Synthesis of 1,2,3-triazole-fused heterocycles viaPd-catalyzed cyclization of 5-iodotriazoles†‡
Jacqueline M.
Schulman
,
Adam A.
Friedman
,
Jane
Panteleev
and
Mark
Lautens
*
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Canada M5S 3H6. E-mail: mlautens@chem.utoronto.ca; Fax: 1 416 946 8185; Tel: 1 416 978 6083
First published on 14th November 2011
Abstract
A convenient approach toward polycyclic frameworks containing fused 1,2,3-triazoles is described. The synthesis consists of a Cu-catalyzed cycloaddition and an intramolecular Pd-catalyzed direct arylation or Heck reaction, and affords the products in good to excellent yields.
There is increasing interest in triazole-containing compounds, resulting from their intriguing physical and biological properties.1 1,4-Disubstituted triazoles are readily accessible via the copper-catalyzed azide-alkyne cycloaddition (CuAAC), as pioneered by Meldal and Sharpless.2,3 In 2009, Fokin reported a synthesis of 5-iodo-1,2,3-triazoles via the regioselective reaction of iodoalkynes with azides, using catalytic copper(I) iodide and a tris((1,2,3-triazolyl)methyl)amine ligand, such as tris((1-benzyl-1H-1,2,3-triazolyl)methyl)amine (TBTA).4,5 In addition, the 5-iodo-1,2,3-triazole products of the cycloaddition are useful synthetic intermediates that can be further functionalized.6
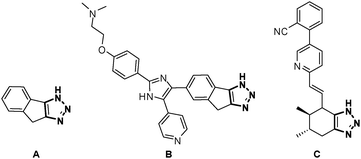
As compounds containing fused triazoles become increasingly common in pharmaceutical targets and biologically active substances, such as chemotherapeutic (A, B) and cardiovascular agents (C, Fig. 1),7–9 new strategies to synthesize this class of molecules are highly desirable. Different methods have been applied toward the synthesis of compounds containing fused triazoles.10 Ackermann recently reported a dehydrogenative intramolecular coupling of 1,4-disubstituted triazoles to obtain tri- and tetracyclic triazoles.11 A recent report by our group utilized an intramolecular direct arylation of 5-iodotriazoles to synthesize unique heterocycles.12 With this method in hand, we were interested in expanding the work to include bicyclic and tricyclic heterocycles. We report an efficient and high-yielding method of synthesizing three unique classes of fused triazole-containing heterocycles.
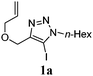
The substrates for the CuAAC reaction were obtained through iodination of terminal alkynes with N-iodomorpholine.13 The CuAAC reaction proceeded efficiently to provide differently substituted 5-iodo-1,2,3-triazoles, which could then undergo a C–H bond functionalization or a Heck reaction to produce the desired fused triazole (Scheme 1).14,15
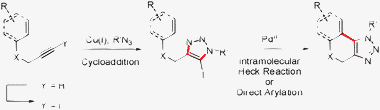 | ||
| Scheme 1 Synthetic sequence for the synthesis of fused triazole-containing polycycles. | ||
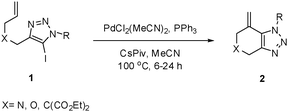
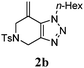
With 5-iodo-1,2,3-triazole 1a in hand, we investigated the influence of solvent, base, catalyst and ligand on the intramolecular Heck reaction. Acetonitrile (MeCN) proved to be the optimal solvent for the reaction, while a base screen demonstrated that CsOPiv was crucial for the reaction to proceed.
A catalyst system consisting of PdCl2(MeCN)2 (5 mol%), along with PPh3 (10 mol%) gave optimal results. After 6 h at 100 °C, the product 2a was obtained in 92% isolated yield.
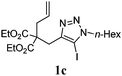
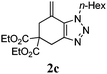
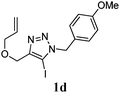
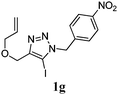
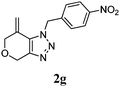
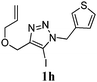
We then investigated the substrate scope of the Heck reaction (Table 1). High to quantitative yields were obtained with oxygen- and nitrogen-containing heterocycles, as well as with a carbocycle (Entries 1–3) bearing an alkyl protecting group on the triazole. Several N-aryl triazoles were reacted as well (Entries 4–7). Both electron-rich and -neutral Heck products were synthesized efficiently (Entries 4–6), whereas the electron-poor substrate 1g bearing a nitro substituent gave a moderate yield (Entry 7). Finally, annulation onto the N-tether of the triazole 1i proceeded in quantitative yield (Entry 9).
| Entry | Substrate | Product | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: 5-iodo-1,2,3-triazole 1 (1 equiv.), PdCl2(MeCN)2 (5 mol%), PPh3 (10 mol%), and CsOPiv (2 equiv.) were dissolved in solvent (0.0625 M) and heated to 100 °C. b Isolated yields. c Reaction performed using Pd(OAc)2 (5 mol%). | |||
| 1 |
|

