Carbon nanotube coated snowman-like particles and their electro-responsive characteristics†
Ke
Zhang
,
Ying Dan
Liu
and
Hyoung Jin
Choi
*
Department of Polymer Science and Engineering, Inha University, Incheon, 402-751, Korea. E-mail: hjchoi@inha.ac.kr
First published on 4th November 2011
Abstract
We report the fabrication of core–shell structured snowman-like microparticles coated with multi-walled carbon nanotubes and their electro-responsive electrorheological behavior under an applied electric field strength when dispersed in silicone oil. It is observed that they form a chain-like structure, possessing microfluidic potential applications with their solid-like property.
Among various applications of carbon nanotubes (CNT), the fabrication of nano-and micro-structured CNT–polymer hybrids1,2 including nanodevices,3 sensors4 and superhydrophobic materials,5etc. has attracted a lot of attentions due to their fascinating electrical,6 thermal7 and mechanical characteristics.8 In particular, the CNT–particle hybrid obtained by the incorporation of CNT onto the surface of microparticles to form the interactive “net” structure on the spheres via various methods such as layer-by-layer self-assembly,9 adsorption,10 chemical covalent bonding11 has been reported. Furthermore, an electrorheological (ER) phenomenon has been recently reported as the potential application of the CNT–particle hybrids.
The electro-responsive ER fluids are in general composed of particles having high dielectric constant or conductivity and dispersed in an insulating oil, exhibiting the field-induced rheological properties with a rapid and reversible change in the suspension microstructures under an applied electric field.12–19 This transition occurs within a millisecond in their phase from a liquid-like to a solid-like state to form the fibrillar structure spanning the gap between the field-generating electrodes and reversibly going back to a liquid-like state upon the removal of the field, along with its magnetically analogous magnetorheological fluids under applied magnetic field strengths.20 By controlling the electric field, the desirable characteristics of the ER fluids, such as short response time, and significant change in shear viscosity and yield stress, make them widely usable in various engineering applications including engine mounts, torque transducers, shock absorbers, vibration attenuators, control systems, as well as ER polishing. Note that the ER phenomenon is known to be generated from the field induced polarization of the dispersed electro-responsive particles relative to the continuous phase, which is related to the material parameters, including the dielectric response of both liquid and solid particles, particle volume fraction and the rheological properties.
In addition to various ER materials of inorganic, non-metallic, organic, and polymeric semiconducting materials, CNT and its hybrid materials have been also adopted as ER fluids. Especially in the case of the CNT–particle hybrids, their ER phenomena are demonstrated to be not only related to the type of the CNT21 but also dependent on the fabrication method.9,12,13 Compared to the single-walled CNT (SWNT)-coated particles,21 multi-walled CNT (MWNT)-modified particles dispersed in the fluid exhibited the thicker columns between the electrodes, which is considered to be related to the different nature of the electric properties of the CNT. On the other hand, the conductivity of the CNT–particle hybrids could be achieved by controlling the introduction of the CNT's layer via a layer-by-layer self-assembly method9 to satisfy the requirement of an ER system. Though the ER phenomena of the CNT–particle hybrids with a regular shape and size distribution have been investigated, not only the ER behavior of the CNT-snowman-like (SL) microparticle hybrids but also a description on how to achieve the effective coating of CNT onto the SL particles surface by a surfactant are seldom reported. Note that recently Shin et al.22 reported dumbbell-shaped particles covered by CNT and studied their ER property.
In this study, we synthesized the SL microparticles by a seed polymerization method and incorporated carboxylic acid functionalized MWNT onto the SL particles' surface with the help of a cationic surfactant cetyltrimethylammonium bromide (CTAB). Surface morphology, thermal property and electrical conductivity of the MWNT–SL particles were characterized by SEM, TGA and four-probe type of the resistance meter, respectively. The ER behavior of the MWNT–SL particles was also observed by an optical microscope (OM).
At first, the SL particles were prepared by a two-step process, in which cross-linked poly(methyl methacrylate) (PMMA) seeds were synthesized via a dispersion polymerization method and then the SL particles were fabricated after the swelling process by seed polymerization.23 (Detailed experimental procedure can be found in ESI†.) Masses of the used materials for preparation of cross-linked PMMA seeds are listed in Table S1 (ESI†), and the recipes for the seed emulsion polymerization are shown in Table S2 (ESI†).
The obtained SL particles were adopted as core materials for the coating by carboxylic acid functionalized MWNT (c-MWNT). The fabrication process of the MWNT adsorbed SL particles is illustrated in Fig. 1. (Detailed experimental procedure can be found in ESI†.) After the c-MWNT adsorption onto the SL particles, the color of the particle powder (shown in Fig. S1 (ESI†)) changed from its original white to gray or black depending on the amount of MWNT adsorbed. And its conductivity significantly increased from 1.02 × 10−10 S cm−1 (pure SL particles) to 4.06 × 10−8 S cm−1 and 6.10 × 10−4 S cm−1 with 0.96 wt% and 5.65 wt% MWNT coating, respectively.
