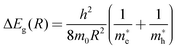A controlled anion exchange strategy to synthesize Bi2S3 nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity†
Hefeng
Cheng
a,
Baibiao
Huang
*a,
Xiaoyan
Qin
a,
Xiaoyang
Zhang
a and
Ying
Dai
b
aState Key Lab of Crystal Materials, Shandong University, Jinan 250100, People's Republic of China. E-mail: bbhuang@sdu.edu.cn
bSchool of Physics, Shandong University, People's Republic of China
First published on 7th November 2011
Abstract
Bi2S3 nanocrystals/BiOCl hybrid architectures with tunable band gaps were synthesized by a controlled anion exchange approach and they displayed highly efficient visible light photoactivities, which is associated with suitable energetics and structural topotactic relationship that can benefit the interfacial charge transfer.
Semiconductor nanocrystals (NCs), which display a range of novel chemical/physical properties, have drawn ever growing interest for their applications in energy conversion, supercapacitors and photocatalysis.1–3 In particular, derived from the dramatic quantum confinement, narrow band gap (NBG) semiconductor NCs (e.g., CdSe and PbS)3 with tunable band gaps have opened up new horizons for harvesting solar light that predominately concentrates in the visible (Vis) and the infrared (IR) region. Such an intriguing size-dependent feature of NBG NCs allows one to tune the light absorption and tailor the band edges; thus the photoinduced charges can be interfacially separated by coupling with other wide band gap (WBG) semiconductors such as TiO2 with favorable energetics.
As a lamellar binary semiconductor, bismuth sulfide (Bi2S3) has significant applications in photovoltaics, thermoelectrics and electrochemical hydrogen storage.4 Moreover, the size quantization enables Bi2S3 nanoparticles (1.3 eV for bulk) to show tunable photosensitization and considerable photoactivity in the visible region.5 However, most of the reported approaches to Bi2S3 NCs require high reaction temperatures and capping agents involving oleylamine (OAm) as stabilizers, which increases the complexity and infeasibility. Recently, ion exchange, which permits the exchange of the component ions with the incoming species, has paved a new way to engender various inorganic nanostructures from the existing materials.6 Furthermore, controlled partial ion exchange gives birth to NC heterostructures, which bring about the close interconnection between the semiconductors to benefit the separation of carriers.6e,f Therefore, developing a wet-chemical ion exchange route to Bi2S3 NCs sensitized hybrids, which could tailor the visible light response and facilitate the interfacial charge transfer, is highly desirable for practical applications yet it remains a challenge.
In the present work, a novel Bi2S3 NCs/BiOCl hybrid has been synthesized by a facile anion exchange route. In addition to providing Bi3+ ions, BiOCl architectures herein also play a role of a framework to construct the Bi2S3 NCs/BiOCl hybrid. Controlled release of S2− ions from various sulfur sources to react with BiOCl by ion exchange leads to the Bi2S3 NCs with tunable sizes, thus regulating the visible light absorption of the hybrids. The as-prepared Bi2S3 NCs/BiOCl hybrid showed highly efficient visible light photocatalytic activities in decontamination of noxious pollutants, which is considered to be interrelated with the effective interfacial charge transfer.
First of all, hierarchical mesoporous BiOCl architectures with an average diameter of 1–2 μm (Fig. S1–S2, ESI†) were synthesized using Bi(NO3)3 and [C12Mim]Cl ion liquid under solvothermal treatment (ESI†) as described elsewhere.7
Due to the rather lower solubility of Bi2S3 (Ksp = 1.0 × 10−97) relative to BiOCl (Ksp = 1.8 × 10−31), BiOCl can adopt a thermodynamically favored direction to yield Bi2S3, and a partial anion exchange reaction could give birth to the Bi2S3/BiOCl hybrid. In our experiments, such a transformation process was realized through introducing the as-prepared BiOCl architectures into the appropriate sulfur source such as thiourea, cysteine and thiacetamide (TAA) solutions followed by ion exchange treatments (ESI†). XRD characterization (Fig. S3, ESI†) was carried out to identify the phase structure of the hybrids; however, no diffraction peaks assigned to Bi2S3 were found, which may result from its high dispersity and low content.6e The structural information of the hybrids was further investigated by Raman spectra, which was shown in Fig. 1A. Two typical vibrational peaks at 141 and 200 cm−1 are observed for the BiOCl sample, which are assigned to A1g and Eg of Bi–X stretching mode, respectively.9 The broad peak regions (70–150 cm−1) in both bulk Bi2S3 (ESI†) and Bi2S3/BiOCl hybrids indicate the presence of Bi2S3 species, which are in good agreement with the reported values.10
