An unprecedented organic–inorganic hybrid based on the first {Nb10V4O40(OH)2}12−clusters and copper cations†
Peng
Huang
,
Chao
Qin
,
Xin-Long
Wang
*,
Chun-Yi
Sun
,
Guang-Sheng
Yang
,
Kui-Zhan
Shao
,
Yan-Qing
Jiao
,
Kun
Zhou
and
Zhong-Min
Su
*
Institute of Functional Material Chemistry, Key Lab of Polyoxometalate, Science of Ministry of Education, Faculty of Chemistry, Northeast Normal University, Changchun, 130024 Jilin, People's Republic of China. E-mail: zmsu@nenu.edu.cn; wangxl824@nenu.edu.cn; Fax: +86 431-85684009; Tel: +86 431-85099108
First published on 4th November 2011
Abstract
An unprecedented organic–inorganic hybrid {[Cu6L6(H2O)3][Nb10V4O40(OH)2]}2·13H2O (1) (L = 1,10-phenanthroline) containing the unreported {Nb10V4O40(OH)2}12− building blocks has been successfully synthesized and its photoluminescent properties, IR spectra, thermogravimetric analyses and single-crystal X-ray diffraction were investigated.
Polyoxometalates (POMs) are an outstanding class of nanosized metal oxoanions with wide structural diversity and interesting properties that have potential applications in catalysis, electrical conductivity, photochemistry, medicine and magnetism.1 However, up to now, the development of polyoxometalate chemistry is still dominated by the polyoxotungstates, polyoxomolybdates and polyoxovanadates, because their polyanions can be easily formed over a wide pH range and sometimes can be obtained simply by acidification and fusion of their oxoanion solutions at standard pressure and ambient temperature.2 As a unique branch of POMs, polyoxoniobates (PONs) also display multiple applications in virology, nuclear waste treatment and the base-catalyzed decomposition of biocontaminants.3 However, in comparison with other polyoxometalates, the development of polyoxoniobates is still in its infancy and primarily controlled by the hexaniobate Lindqvist anion [Nb6O19]8−. The feature that the [Nb6O19]8− anion exists only under alkaline conditions and forms Nb2O5 precipitates under acidic conditions has severely restricted the development of polyoxoniobates.4 Fortunately, in recent years, polyoxoniobate chemistry has gained great progress due to the pioneering work of Nyman and co-workers on the heteropolyniobates,5 and further developed by the series of research by Casey and co-workers.6 The other representative examples are as follows: [Nb20O54]8−,7[{Cu(H2O)L}2(CuNb11O35H4)]5− (L = 1,10-phenanthroline, 2,2′-bipyridine),1k[H9Nb24O72]15−,8[HNb27O76]16− and [H10Nb31O92(CO3)]23−.9 In addition, niobium-substituted POMs synthesized by the reaction of lacunary polyoxotungstates and K7HNb6O19·13H2O in acidic solution have also made great progress, such as the trisubstituted heteropolytungstates {MW9Nb3O40} (M = SiIV, GeIV), dimer {H15Ge2W18Nb8O88}, and tetramer {Ge4W36Nb16O166} cluster.10 Lately, the first example of the vanadoniobate cluster with ‘trans-vanadium’ bicapped Keggin-type {VNb12O40(VO)2} was reported by Hu's group.11 These reported polyoxoniobates may assist toward the fundamental understanding of chemistry and are expected to find potential application in catalysis.
