Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids
Ying
Zhou
ab and
Juyoung
Yoon
*a
aDepartment of Chemistry and Nano Science and Department of Bioinspired Science (WCU), Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr; Fax: +82-2-3277-2384; Tel: +82-2-3277-2400
bSchool of Chemical Science and Technology, Yunnan University, Kunming 650091, P. R. China
First published on 28th July 2011
Abstract
Due to the biological importance of amino acids, the development of optical probes for these molecules has been an active research area in recent years. This tutorial review focuses on recent contributions since the year 2000 concerning the fluorescent or colorimetric sensors for amino acids, and is organized according to their structural classification and reaction types. For reaction based chemosensors, the works are classified according to the mechanisms between sensors and amino acids, including imine formation, Michael addition, thiazinane or thiazolidine formation, cleavage of a sulfonate ester, cleavage of disulfide, metal complexes-displace coordination and others.
 Ying Zhou | Ying Zhou was born in Shenyang, China, in 1978. She received her PhD in 2008 from Dalian University of Technology under the supervision of Prof. Xuhong Qian. Subsequently she joined the group of Juyoung Yoon at Ewha Womans University as a postdoctoral fellow. In 2010, she became a staff member of Yunnan University. Her recent research focuses on the fluorescence chemosensors and the organic electroluminescent materials. |
 Juyoung Yoon | Juyoung Yoon was born in Pusan, Korea, in 1964. He received his PhD (1994) from The Ohio State University. After completing postdoctoral research at UCLA and at Scripps Research Institute, he joined the faculty at Silla University in 1998. In 2002, he moved to Ewha Womans University, where he is currently a Professor of Department of Chemistry and Nano Science and Department of Bioinspired Science. His research interests include investigations of fluorescent chemosensors, molecular recognition and organo EL materials. |
1. Introduction
Amino acids (AA), the key constituents of proteins, are small molecules with various functional side chain groups, which result in different roles of amino acids in physiological processes.1 In this family, lysine (Lys) is closely related to the Krebs–Henseleit cycle and polyamine synthesis, and an appropriate amount of lysine in the diet is essential for the metabolic functions and weight gain of animals;2 histidine (His) is essential for the growth and repair of tissue as well as for the control of metal elements transmission in biological bases;3 tryptophan (Trp) plays a crucial part in biological processes such as protein biosynthesis, animal growth, and plant development.4 In addition, the deficiency of some amino acids causes various abnormalities, for example, deficiency of cysteine (Cys) results in slow growth, hair depigmentation, edema, lethargy, liver damage, muscle and fat loss.5As the result of increasing attention paid to human health, and diagnosis and treatment of disease, many efforts have been directed to the development of new methods toward amino acid analysis. Currently, the most used analytical procedures to detect and characterize amino acids are based on spectroscopic,6 chromatographic7 or electrochemical approaches.8 However, each approach has some drawbacks such as the need of apparatus and trained personnel, operational convenience, analysis cost, test speed and detection limit, etc. Although there are numerous systems for their detection, there is still a need to develop novel and simple fluorescent and colorimetric chemosensors for visual discrimination due to their ease of use in solution as well as the high sensitivity and selectivity.9 Therefore, the interest in developing optical chemosensors specifically recognizing a target amino acid has grown steadily, and the ability to easily and quickly obtain fingerprints for different amino acids by chemosensors will still constitute a breakthrough.
Recent advances in the field of amino acid sensing have focused on the use of fluorescent and colorimetric methods for the enantioselective discrimination of amino acids. Indicator-displacement assays, metal complexes coordination, specific reactions between probes and amino acids and other ways have been widely utilized. Since the chemosensors for Cys have been recently reviewed,10 the very latest contributions for Cys selective chemosensors are included here. In this tutorial review, we focus on the fluorescent and colorimetric chemosensors developed since the year 2000 for the detection of basic amino acids. These sensors are classified according to their structural classifications. We hope to provide a general overview of the design and applications of these chemosensors for the detection of amino acids.
2. Fluorescent and colorimetric chemosensors for detecting amino acids
2.1 Fluorescent and colorimetric chemosensors based on metal complexes
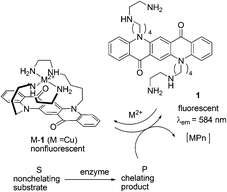 | ||
| Fig. 1 Principle of the fluorescence enzyme assay using 1. | ||
Reymond and co-workers further explored a water soluble fluorescein-based ligand 2 forming a non-fluorescent complex with Cu2+ (Fig. 2).12 This complex served as a fluorescent sensor for most amino acids in the 10−3 M concentration range. Cysteine resulted in a full return of fluorescence upon addition of one equivalent relative to Cu2+. Since the signal response was very fast, the sensor can be used to detect the hydrolytic activity of various proteases (trypsin, chymotrypsin, subtilisin) on bovine serum albumin as a whole protein substrate, and more generally to follow reactions releasing or removing free amino acids in real time.