 | ||
| Fig. 1 (A) Raman spectra and (B) UV–Vis diffuse reflectance spectra of the samples: (a) BiOCl, (b–e) Bi2S3/BiOCl hybrids synthesized using thiourea, cysteine, TAA at room temperature and TAA at 60 °C, respectively, and (f) bulk Bi2S3. | ||
Fig. 1B presents the UV–Vis diffuse reflectance spectra of the as-prepared samples. The white BiOCl only exhibits UV light response with an absorption edge around 380 nm, while the black Bi2S3 shows intense absorption over the visible range even extending to the infrared region. The fitted direct band gap of Bi2S3 was determined to be 1.33 eV, which is equal to its bulk value. It is noted that the Bi2S3/BiOCl hybrids show tunable visible light absorption in a large range, which is attributed to the quantum confinement of Bi2S3 NCs. The band gaps of Bi2S3 NCs/BiOCl hybrids synthesized using thiourea, cysteine, TAA at room temperature and TAA at 60 °C are estimated to be about 3.35, 2.90, 2.20 and 1.60 eV, respectively (Fig. S4, ESI†). On the basis of the effective mass approximation model, the blue shift of Bi2S3 NCs relative to the bulk is dominated by the confinement of electrons and holes, as described by the following equation:11
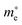 and
and 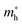 are the effective masses of electrons and holes, respectively. From the band gap shift as a function of crystal radius (Fig. S5, ESI†), the calculated sizes of the as-prepared Bi2S3 NCs in the Bi2S3/BiOCl hybrids range from 3.1 to 8.5 nm, which are summarized in Table 1. As the relative reactivity follows the order TAA > cysteine > thiourea,8 the higher reactivity of the sulfur source engenders the faster growth rate of Bi2S3, thereby leading to the larger size of Bi2S3 NCs with a smaller blueshift. In addition, higher temperature (60 °C) favors the decomposition of TAA, and as a consequence a Bi2S3 NCs/BiOCl hybrid with larger Bi2S3 size was obtained. Since the size of Bi2S3 NCs is much smaller than its Bohr excitation radius (ca. 24 nm),11 remarkable quantum size confinement is thus observed.
are the effective masses of electrons and holes, respectively. From the band gap shift as a function of crystal radius (Fig. S5, ESI†), the calculated sizes of the as-prepared Bi2S3 NCs in the Bi2S3/BiOCl hybrids range from 3.1 to 8.5 nm, which are summarized in Table 1. As the relative reactivity follows the order TAA > cysteine > thiourea,8 the higher reactivity of the sulfur source engenders the faster growth rate of Bi2S3, thereby leading to the larger size of Bi2S3 NCs with a smaller blueshift. In addition, higher temperature (60 °C) favors the decomposition of TAA, and as a consequence a Bi2S3 NCs/BiOCl hybrid with larger Bi2S3 size was obtained. Since the size of Bi2S3 NCs is much smaller than its Bohr excitation radius (ca. 24 nm),11 remarkable quantum size confinement is thus observed.
The morphology of the hybrids was studied and a typical scanning electron microscopy (SEM) image of the Bi2S3 NCs/BiOCl hybrid synthesized using TAA at room temperature is illustrated in Fig. 2a, in which quasi uniform architectures are observed. It is found that during the course of anion exchange reaction, the morphology of BiOCl architectures with hierarchy was retained in the final Bi2S3 NCs/BiOCl hybrid with high fidelity (Fig. S6, ESI†). Chemical element mapping analysis (Fig. 2b–d) shows that the elements including bismuth, chlorine and sulfur are homogenously distributed over the hybrid, which demonstrates the nanoscale hybridization between Bi2S3 NCs and BiOCl architectures. The energy-dispersive X-ray spectrum (EDS, Fig. 2e) confirms the presence of Bi, Cl, O and S elements in the Bi2S3 NCs/BiOCl hybrid, and the relative atom ratios are 32.22%, 26.43%, 41.04% and 0.31%, respectively (Fig. S7, ESI†). Moreover, as seen from the typical transmission electron microscopy (TEM) image in Fig. 2f, the sample exhibits hierarchical architectures with a size of 1–2 μm built by nanosheets. As seen from the high resolution TEM (HRTEM) image (Fig. 2g), the adjacent lattice spacing of ca. 0275 nm is in agreement with the (110) plane of tetragonal BiOCl. It is worth noting that some nanoparticles (as illustrated) with the size in the range of 4–6 nm are anchored on the surface of BiOCl nanosheets, which corresponds to the Bi2S3 NCs with a calculated size value.