Inspired by the prominent work of Finke, Hill, Liu and their co-workers,4a,10 we considered that vanadium might be a good candidate for construction of niobium-substituted POMs due to the similar ion radii and coordination numbers of V and Nb. Herein, we report the synthesis and structural characterization of an unprecedented compound {[Cu6L6(H2O)3][Nb10V4O40(OH)2]}2·13H2O (1) (L = 1,10-phenanthroline), which contains the unique polyoxoanion unit {Nb10V4O40(OH)2}12−.‡
Compound 1 was obtained from heating a mixture of K7HNb6O19·13H2O, Cu(Ac)2·3H2O, NaVO3·2H2O, 1,10-phenanthroline, and NaOH at 58 °C for 8 h.† Compared with other transition metal cations, copper-amine cations have even more potential in isolating new niobate cluster geometries. The possible reasons are as follows: their strong affinity for organic amine or aromatic N-ligands originating from the distinct Jahn–Teller effect of the Cu2+ with d9 configuration, their flexible coordination modes, and their stability in alkaline solutions.11 Compound 1 is stable in air and insoluble in acidic and alkaline solutions. In 1, the oxidation states of all Nb, V and Cu centers are +5, +5 and +2, respectively, based on the charge balance consideration and bond valence sum calculations.12 The phase purity of 1 is confirmed by the agreement between the experimental X-ray powder diffraction (XRPD) pattern and the simulated pattern based on structural analysis (Fig. S5, ESI†). X-Ray structural analysis indicates that 1 contains two identical {Nb10V4O40(OH)2}12−clusters decorated by twelve {CuL} fragments. The surrounding twelve {CuL} fragments are linked to the clusters through twenty-four terminal oxygens and eight bridging oxygen atoms (Fig. 1 and Fig. S1, ESI†). The {Nb10V4O40(OH)2}12− unit is derived from two fundamental building blocks, namely, the {Nb10O32} cluster and the {VO4} tetrahedron (Fig. 2e). Although the decaniobate ion, first reported in 1977 by Edward J. Graeber and B. Morosin (Fig. 2b),13 is a well-known polyoxoniobate (PON), the structure of the decaniobate ion described here has never been observed to date (Fig. 2d). The new decaniobate cluster is made up of four additional {NbO6} octahedra and an interesting {Nb6O20} unit (Fig. 2c). Each {NbO6} octahedron is considerably distorted around the niobium center, with a spread of Nb–O bond distances which range from 1.767(13) to 2.220(9) Å. Similar distortions of {NbO6} octahedra were also observed in classical Lindqvist hexaniobate (Fig. 2a). Four such {NbO6} octahedra bond to the {Nb6O20} fragment by the edge-sharing mode into a unique {Nb10O32} unit. Compared with classical Lindqvist hexaniobate, the Nb atoms of the new {Nb6O20} unit display six-coordinate and seven-coordinate environments (Fig. S2, ESI†). The formation of the unprecedented {Nb10O32} structure is attributed to the seven-coordinate Nb. To our knowledge, there is no similar species reported in polyoxotungstates, polyoxomolybdates and polyoxovanadates chemistry. The {Nb10O32} unit has four types of O atoms: nine terminal oxygen atoms (Ot), ten bridging μ2-oxygen atoms, six bridging μ3-oxygen atoms, and one central μ6-oxygen atom. The Nb–Ot, Nb–μ2-O, Nb–μ3-O, and Nb–μ6-O bond lengths in {Nb10O32} anions range from 1.716(12)–1.810(11), 1.908(10)–2.068(10), 1.982(10)–2.219(10), and 2.321(9)–2.589(9) Å, respectively, and the Nb⋯Nb distances are in the range 3.261(2) to 3.359(2) Å. Each {Nb10O32} cluster is further connected to four {VO4} tetrahedra by a corner-sharing mode into a novel {Nb10V4O40(OH)2}12−cluster (Fig. 2e and Fig. S2, ESI†). All V ions of the {Nb10V4O40(OH)2}12− unit are tetrahedrally coordinated. The V–O bond lengths range from 1.644(16)–1.88(4) Å. There are two different copper coordination environments in compound 1 (Fig. S2, ESI†). The Cu1, Cu3, Cu4, Cu6, Cu7, Cu9, Cu10 and Cu12 cations exhibit five-coordinate square pyramidal geometry, in which the square plane is defined by two N atoms from a 1,10-phenanthroline ligand and two terminal O atoms from the {Nb10V4O40(OH)2}12− unit (Cu–N: 1.982(15)–2.018(14) Å, Cu–O: 1.875(11)–1.945(13) Å), and the apical site is occupied by one coordinate water (Cu–OW: 2.221(16)–2.295(14) Å). While Cu2, Cu8, Cu11 and Cu5 cations show a four-coordinate square-planar geometry defined by two N atoms from a 1,10-phenanthroline ligand and two bridging O atoms from the {Nb10V4O40(OH)2}12− unit (Cu–N: 1.993(14)–2.003(13) Å, Cu–O: 1.878(10)–1.930(10) Å).1i The adjacent {Nb10V4O40(OH)2}12− units form a dipolymer through a Cu–Ob–Cu bond. Furthermore, the {[Cu6L6(H2O)3][Nb10V4O40(OH)2]}2·13H2O molecules close-pack in the solid-state to form a 3D supramolecular network via π–π stacking interaction between the neighboring 1,10-phenanthroline ligands with the distance of 3.472 Å (face to face) (Fig. 3). Interestingly, there exist one-dimensional quadrangular channels of approximately 6.9 × 10.9 Å along the [−1 1 −2] direction that are occupied by the free water molecules. PLATON analysis shows that the effective free volume of 1 is about 31.5% of the crystal volume (2182.3 Å3 out of the 6939 Å3).