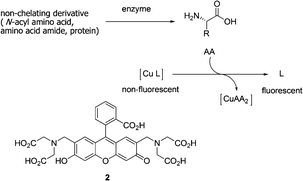 | ||
| Fig. 2 Principle of a fluorescence assay for amino acids using copper-ligand complexes. | ||
In 2003, Fabbrizzi et al. reported an off–on chemosensing ensemble for selective recognition of the ambidentate imidazole residue of histidine over the carboxylate group of natural amino acids via the indicator-displacement assays (IDAs) (Fig. 3).13 Coumarine 343, fluorescein, and eosine Y were used as the indicators. Titration of each indicator with the [Cu2+2(3)]4+ receptor complex at pH = 7 resulted in a complete quenching of the emission. The [Cu2+2(3)]4+/coumarine ensemble does not discriminate His and glycine (Gly), while the fluorescein-containing ensemble satisfactorily discriminates His with full recovery of fluorescence from Gly. The highest sensing selectivity is observed with the [Cu2+2(3)]4+/eosine Y ensemble. The observed trend of stability of other amino acids (Gly > alanine (Ala) > phenylalanine (Phe) > valine (Val) > Leu > proline (Pro)) seems to be related to the increasing steric repulsive effects exerted by the substituent.
![The structures of [Cu2+2(3)]4+, Coumarine 343, fluorescein, and eosine Y.](/image/article/2012/CS/c1cs15159b/c1cs15159b-f3.gif) | ||
| Fig. 3 The structures of [Cu2+2(3)]4+, Coumarine 343, fluorescein, and eosine Y. | ||
In light of their previous work, Fabbrizzi and co-workers reported a dicopper(II) octamine cage as a selective receptor for L-glutamate ion in water at pH 7, establishing Cu2+/–COO− coordinative interactions (Fig. 4).14L-Glutamate (Glu) was able to displace the quenched rhodamine indicator (7) from the cage, whose fluorescence was then fully restored. Addition of 5 caused the fluorescence intensity of 7 to decrease, until complete quenching occurred. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct between the dimetallic cage 5 and the indicator 7, whose association constant log
1 adduct between the dimetallic cage 5 and the indicator 7, whose association constant log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kass is 7.0 ± 0.2, was formed. The solution was titrated with a solution of L-glutamic acid. And, upon titration, fluorescence of 7 at 571 nm was progressively restored. A value of log
Kass is 7.0 ± 0.2, was formed. The solution was titrated with a solution of L-glutamic acid. And, upon titration, fluorescence of 7 at 571 nm was progressively restored. A value of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kass = 6.9 ± 0.2 was obtained for the adduct-formation equilibrium.
Kass = 6.9 ± 0.2 was obtained for the adduct-formation equilibrium.
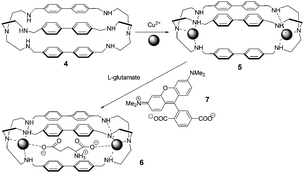 | ||
| Fig. 4 Cascade mechanism for the consecutive inclusion of two Cu2+ ions and a dicarboxylate ion (e.g., glutamate) within a bis-tren cage with diphenyl spacers. | ||
In 2005, coordinatively unsaturated metal complexes were reported by Anslyn et al. (Fig. 5).15 The receptors [Cu(8)]2+ and [Cu(9)]2+ were found to discriminate His from other zwitterionic α-amino acids by means of IDAs using 5(6)-carboxyfluorescein as an indicator. The titrations of each of the complexes into solutions of the indicator caused an increase in the absorbance at 494 nm resulting in a visual color change from a bright yellow green to a dark yellow brown in buffered MeOH/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The colorimetric detection of His was achieved by using this IDA method, which appears to owe its selectivity to a unique process involving disruption of the host complex to form a 2
1) solution. The colorimetric detection of His was achieved by using this IDA method, which appears to owe its selectivity to a unique process involving disruption of the host complex to form a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 His/Cu2+ complex rather than simple indicator displacement.