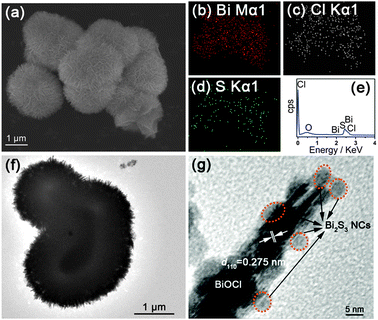 | ||
| Fig. 2 (a) Typical SEM image, (b–d) chemical element mapping data, (e) EDS, (f) TEM image and (g) HRTEM image of the Bi2S3 NCs/BiOCl sample synthesized using TAA at room temperature. | ||
As the quantum confinement of Bi2S3 NCs enables Bi2S3/BiOCl hybrids to display tunable photoabsorption in the visible region, the visible light photocatalytic activities of the hybrids were carried out by decomposition of 2,4-dichlorophenol (2,4-DCP) solution, which eliminates the influences such as self-photodegradation. As shown in Fig. 3a, bulk Bi2S3 and BiOCl could decompose 40.8% and 47.1% of 2,4-DCP under irradiation for 150 min, respectively. As a reference photocatalyst, N-doped P25 could only decompose 12.1% of 2,4-DCP molecules. While in the case of the Bi2S3 NCs/BiOCl hybrid synthesized using TAA as the sulfur source at room temperature, the photocatalytic decomposition efficiency can reach as high as 82.3% (Fig. S8, ESI†). Given the pseudo-first-order photodecomposition of the pollutants, the 2,4-DCP decomposition rate over the Bi2S3 NCs/BiOCl hybrid (0.693 h−1) is faster than that of N-doped P25 (0.0516 h−1) by a factor of thirteen. Besides, the correlation between the Bi2S3 quantum sizes and the photocatalytic performances of Bi2S3 NCs/BiOCl hybrids was also investigated (Fig. 3b). It is found that the 4.7 nm Bi2S3 NCs/BiOCl hybrid exhibits the best photocatalytic performance. However, Bi2S3 NCs with larger (8.5 nm) or smaller (3.1 and 3.5 nm) sizes render the Bi2S3 NCs/BiOCl hybrids with inferior photocatalytic performances. Apart from decomposing 2,4-DCP, the Bi2S3 NCs/BiOCl hybrid also exhibits highly photocatalytic efficiency in methyl orange (MO) degradation (ESI†).
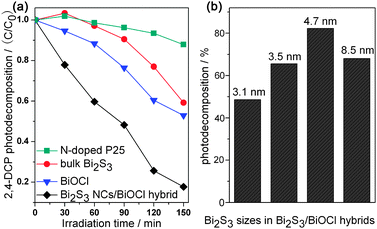 | ||
| Fig. 3 (a) Photocatalytic decomposition of 2,4-DCP over different samples under visible light irradiation (λ ≥ 400 nm), and (b) the influence of Bi2S3 size on the photocatalytic efficiencies of the Bi2S3 NCs/BiOCl hybrid. | ||
It is of great interest to explore the plausible reaction mechanism of the Bi2S3 NCs/BiOCl hybrid, as is shown in Fig. 4. Through ion exchange reaction between BiOCl architectures and sulfur sources, Bi2S3 was in situ anchored on the surface of BiOCl nanosheets. The structural topotactic relationship,4c originating from the close matching of lattice constants of tetragonal BiOCl (a = b = 0.388 nm) and orthorhombic Bi2S3 (c = 0.398 nm), could favor the formation of Bi2S3/BiOCl heterostructures and facilitate the interfacial charge transfer. Moreover, band position calculations (ESI†) suggest that BiOCl and Bi2S3 have the staggered energy potentials that can reduce the recombination of the photogenerated carriers between the two semiconductors. As the sizes of Bi2S3 NCs decrease, the quantum confinement makes the Bi2S3 NCs/BiOCl hybrids absorb less visible light; while the conduction band (CB) of Bi2S3 shifts to more negative potentials to result in the larger CB energy difference (ΔEC) between BiOCl and Bi2S3, which favors the electrons transfer.5 Upon the interrelationship of the two above mentioned factors, the optimized condition is observed for a 4.7 nm Bi2S3 NCs/BiOCl hybrid, which shows the superior photocatalytic performance. Under visible light irradiation, Bi2S3 NCs are excited and the as-photoinduced electrons migrate to the surface of BiOCl, which could be further trapped by molecular oxygen in solution to form O2− and other oxidative species.12 As an extension, a Bi2S3 NCs/BiOBr hybrid was also prepared by a partial anion exchange reaction and showed highly efficient visible light photocatalytic activity (ESI†).