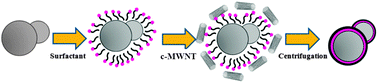 | ||
| Fig. 1 Preparation route for the c-MWNT–SL particles. | ||
Surface morphologies of the PMMA seeds, pure SL particles and c-MWNT adsorbed SL particles are observed by SEM as shown in Fig. 2. From Fig. 2(a), it can be seen that the PMMA seeds obtained by dispersion polymerization are monodisperse with the diameter of 5 μm and have plicate surface morphology. During the experiment, the PMMA seeds adsorbed a large amount of monomer and were further polymerized by the seeded polymerization to form SL particles as exhibited in Fig. 2(b). Although it was observed by SEM incidentally, it is found that the sizes of SL particles are relatively uniform. After the adsorption of the c-MWNT by the aid of a surfactant, the snowman-like particles are observed to be well wrapped by the CNT (Fig. 2(c)). Because the hydrophobic tail groups of the CTAB could be adsorbed onto the particles' surface the hydrophilic head groups are adsorbed onto the c-MWNT by the electrostatic force in the solvent. In this study, experimental results show that 5.65 wt% c-MWNT can be adsorbed onto the SL particle surface (SEM image in Fig. S2 (ESI†)) at most by the aid of surfactant CTAB, which is determined by thermogravimetric analysis (TGA) (Fig. S3 (ESI†)).
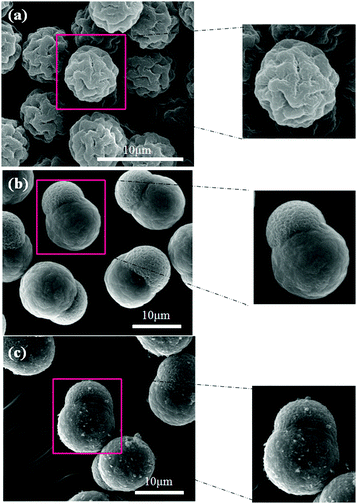 | ||
| Fig. 2 SEM images of PMMA seeds (a), pure SL particles (b) and c-MWNT (0.96 wt%) adsorbed SL particles (c). | ||
Chemical composition of both pure SL particles and c-MWNT adsorbed SL particles was analyzed by an energy dispersive spectroscopy (EDS) as shown in Fig. S4 (ESI†). The ratios of the C, O and Pt elements of SL particles and c-MWNT–SL particles are shown in Table S3 (ESI†).
Fig. 3 shows the flow curves obtained from the controlled shear rate (CSR) test for 10% vol suspension of c-MWNT (0.96 wt%) adsorbed SL particles under different electric field strengths. The c-MWNT adsorbed SL particle based ER fluid behaves like a Newtonian fluid without an electric field, in which the shear stress increases linearly with a shear rate. However, when exposed to an electric field, shear stress abruptly increased as well as the plateau appeared in the low shear rate region, which is attributed to formation of the chain-like structure among the polarized particles showing typical characteristics of Bingham fluid behavior.24 In a low ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) region, the electrostatic interactions among particles induced by external electric fields are dominant compared to the hydrodynamic interactions induced by the external flow field. The chainlike structures of the particles were deformed and started to break down with a further increase in
region, the electrostatic interactions among particles induced by external electric fields are dominant compared to the hydrodynamic interactions induced by the external flow field. The chainlike structures of the particles were deformed and started to break down with a further increase in ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . Above the critical points of
. Above the critical points of ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) where the destruction rate of the column structures becomes faster than the column reforming rate by the electric field, the flow curves behave much like those without an electric field. The fibrillar structures are then broken into particles or particle clusters by shearing due to dominant hydrodynamic interactions and eventually the ER effect virtually disappears.
where the destruction rate of the column structures becomes faster than the column reforming rate by the electric field, the flow curves behave much like those without an electric field. The fibrillar structures are then broken into particles or particle clusters by shearing due to dominant hydrodynamic interactions and eventually the ER effect virtually disappears.