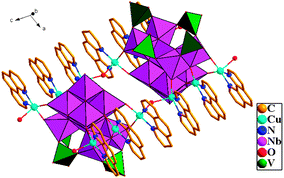 | ||
| Fig. 1 Polyhedral/ball-and-stick views of the anions of 1 (H atom and water molecules are omitted for clarity). | ||
![(a) The classical Lindqvist [Nb6O19]8− fragment. (b) The decaniobate ion reported by Edward J. Graeber and B. Morosin. (c and d) The new Lindqvist {Nb6O20} cluster and the new {Nb10O32} cluster in this communication. (e) Summary of the formation of {Nb10V4O40(OH)2}12−.](/image/article/2012/CC/c1cc15684e/c1cc15684e-f2.gif) | ||
| Fig. 2 (a) The classical Lindqvist [Nb6O19]8− fragment. (b) The decaniobate ion reported by Edward J. Graeber and B. Morosin. (c and d) The new Lindqvist {Nb6O20} cluster and the new {Nb10O32} cluster in this communication. (e) Summary of the formation of {Nb10V4O40(OH)2}12−. | ||
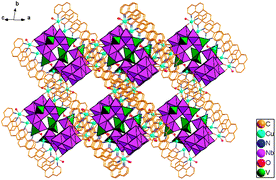 | ||
| Fig. 3 Polyhedral representation of the three-dimensional supramolecular structure in compound 1 (H atom and water molecules are omitted for clarity). | ||
The IR spectrum of 1 is recorded between 400 and 4000 cm−1 with a KBr pellet, which is very useful for identification of characteristic vibration bands of PONs and organic components in products. In the IR spectrum of compound 1, the characteristic peaks at 903, 715, 622 and 442 cm−1 are assigned to the ν(Nb–Ob), ν(Nb–O–M) and ν(V–O) (M = NbV or VV) stretches. Bands in the 1423–1514 cm−1 region are attributed to the 1,10-phenanthroline groups. The peak at 3423 cm−1 is assigned to the water molecules.14
In order to characterize the compound more fully, a TGA experiment was carried out to investigate its thermal stability. A weight loss of ca. 4.99% occurred from 42 to 100 °C, corresponding to the loss of all lattice and coordinated water molecules (4.97%), followed by two-step continuous weight loss of 32.13% in the range 100 and 900 °C consistent with the removal of twelve 1,10-phenanthroline ligands (calcd 34.57%). The whole weight loss of 37.12% is consistent with the calculated value of 39.54%.
The solid-state luminescence property of 1 was investigated at room temperature. Compound 1 exhibits an intense emission maximum at ca. 424 nm upon excitation at ca. 365 nm (Fig. 4). As we know, the strongest emission peak for the free 1,10-phenanthroline ligand is at 362 nm and 380 nm (λex = 227 nm).15,16 The K7HNb6O19·13H2O exhibits a broad emission band at around 320 nm (λex = 227 nm), which can be assigned to the O–Nb charge transfer.11 Thus, the emission of 1 may be attributed to the mixture of the intraligand πL*–πL transitions from the 1,10-phenanthroline and the O → Nb and O → V charge transfer of the {Nb10V4O40(OH)2}12− unit.15
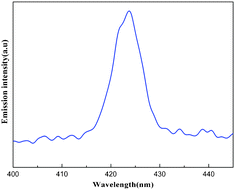 | ||
| Fig. 4 Solid-state emission spectrum of compound 1 at room temperature. | ||
In summary, we have synthesized and structurally characterized an unprecedented organic–inorganic hybrid, {[Cu6L6(H2O)3][Nb10V4O40(OH)2]}2·13H2O, which contains the unique polyniobovanadate {Nb10V4O40(OH)2}12−. The successful synthesis of 1 adds a new number of Nb-based POMs and unveils the potential for further development of polyoxoniobate chemistry. In addition, it provides us an enlightening synthetic strategy for the introduction of other transition metal cations and large aromatic or chiral ligands into this system to construct organic–inorganic hybrid multidimensional PONs or chiral PONs. In the following exploration, we will continue to investigate the pertinent synthetic chemistry.
