the brain increases up to a level of 25% higher than the initial signal intensity at 20 min after SA-MNPs injection. This increment is originated from SA-MNPs in both blood vessels and brain tissues. The majority of nanoparticles in the blood are removed by the urinary system and uptaken in reticuloendothelial system (RES) organs including liver and spleens.11 Such effects cause the drop in MRI signal. Afterwards, the T2 value remains ∼6% higher than the pre-injection value, which indicates the presence of SA-MNPs in the brain tissues.
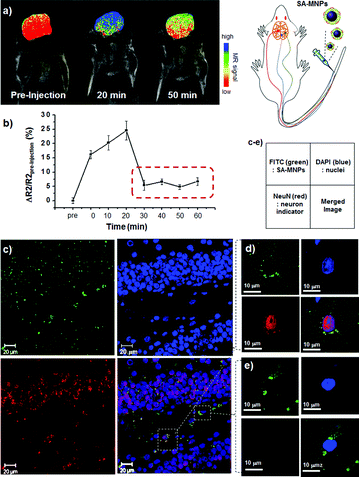 | ||
| Fig. 3 In vivo internalization of SA-MNPs into the brain. (a, b) Time dependent MRI signal changes in the brain after the SA-MNPs injection. (a) The color-mapped MR image of mouse over time and (b) the graph of MR signal at the brain (ΔR2/R2pre-injection) vs. time. The mouse brain image is color-coded where red and blue are low to high MRI signal and green is the intermediate signal. In Fig. 3b, the red-dotted box shows the steady presence of SA-MNPs in the brain. Confocal images of (c) hippocampal brain tissue and magnified confocal images of (d) neuron and (e) neuroglia. Green color corresponds to FITC labelled SA-MNPs. The mouse brain tissue is excised and incubated with DAPI (blue, nucleus marker) and anti-NeuN (red, neuron marker). In the magnified images (d, e), green SA-MNPs exist in the cytosol of both (d) neuron and (e) neuroglial cells. The small box is the legend for the optical images. | ||
Further microscopic optical analysis with a confocal microscopy is carried out to obtain the detailed information on the distribution of the SA-MNPs in the brain. Sectioned brain tissues are incubated with a cell nucleus marker (DAPI in blue) and a neuronal marker (anti-NeuN in red). In the image of the hippocampal region at low magnification (Fig. 3c), green dots are clearly visible throughout the tissue. Among cells, anti-NeuN stained cells are indicated as neurons and unstained cells are neuroglias which include astrocytes. In the merged image, green or yellow spots are observed around cells. The merged red and green regions appear yellow. To see the detailed location of SA-MNPs, images of individual cells are magnified. The magnified images that display green dots are present in the cytosol of both neurons (Fig. 3d) and neuroglia (Fig. 3e). In the merged confocal images, the green dots are not observed in the nuclei, which is similar to the results of the in vitro system. These results confirm that SA-MNPs penetrate into the BBB and neuronal cell membranes and are present in the cytosols of brain cells of mice. The amount of SA-MNPs in the brain is estimated by the radioisotope 125I labeling study. At week 2, the amount of SA-MNPs in major organs including the brain, lung, heart and liver is measured as 6.0, 6.5, 8.6 and 29.6 ng g−1, respectively (ESI†), and the largest portion of SA-MNPs exists in the liver. Observed SA-MNPs in brain tissue is 20% of that in liver and such a result is consistent with the previously reported values (5–30%) of BBB permeable nanoparticle agents.12
It has been reported that external introduction of chemicals or mechanical stress can disrupt the tight junctions of the BBB and lead to the malfunction of the BBB.2 To evaluate the potential effects of SA-MNPs on the BBB disruption, a functional test of the BBB is carried out with Evans Blue (EB, M.W.: 961 Da) which cannot pass across the BBB under normal conditions. A negative control group is saline treated mice. As a positive control, mice are put in warm water (ca. 42 °C) for 45 min, so that the BBB breakdown is artificially induced by hyperthermia. For all three groups, cross sections of the mice brain are examined 30 min after the EB treatments (Fig. 4). In a saline treated group with intact BBB, no blue color from EB is seen (Fig. 4a). Meanwhile, the hyperthermia group shows blue in the brain tissue, representing the destruction of the BBB (Fig. 4b). The SA-MNPs treated group does not show blue color in the brain (Fig. 4c). Therefore, SA-MNPs pass through the BBB without leading to any malfunctions of it. In addition, when the cellular toxicity of SA-MNPs on the neurons is tested, no recognizable effects on the viability of cells are observed (ESI†).
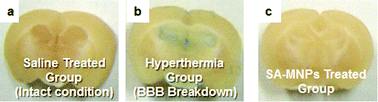 | ||
| Fig. 4 The examination of the intact BBB penetration of SA-MNPs in mice. The cross-sectioned images of brains from mice are shown, which included (a) saline treated (intact conditions, negative control), (b) hyperthermia (BBB breakdown, positive control) and (c) SA-MNPs treated groups. EB is injected via the tail vein. | ||
We obtain BBB permeable magnetic nanoparticles by simple adoption of SAs on the MNPs' surface. Free cationic SAs are reported to enter the brain via adsorptive-transcytosis.4,13 To enhance the brain transportation ability via adsorptive-transcytosis, SAs on the MNPs' surface are partially cationized via cross-linking processes (ESI†). Such an SA assisted inorganic BBB penetrating strategy is not limited to magnetic nanoparticles but can be easily extended to different nanoparticles such as quantum dots and gold nanoparticles as well. BBB permeable inorganic nanoparticles can be useful for imaging and therapeutics for CNS, which have been otherwise difficult to achieve so far.