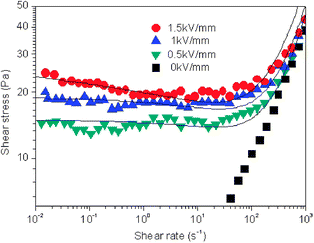 | ||
| Fig. 3 Flow curves of a 10 vol% c-MWNT adsorbed SL particle-based ER fluid under various electric field strengths. Solid lines are generated by fitting by the CCJ model. | ||
Flow curves of the ER fluids under fixed electric field strengths are confirmed to better explain via constitutive rheological equation the Cho–Choi–Jhon (CCJ) model (eqn (1)), under an applied electric field, which can be can be written as
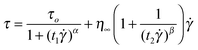 | (1) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≥ 0 above
≥ 0 above ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) crit at which the shear stress becomes a minimum. The first term in eqn (1) suggests the shear stress behavior at a low shear rate region especially in the case of the decline in shear rate and the second term as a function of shear rate explains well the shear stress movement at a high shear rate region. The spacious flexibility of eqn (1) includes diverse ER materials such as Na-fluorohectorite,25polyaniline/nano-silica nanocomposite,26laponite27 and PMMA/MWNT nanocomposite.28 The solid lines in Fig. 3 are values calculated from the CCJ model, in which it is found that the CCJ not only covers the stress in the low shear rate region but also provides more accurate value for the yield stress. The yield stresses and the optimum parameters for the CCJ model are summarized in Table S4 (ESI†).
crit at which the shear stress becomes a minimum. The first term in eqn (1) suggests the shear stress behavior at a low shear rate region especially in the case of the decline in shear rate and the second term as a function of shear rate explains well the shear stress movement at a high shear rate region. The spacious flexibility of eqn (1) includes diverse ER materials such as Na-fluorohectorite,25polyaniline/nano-silica nanocomposite,26laponite27 and PMMA/MWNT nanocomposite.28 The solid lines in Fig. 3 are values calculated from the CCJ model, in which it is found that the CCJ not only covers the stress in the low shear rate region but also provides more accurate value for the yield stress. The yield stresses and the optimum parameters for the CCJ model are summarized in Table S4 (ESI†).
The OM images show the ER behaviors of the 10% vol c-MWNT (0.96 wt%)–SL particles dispersed in silicone oil between two electrodes before (Fig. 4(a)) and after applying electric field (Fig. 4(b)). It is found that the c-MWNT–SL particles of the ER fluid are randomly dispersed in the oil medium showing a liquid-like behavior when they were placed in the gap without an applied electric field (Fig. 4(a)). Interestingly, as soon as a high dc voltage of 0.5 kV mm−1 was applied to the sample, they began to move rapidly toward the electrodes and then formed a fibrillated structure spanned within electrodes, exhibiting a typical ER behavior.29 And the structure remained stable as long as the electrical field was applied. The alignment of the CNT–particle hybrids induced by the applied electric field demonstrated different states, such as thicker columns (MWNT-coated particles),21 thinner columns (SWNT-coated particles)21 and “broken bridges”.30 It was assumed that the state of the column formed induced by the applied electric field might affect the ER properties of the CNT–particle hybrids. Due to an anisotropic snowman-like shape, not only less number of particles will be placed between two electrodes compared to spherical microparticles but also the polarized interaction between particles will be reduced due to unsymmetric polarization density,13,21 while their further study on ER properties induced by the particle shape and MWNT loading is underway for a future work.
 | ||
| Fig. 4 The OM images of the c-MWNT (0.96 wt%)–SL particles dispersed in silicone oil with no applied electric field (a) and under an applied electric field (0.5 kV mm−1) (b). | ||
In this study, we decorated the SL particles by c-MWNT under the existence of a cationic surfactant by a simple and convenient method. The observations of SEM, TGA and EDS indicated that the c-MWTN was successfully adsorbed onto the surface of the SL particles due to the presence of the electrostatic interaction between the carboxylic acid functionalized MWNT and the cationic surfactant. The ER fluid of c-MWNT–SL particles dispersed in a silicone oil exhibited a typical ER behavior induced by the applied electric field.
This research was supported by Ministry of Knowledge Economy, Korea and Kolon (2011).
Notes and references
- (a) J. Zhu, S. Wei, J. Ryu, M. Budhathoki, G. Liang and Z. Guo, J. Mater. Chem., 2010, 20, 4937 RSC; (b) J. M. Thomassin, I. Molenberg, I. Huynen, A. Debigne, M. Alexandre, C. Jerome and C. Detrembleur, Chem. Commun., 2010, 46, 3330 RSC.
- E. Schopf, R. Broyer, L. Tao, Y. Chen and H. D. Maynard, Chem. Commun., 2009, 4818 RSC.
- S. V. N. T. Kuchibhatla, A. S. Karakoti, D. Bera and S. Seal, Prog. Mater. Sci., 2007, 52, 699 CrossRef CAS.
- L. Zhang, L. Tao, B. Li, L. Jing and E. Wang, Chem. Commun., 2010, 46, 1476 RSC.
- Y. Li, X. J. Huang, S. H. Heo, C. C. Li, Y. K. Choi, W. P. Cai and S. O. Cho, Langmuir, 2007, 23, 2169 CrossRef CAS.