|
92 |
| 2 |

|
|
99 |
| 3 |
|
|
89 |
| 4 |
|

|
92 |
| 5 |

|

|
99 |
| 6 |

|

|
99 |
| 7 |
|
|
61 |
| 8 |
|
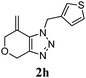
|
84 |
| 9 |
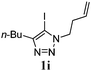
|

|
99c |
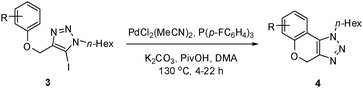
In addition to the coupling with alkenes, we were interested in examining substrates capable of cyclization through direct annulation. The optimized conditions ideal for the Heck reaction, as well as our previous arylation conditions,16 failed to yield any direct arylation product with substrate 3a. Working with this substrate, we found that the optimal temperature for the reaction was 130 °C, as lower temperatures resulted in poorer conversion, and higher temperatures caused decomposition of the product.
Dimethylacetamide (DMA) was superior over other solvents, and K2CO3 gave a considerably higher yield than other bases. Among the ligands examined, P(p-FC6H4)3 (5 mol%) was the most effective, when combined with PdCl2(MeCN)2 (5 mol%). As reported by Fagnou, pivalic acid was an essential additive in this reaction, and without it, the yield decreased significantly.17,18
With these optimal conditions, product 4a was obtained in 88% isolated yield.
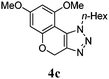
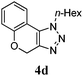
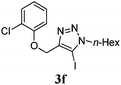
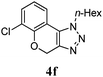
We then probed the scope of the intramolecular direct arylation (Table 2). Electron-rich substrates reacted efficiently (Entries 1–3). Direct arylation with electron-neutral substrates proceeded in a moderate yield (Entry 4), while electron-poor products were afforded in fair to good yields (Entries 5 and 6). Substitution was tolerated at ortho, meta and para positions of the aryl nucleophile.
| Entry | Substrate | Product | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: 5-iodo-1,2,3-triazole (1 equiv.), PdCl2(MeCN)2 (5 mol%), P(p-FC6H4)3 (5 mol%), K2CO3 (3 equiv.) and PivOH (30 mol%) were dissolved in solvent (0.26 M) and heated to 130 °C. b Isolated yields. | |||
| 1 |
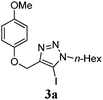
|
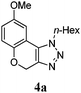
|
88 |
| 2 |