The authors gratefully acknowledge the financial support from the NNSF of China (No. 21001022, 21171033, 21131001), The National Grand Fundamental Research 973 Program of China (2010CB635114), Program for New Century Excellent Talents in Chinese University (NCET-10-0282), PhD Station Foundation of Ministry of Education (20100043110003), The Foundation for Author of National Excellent Doctoral Dissertation of P.R. China (FANEDD) (No. 201022), The Science and Technology Development Planning of Jilin Province (201001169,20100182), The Fundamental Research Funds for the Central Universities (09ZDQD003 and 10CXTD001).
Notes and references
- (a) C. L. Hill, J. Mol. Catal. A: Chem., 2007, 262, 2 CrossRef CAS; (b) E. Coronado, C. Gimenez-Saiz and C. J. Gomez-Garcia, Coord. Chem. Rev., 2005, 249, 1776 CrossRef CAS; (c) B. Hasenknopf, Front. Biosci., 2005, 10, 275 CrossRef CAS; (d) D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC; (e) D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS; (f) M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, 1983 Search PubMed; (g) M. T. Pope and A. Müller, Angew. Chem., 1991, 103, 56 CrossRef CAS; (h) R. P. Bontchev and M. Nyman, Angew. Chem., 2006, 118, 6822 ( Angew. Chem., Int. Ed. , 2006 , 45 , 6670 ) CrossRef; (i) N. V. Izarova, N. Vankova, A. Banerjee, G. B. Jameson, T. Heine, F. Schinle, O. Hampe and U. Kortz, Angew. Chem., Int. Ed., 2010, 49, 7807 CrossRef CAS; (j) X. L. Wang, C. Qin, E. B. Wang, Z. M. Su, Y. G. Li and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 7411 CrossRef CAS; (k) J. Y. Niu, G. Chen, J. W. Zhao, P. T. Ma, S. Z. Li, J. P. Wang, M. X. Li, Y. Bai and B. S. Ji, Chem.–Eur. J., 2010, 16, 7082 CAS; (l) J. Y. Niu, P. T. Ma, H. Y. Niu, J. Li, J. W. Zhao, Y. Song and J. P. Wang, Chem.–Eur. J., 2007, 13, 8739 CrossRef CAS.
- (a) W. G. Klemperer, T. A. Marquart and O. M. Yaghi, Angew. Chem., 1992, 104, 51 ( Angew. Chem., Int. Ed. Engl. , 1992 , 31 , 49 ) CrossRef CAS; (b) U. Kortz, A. Müller, J. van Slageren, J. Schnack, N. S. Dalal and M. Dressel, Coord. Chem. Rev., 2009, 253, 2315 CrossRef CAS; (c) A. Müller, F. Peters, M. T. Pope and D. Gatteschi, Chem. Rev., 1998, 98, 239 CrossRef; (d) T. Yamase, Chem. Rev., 1998, 98, 307 CrossRef CAS; (e) X. L. Wang, C. Qin, E. B. Wang and Z. M. Su, Chem. Commun., 2007, 4245 RSC; (f) S. T. Zheng, H. Zhang and G. Y. Yang, Angew. Chem., Int. Ed., 2008, 47, 3909 CrossRef CAS.
- (a) J. T. Rhule, C. L. Hill and D. A. Judd, Chem. Rev., 1998, 98, 327 CrossRef CAS; (b) M. H. Cjoamg, C. W. Williams, L. Soderhlm and M. R. Antonio, Eur. J. Inorg. Chem., 2003, 2663 Search PubMed; (c) A. J. Russell, J. A. Berberich, G. F. Drevon and R. R. Koepsel, Annu. Rev. Biomed. Eng., 2003, 5, 1 CrossRef CAS; (d) U. Kortz, M. G. Savelieff, B. S. Bassil, B. Keita and L. Nadjo, Inorg. Chem., 2002, 41, 783 CrossRef CAS; (e) A. Besserguenew, M. Dickman and M. Pope, Inorg. Chem., 2001, 40, 2582 CrossRef; (f) F. Bonhomme, J. P. Larentzos, T. M. Alam, E. J. Maginn and M. Nyman, Inorg. Chem., 2005, 44, 1774 CrossRef CAS; (g) C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 5634 CrossRef CAS.