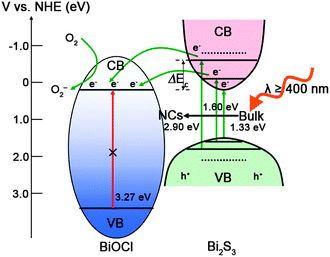 | ||
| Fig. 4 Schematic illustration of band energy positions and the charge transfer process of the Bi2S3 NCs/BiOCl hybrid under visible light irradiation. | ||
In summary, a novel Bi2S3 nanocrystals/BiOCl hybrid with tunable visible light response was synthesized by a controlled anion exchange approach. The Bi2S3 nanocrystals/BiOCl hybrid, which involves suitable energetics and structural topotactic relationship that can benefit the interfacial charge transfer, displays highly efficient visible light photoreactivity. The work illustrates a facile controlled anion exchange access to isogenous “mother–children” Bi2S3 NCs/BiOCl hybrids with tunable photoresponse and photoreactivity, which could allow general modifications to other semiconductor materials.
This work was financially supported by the National Natural Science Foundation of China (Nos. 20973102, 51021062, 51002091), the National Basic Research Program of China (No. 2007CB613302) and Graduate Independent Innovation Foundation of Shandong University (GIIFSDU, 11250071613044).
Notes and references
- A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. C, 2008, 112, 18737 CAS.
- (a) H. Bao, C. Li, X. Cui, Y. Gan, Q. Song and J. Guo, Small, 2008, 4, 1125 CrossRef CAS; (b) L. Cademartiri, F. Scotognella, P. G. O'Brien, B. V. Lotsch, J. Thomson, S. Petrov, N. P. Kherani and G. A. Ozin, Nano Lett., 2009, 9, 1482 CrossRef CAS; (c) L. Li, N. Sun, Y. Huang, Y. Qin, N. Zhao, J. Gao, M. Li, H. Zhou and L. Qi, Adv. Funct. Mater., 2008, 18, 1194 CrossRef CAS.
- (a) L. M. Peter, K. G. U. Wijayantha, D. J. Riley and J. P. Waggett, J. Phys. Chem. B, 2003, 107, 8378 CrossRef CAS; (b) T. Wu, X. G. Zhou, H. Zhang and X. H. Zhong, Nano Res., 2010, 3, 379 CrossRef CAS.
- (a) D. H. Son, S. M. Hughes, Y. Yin and A. P. Alivisatos, Science, 2004, 306, 1009 CrossRef CAS; (b) L. Dloczik and R. Könenkamp, Nano Lett., 2003, 3, 651 CrossRef; (c) P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M.-H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931 CrossRef CAS; (d) S. Yan, S. Ouyang, J. Gao, M. Yang, J. Feng, X. Fan, L. Wan, Z. Li, J. Ye, Y. Zhou and Z. Zou, Angew. Chem., Int. Ed., 2010, 49, 6400 CrossRef CAS; (e) H. F. Cheng, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054 RSC; (f) J. Yu, J. Zhang and M. Jaroniec, Green Chem., 2010, 12, 1611 RSC.
- H. F. Cheng, B. B. Huang, Z. Y. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem.–Eur. J., 2011, 17, 8039 CrossRef CAS.
- D. Pan, Q. Wang, S. Jiang, X. Ji and L. An, J. Phys. Chem. C, 2007, 111, 5661 CrossRef CAS.
- (a) J. E. D. Davies, J. Inorg. Nucl. Chem., 1973, 35, 1531 CrossRef CAS; (b) Y. Y. Liu, W.-J. Son, J. B. Lu, B. B. Huang, Y. Dai and M.-H. Whangbo, Chem.–Eur. J., 2011, 17, 9342 CrossRef CAS.
- Y. Zhu, D. Fan and W. Shen, Langmuir, 2008, 24, 11131 CrossRef CAS.
- (a) B. Pejova and I. Grozdanov, Mater. Chem. Phys., 2006, 99, 39 CrossRef CAS; (b) R. P. Panmand, G. Kumar, S. M. Mahajan, M. V. Kulkarni, D. P. Amalnerkar, B. B. Kale and S. W. Gosavi, J. Appl. Phys., 2011, 109, 033101 CrossRef.
- H. F. Cheng, B. B. Huang, Y. Dai, X. Y. Qin and X. Y. Zhang, Langmuir, 2010, 26, 6618 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, band calculations and Fig. S1–S14. See DOI: 10.1039/c1cc15487g |
| This journal is © The Royal Society of Chemistry 2012 |