|

|
93 |
| 3 |

|
|
75 |
| 4 |

|
|
68 |
| 5 |

|

|
51 |
| 6 |
|
|
79 |
When attempting to construct 4gvia direct arylation of 3g, we observed a mixture of inseparable isomers, which we suspected to be 4g and 5g (Scheme 2). The unexpected product 5g, a 1,2,3-triazole-fused isoindoline, would form from the annulation of the 5-iodo-1,2,3-triazole onto the p-methoxybenzyl protecting group of the triazole, forming a five-membered ring instead. This intriguing result prompted us to examine substrates capable of cyclization only onto the N-tether and to examine a synthetic route for products such as 5g.
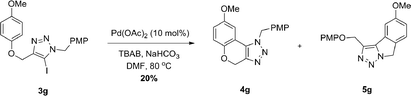 | ||
| Scheme 2 Pd-catalyzed direct arylation of 3g. PMP = p-methoxyphenyl. | ||
When examining the alkyl-substituted substrate 5a, we found that the conditions developed for the intramolecular Heck reaction were effective, giving 1,2,3-triazole-fused isoindoline 6a in a yield of 70%. Reoptimization revealed Pd(OAc)2 to be the best catalyst. Furthermore, increasing the reaction time to 27 h brought the reaction yield to 87%. Similar yields were obtained with other iodotriazole substrates (Scheme 3).
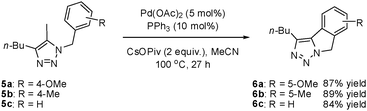 | ||
| Scheme 3 The synthesis of 1,2,3-triazole-fused isoindolines by intramolecular direct arylation. | ||
Isoindolines are useful building blocks and are often found in biologically active compounds.19 Therefore, the development of new methods toward the synthesis of isoindoline-containing molecules is important. Our strategy is unique from previous methods,20 as it involves a 5-halotriazole intermediate, giving us access to these potentially bioactive molecules in three steps from commercially available material.
In conclusion, we have demonstrated an efficient and highly practical approach toward the synthesis of three different heterocyclic motifs containing fused triazoles. In the synthetic sequence, consisting of a CuAAC and a Pd-catalyzed intramolecular annulation, the 5-iodo-1,2,3-triazole proved to be a versatile intermediate. The products were obtained in synthetically useful yields. Our approach gave access to products that may exhibit biological activity and prove useful to the chemical and biological community.
We gratefully acknowledge the Natural Sciences and Engineering Research Council (NSERC) and Merck for an Industrial Research Chair, and the University of Toronto for financial support. J. P. thanks NSERC for a CGSD scholarship, and A. A. F. thanks NSERC for a USRA scholarship.
Notes and references
- (a) K. Ohmatsu, M. Kiyokawa and T. Ooi, J. Am. Chem. Soc., 2011, 133, 1307 CrossRef CAS; (b) H. Nandivada, X. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS; (c) C. W. Tornøe and M. Meldal, Chem. Rev., 2008, 108, 2952 CrossRef.
- (a) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef.
- Reviews on the CuAAC and its applications: (a) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS; (b) V. D. Bock, H. Hiemstra and J. H. Van Maarseveen, Eur. J. Org. Chem., 2006, 51 CrossRef CAS; (c) W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15 CrossRef CAS; (d) J. F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018 CrossRef CAS; (e) P. M. E. Gramlich, C. T. Wirges, A. Manetto and T. Carell, Angew. Chem., Int. Ed., 2008, 47, 8350 CrossRef CAS; (f) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302 RSC.
- J. E. Hein, J. C. Tripp, L. B. Krasnova, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2009, 48, 8018 CrossRef CAS.
- T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS.
- J. Deng, Y.-M. Wu and Q.-Y. Chen, Synthesis, 2005, 2730 CAS.
- L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. Schulz-Pritchard, W. W. Smith, C. A. Janson, M. D. Ryan, G.-F. Zhang, K. O. Johanson, R. B. Kirkpatrick, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, J. Med. Chem., 2005, 48, 5644 CrossRef CAS.
- S. Chackalamannil, M. V. Chelliah, Y. Wang and Y. Xia, WO, 2,008,042,422, 2008 Search PubMed.
- D. Niculescu-Duvaz, I. Niculescu-Duvaz, B. M. J. M. Suijkerbuijk, D. Menard, A. Zambon, A. Nourry, L. Davies, H. A. Manne, F. Friedlos, L. Ogilvie, D. Hedley, A. K. Takle, D. M. Wilson, J.-F. Pons, T. Coulter, R. Kirk, N. Cantarino, S. Whittaker, R. Marais and C. J. Springer, Bioorg. Med. Chem., 2010, 18, 6934 CrossRef CAS.
- E. A. Shafran, V. A. Bakulev, Y. A. Rozin and Y. M. Shafran, Chem. Heterocycl. Compd., 2008, 44, 1040 CrossRef CAS.
- L. Ackermann, R. Jeyachandran, H. K. Potukuchi, P. Novák and L. Büttner, Org. Lett., 2010, 12, 2056 CrossRef CAS.
- J. Panteleev, K. Geyer, A. Aguilar-Aguilar, L. Wang and M. Lautens, Org. Lett., 2010, 12, 5092 CrossRef CAS.
- (a) R. V. Rice and G. D. Beal, US Patent, 2,290,710, 1943 Search PubMed; (b) M. Koyama, N. Ohtani, F. Kai, I. Moriguchi and S. Inouye, J. Med. Chem., 1987, 30, 552 CrossRef CAS.
- Reviews on C–H functionalization: (a) Handbook of C–H Transformations, ed. G. Dyker, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (c) P. Thansandote and M. Lautens, Chem.–Eur. J., 2009, 15, 5874 CrossRef CAS; (d) G. P. McGlacken and L. M. Bateman, Chem. Soc. Rev., 2009, 38, 2447 RSC; (e) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS.
- Reviews on the Heck reaction: (a) R. F. Heck, Org. React., 1982, 27, 345 CAS; (b) A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef; (c) E.-i. Negishi, C. Copéret, S. M. Ma, S. Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS; (d) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS.
- Our original arylation conditions consisted of Pd(OAc)2 (10 mol%), TBAB (40 mol%), NaHCO3 (2.5 equiv.), DMF (0.07 M), 80 °C.
- M. Lafrance, D. Lapointe and K. Fagnou, Tetrahedron, 2008, 64, 6015 CrossRef CAS.
- Without PivOH, the 1H NMR yield of the reaction was 25%.
- (a) G. Cignarella and P. Sanna, J. Med. Chem., 1981, 24, 1003 CrossRef CAS; (b) P. Sanna, G. Cignarella, V. Ananiaa, R. M. S. Siri and M. S. Desole, Farmaco Ed. Sci., 1985, 40, 777 CAS.
- (a) C. Chowdhury, S. B. Mandal and B. Achari, Tetrahedron Lett., 2005, 46, 8531 CrossRef CAS; (b) V. Fiandanese, G. Marchese, A. Punzi, F. Iannone and G. G. Rafaschieri, Tetrahedron, 2010, 66, 8846 CrossRef CAS; (c) Z. Huo and Y. Yamamoto, Tetrahedron Lett., 2009, 50, 3651 CrossRef CAS; (d) L. Ackermann, R. Vicente and R. Born, Adv. Synth. Catal., 2008, 350, 741 CrossRef CAS; (e) Y.-N. Niu, Z.-Y. Yan, G.-L. Gao, H.-L. Wang, X.-Z. Shu, K.-G. Ji and Y.-M. Liang, J. Org. Chem., 2009, 74, 2893 CrossRef CAS.
Footnotes |
| † This article is part of the ChemComm ‘Advances in catalytic C–C bond formation via late transition metals’ web themed issue. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c1cc16110e |
| This journal is © The Royal Society of Chemistry 2012 |