- (a) S. J. Li, S. X. Liu, C. C. Li, F. J. Ma, D. D. Liang, W. Zhang, R. K. Tan, Y. Y. Zhang and L. Xu, Chem.–Eur. J., 2010, 16, 13435 CrossRef CAS; (b) I. Lindqvist, Ark. Kemi, 1953, 5, 247 CAS; (c) G. M. Rozantsev, O. I. Dotsenko and G. V. Taradina, Russ. J. Coord. Chem., 2000, 26, 247 CAS; (d) T. M. Alam, M. Nyman, B. R. Cherry, J. M. Segall and L. E. Lybarger, J. Am. Chem. Soc., 2004, 126, 5610 CrossRef CAS; (e) T. Ozeki, T. Yamase, H. Naruke and Y. Sasaki, Bull. Chem. Soc. Jpn., 1994, 67, 3249 CrossRef CAS.
- (a) M. Nyman, F. Bonhomme, T. M. Alam, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996 CrossRef CAS; (b) M. Nyman, F. Bonhomme, T. M. Alam, J. B. Parise and G. M. B. Vaughan, Angew. Chem., 2004, 116, 2847 ( Angew. Chem., Int. Ed. , 2004 , 43 , 2787 ) CrossRef; (c) M. Nyman, A. J. Celestian, J. B. Parise, G. P. Holland and T. M. Alam, Inorg. Chem., 2006, 45, 1043 CrossRef CAS.
- (a) C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., 2008, 120, 5716 ( Angew. Chem., Int. Ed. , 2008 , 47 , 5634 ) CrossRef; (b) C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., 2008, 120, 8375 ( Angew. Chem., Int. Ed. , 2008 , 47 , 8251 ) CrossRef; (c) E. M. Villa, C. A. Ohlin, J. R. Rustad and W. H. Casey, J. Am. Chem. Soc., 2009, 131, 16488 CrossRef CAS.
- M. Maekawa, Y. Ozawa and A. Yagasaki, Inorg. Chem., 2006, 45, 9608 CrossRef CAS.
- R. P. Bontchev and M. Nyman, Angew. Chem., 2006, 118, 6822 ( Angew. Chem., Int. Ed. , 2006 , 45 , 6670 ) CrossRef.
- R. Tsunashima, D. L. Long, H. N. Miras, D. Gabb, C. P. Pradeep and L. Cronin, Angew. Chem., 2010, 112, 117 ( Angew. Chem., Int. Ed. , 2010 , 49 , 113 ) Search PubMed.
- (a) R. G. Finke and M. W. Droege, J. Am. Chem. Soc., 1984, 106, 7274 CrossRef CAS; (b) D. A. Judd, Q. Chen, C. F. Campana and C. L. Hill, J. Am. Chem. Soc., 1997, 119, 5461 CrossRef CAS; (c) G. S. Kim, H. Zeng and C. L. Hill, Bull. Korean Chem. Soc., 2003, 24, 1005 CrossRef CAS; (d) G. S. Kim, H. D. Zeng, D. VanDerveer and C. L. Hill, Angew. Chem., 1999, 111, 3413 ( Angew. Chem., Int. Ed. , 1999 , 38 , 3205 ) CrossRef.
- G. L. Guo, Y. Q. Xu, J. Cao and C. W. Hu, Chem. Commun., 2011, 47, 9411 RSC.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B, 1985, 41, 244 CrossRef.
- E. J. Graeber and B. Morosin, Acta Crystallogr., Sect. B, 1977, 33, 2137 CrossRef.
- C. Y. Sun, Y. G. Li, E. B. Wang, D. R. Xiao, H. Y. An and L. Xu, Inorg. Chem., 2007, 46, 1563 CrossRef CAS.
- J. Y. Niu, X. Fu, J. W. Zhao, S. Z. Li, P. T. Ma and J. P. Wang, Cryst. Growth Des., 2010, 10, 7 Search PubMed.
- M. Filowitz, R. K. C. Ho, W. G. Klemperer and W. Shum, Inorg. Chem., 1979, 18, 93 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis, measurements, the coordination geometric frameworks, XRPD, TGA and IR data. CCDC 836458. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cc15684e |
‡ Crystallographic data for 1 (H138Nb20V8O103Cu12C144N24), Mr = 6892.98, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 18.429(5) Å, b = 18.778(2) Å, c = 23.173(3) Å, V = 6939(2) Å3, Z = 1, R1 = 0.0941 and wR2 = 0.2719 (Rint = 0.0399) for 21 , a = 18.429(5) Å, b = 18.778(2) Å, c = 23.173(3) Å, V = 6939(2) Å3, Z = 1, R1 = 0.0941 and wR2 = 0.2719 (Rint = 0.0399) for 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 independent reflections [I > 2σ(I)]. CCDC 836458. 366 independent reflections [I > 2σ(I)]. CCDC 836458. |
| This journal is © The Royal Society of Chemistry 2012 |