We thank Dr H.-S. Kwon for the TEM analysis (HVEM, KBSI) and Yonsei-Carl Zeiss Advanced Imaging Center for technical assistance. This work is supported by NCRC (R15-2004-024-00000-0), CRI (2010-0018286), WCU (R32-2009-10217), BK21 projects of Medical Sciences and Chemistry and a faculty research grant of Yonsei University College of Medicine (6-2007-0223).
Notes and references
- N. Erathodiyil and J. Ying, Acc. Chem. Res., 2011, 44, 925 CrossRef CAS; I. Medintz, H. Uyeda, E. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS; R. Rosensweig, J. Magn. Magn. Mater., 2002, 252, 370 CrossRef CAS; R. Mannix, S. Kumar, F. Cassiola, M. Montoya-Zavala, E. Feinstein, M. Prentiss and D. Ingber, Nat. Nanotechnol., 2008, 3, 36 CrossRef CAS; F. Sousa, S. Mandal, C. Garrovo, A. Astolfo, A. Bonifacio, D. Latawiec, R. H. Menk, F. Arfelli, S. Huewel, G. Legname, H.-J. Galla and S. Krol, Nanoscale, 2010, 2, 2826 RSC; T. Schladt, K. Schneider, H. Schild and W. Tremel, Dalton Trans., 2011, 40, 6315 RSC; S. Lesieur, F. Gazeau, N. Luciani, C. Ménager and C. Wilhelm, J. Mater. Chem., 2011, 21, 14387 RSC.
- H. Yang, Pharm. Res., 2010, 27, 1759 CAS; J. M. Provenzale and G. A. Silva, Am. J. Neuroradiol., 2009, 30, 1293 Search PubMed.
- W. M. Pardridge, J. Neurochem., 1998, 70, 1781 CAS.
- J. Kreuter, R. N. Alyautdin, D. A. Kharkevich and A. A. Ivanov, Brain Res., 1995, 674, 171 CrossRef CAS; Z. M. Qian, H. Li, H. Sun and K. Ho, Pharmacol. Rev., 2002, 54, 561 CrossRef CAS; W. Lu, J. Wan, Z. She and X. Jiang, J. Controlled Release, 2007, 118, 38 CrossRef CAS.
- X. Gao, J. Chen, J. Chen, B. Wu, H. Chen and X. Jiang, Bioconjugate Chem., 2008, 19, 2189 CrossRef CAS; D. Arndt-Jovin, S. Kantelhardt, W. Caarls, A. de Vries, A. Giese and T. Jovin, IEEE Trans. NanoBiosci., 2009, 8, 65 CrossRef; S. Santra, H. Yang, J. Stanley, P. Holloway, B. Moudgil, G. Walterd and R. Mericlee, Chem. Commun., 2005, 3144 RSC; G. Xu, K. Yong, I. Roy, S. Mahajan, H. Ding, S. Schwartz and P. Prasad, Bioconjugate Chem., 2008, 19, 1179 CrossRef CAS.
- H. Liu, M. Hu, H. Yang, C. Huan, P. Chu, J. Wua, I. Tseng, J. Wang, T. Yen, P. Chen and K. Wei, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15205 CrossRef CAS.
- J. Weinstein, C. Varallyay, S. Gahramanov, B. Hamilton, W. Rooney, L. Muldoon and E. Neuwelt, J. Cereb. Blood Flow Metab., 2010, 30, 15 Search PubMed.
- J. Lee, K. Lee, S. Moon, Y. Lee, T. Park and J. Cheon, Angew. Chem., Int. Ed., 2009, 48, 4174 CrossRef CAS.
- N. Abbott, L. Rönnbäck and E. Hansson, Nat. Rev. Neurosci., 2006, 7, 41 CrossRef CAS.
- Y. Ma and H. Gu, J. Mater. Sci.: Mater. Med., 2007, 18, 2145 CrossRef CAS.
- N. Khlebtsov and L. Dykmana, Chem. Soc. Rev., 2011, 40, 1647 RSC.
- A. Ambruosi, A. Khalansky, H. Yamamoto, S. Gelperina, D. Begley and J. Kreuter, J. Drug Targeting, 2006, 14, 97 CrossRef CAS; W. Jong, W. Hagens, P. Krystek, A. Sips and R. Geertsma, Biomaterials, 2008, 29, 1912 CrossRef CAS.
- M. Smith and M. Gumbleton, J. Drug Targeting, 2006, 14, 191 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The details of compliance with ethical guidelines for our experiments with live animals and supplementary data including surface charge, hydrodynamic size, cellular toxicity and biodistribution of SA-MNPs. See DOI: 10.1039/c1cc15113d |
| This journal is © The Royal Society of Chemistry 2012 |