- M. F. Yu, B. S. Files, S. Arepalli and R. S. Ruoff, Phys. Rev. Lett., 2000, 84, 5552 CrossRef CAS.
- E. N. Konyushenko, J. Stejskal, M. Techova, J. Hradil, J. Kovarova, J. Prokes, M. Cieslar, J. Y. Hwang, K. H. Chen and I. Sapurina, Polymer, 2006, 47, 5715 CrossRef CAS.
- A. Godaraa, L. Mezzoa, F. Luizia, A. Warrierb, S. V. Lomovb, A. W. van Vuureb, L. Gorbatikhb, P. Moldenaersc and I. Verpoestb, Carbon, 2009, 47, 2914 CrossRef.
- Y. S. Kim, B. S. Kim and K. D. Suh, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1058 CrossRef.
- H. J. Jin, H. J. Choi, S. H. Yoon, S. J. Myung and S. E. Shim, Chem. Mater., 2005, 17, 4034 CrossRef CAS.
- S. J. Han, B. Kim and K. D. Suh, Mater. Lett., 2007, 61, 3995 CrossRef CAS.
- F. Liu, G. Xu, J. Wu, Y. Cheng, J. Guo and P. Cui, Colloid Polym. Sci., 2010, 288, 1739 CAS.
- J. Y. Hong, M. Choi, C. Kim and J. Jang, J. Colloid Interface Sci., 2010, 347, 177 CrossRef CAS.
- J. Yin, X. Zhao, L. Xiang, X. Xia and Z. Zhang, Soft Matter, 2009, 5, 4687 RSC.
- M. Stenicka, V. Pavlinek, P. Saha, N. V. Blinova, J. Stejskal and O. Quadrat, Colloid Polym. Sci., 2009, 287, 403 CAS.
- H. Yilmaz, H. I. Unal and B. Sari, J. Appl. Polym. Sci., 2007, 103, 1058 CrossRef CAS.
- P. Hiamtup, A. Sirivat and A. M. Jamieson, J. Mater. Sci., 2010, 45, 1972 CrossRef CAS.
- W. L. Zhang, B. J. Park and H. J. Choi, Chem. Commun., 2010, 46, 5596 RSC.
- K. P. S. Parmar, Y. Meheust, B. Schjelderupsen and J. O. Fossum, Langmuir, 2008, 24, 1814 CrossRef CAS.
- (a) B. J. Park, F. F. Fang and H. J. Choi, Soft Matter, 2010, 6, 5246 RSC; (b) I. Bica, Ind. Eng. Chem. Res., 2010, 16, 359 CrossRef CAS.
- B. Kim, Y. H. Lee, S. J. Han, J. H. Ryu and K. D. Suh, Colloids Surf., A, 2007, 298, 245 CrossRef CAS.
- K. Shin, S. Lee, J. J. Kim and K. D. Suh, Macromol. Rapid Commun., 2010, 31, 1987 CrossRef CAS.
- Y. D. Liu, F. F. Fang and H. J. Choi, Langmuir, 2010, 26, 12849 CrossRef CAS.
- Q. Cheng, V. Pavlinek, Y. He, C. Li and P. Saha, Colloid Polym. Sci., 2009, 287, 435 CAS.
- Y. Meheust, K. P. S. Parmar, B. Schjelderupsen and J. O. Fossum, J. Rheol., 2011, 55, 809 CrossRef CAS.
- Y. D. Liu, F. F. Fang, H. J. Choi and Y. Seo, Colloids Surf., A, 2011, 381, 17 CrossRef CAS.
- B. Wang, M. Zhou, Z. Rozynek and J. O. Fossum, J. Mater. Chem., 2009, 19, 1816 RSC.
- S. J. Park, M. S. Park, S. T. Lim, H. J. Choi and M. S. Jhon, Macromol. Rapid Commun., 2005, 26, 1563 CrossRef CAS.
- M. S. Choi, Y. H. Cho, H. J. Choi and M. S. Jhon, Langmuir, 2003, 19, 5875 CrossRef.
- S. T. Kim, J. Y. Lim, B. J. Park and H. J. Choi, Macromol. Chem. Phys., 2007, 208, 514 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, Table S1 (recipes for the preparation of monodisperse cross-linked PMMA seeds), Table S2 (recipes for the preparation of snowman-like particles), Table S3 (ratio of the C, O and Pt elements obtained by EDS analysis (in atomic %)), Table S4 (the optimal parameters in the CCJ model obtained from the flow curve of c-MWNT-coated particle based ER fluids at various electric field strengths), photographs of the particle powder, TGA curves, EDS spectrum, SEM image. See DOI: 10.1039/c1cc16140g |
| This journal is © The Royal Society of Chemistry 2012 |