1 His/Cu2+ complex rather than simple indicator displacement.
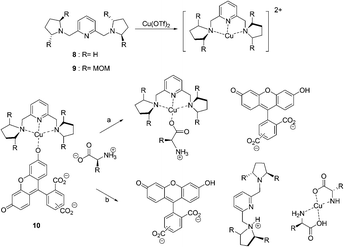 | ||
| Fig. 5 The structures of 8, 9 and two pathways for the displacement of indicator 10 from a Cu2+-containing receptor by an L-amino acid guest. | ||
In the same year, Anslyn et al. reported another operationally simple sensing scheme based on competitive dynamic metal coordination (Fig. 6).16 Pyrocatechol violet (PV) was used in this indicator displacement assay to effectively compete with the amino acid guest for open coordination sites on (S,S)-11–Cu2+. Titration of (S,S)-11–Cu2+ with PV gave a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with a shift from 445 to 645 nm, resulting in a change in color from pale yellow to intense blue. Addition of amino acid to the (S,S)-11–Cu2+/PV complex resulted in the reversal of the spectral change, signaling PV displacement. Relationships between ee and absorbance were obtained in 1
1 complex with a shift from 445 to 645 nm, resulting in a change in color from pale yellow to intense blue. Addition of amino acid to the (S,S)-11–Cu2+/PV complex resulted in the reversal of the spectral change, signaling PV displacement. Relationships between ee and absorbance were obtained in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/H2O (50 mM HEPES buffer pH = 7.0). The resultant ee versus A relationships are remarkably linear (R2 > 0.99), demonstrating uniform sensitivity over the entire ee range.
1 MeOH/H2O (50 mM HEPES buffer pH = 7.0). The resultant ee versus A relationships are remarkably linear (R2 > 0.99), demonstrating uniform sensitivity over the entire ee range.
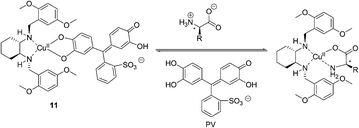 | ||
| Fig. 6 The proposed binding mode of host 11, indicator (PV) with amino acids. | ||
Anslyn and co-workers further reported the discrimination of enantiomeric and structurally similar amino acids through a series of IDAs based on dynamic metal coordination (Fig. 7).17 The selected indicators, chromophores pyrocatechol violet (PCV), chromoxane cyanin R (CCR) and chrome azurole S (CAS), undergo large red shifts in their absorbance spectra upon Cu2+ coordination, thus providing a highly sensitive colorimetric output. The sense of enantioselectivity exhibited in each IDA was found to be a general property of the chiral receptor, with complexes 12–Cu2+and 13–Cu2+ preferring L configurations while 14–Cu2+ favoring D configurations. Because free CAS is yellow in color and Cu2+-bound CAS is blue, the ratio of the bound to the free indicator, which depends on the stability of the receptor–amino acid complex, can be assessed by visual inspection (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH
1 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 50 mM HEPES buffer, pH = 7.8). All of the IDAs conformed to the relative chemoselective ordering for complex stability, as Trp > Phe > Leu ≈ Val > tert-leucine (Tle).
H2O, 50 mM HEPES buffer, pH = 7.8). All of the IDAs conformed to the relative chemoselective ordering for complex stability, as Trp > Phe > Leu ≈ Val > tert-leucine (Tle).
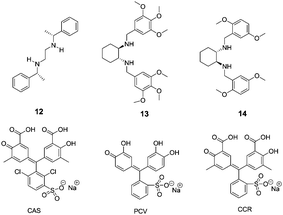 | ||
| Fig. 7 The structures of compounds 12–14 and indicators (CAS, PCV, CCR). | ||
Anslyn et al. extended the scope of the previous study by an enantioselective indicator displacement assay (eIDA) (Fig. 8).18 One of the two chiral receptors ([Cu2+(15)]2+ or [Cu2+(16)]2+) and chrome azurol S (CAS), as the indicator, were used to enantioselectively discriminate 13 α-amino acids in aqueous medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH
1 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, HEPES pH = 7.5). [Cu2+((R,R)-15)]2+ was generally found to bind more strongly to L-α-amino acids, however in the case of aspartate, asparagine, and histidine, the D-enantiomer was preferred by receptor [Cu2+((R,R)-15)]2+. Enantiomeric excess was determined for true test samples using ee calibration curves to demonstrate the capabilities of eIDAs.
H2O, HEPES pH = 7.5). [Cu2+((R,R)-15)]2+ was generally found to bind more strongly to L-α-amino acids, however in the case of aspartate, asparagine, and histidine, the D-enantiomer was preferred by receptor [Cu2+((R,R)-15)]2+. Enantiomeric excess was determined for true test samples using ee calibration curves to demonstrate the capabilities of eIDAs.
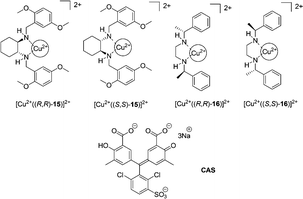 | ||
| Fig. 8 The structures of copper complexes of 15–16 and indicators (CAS). | ||
In 2000, two modified cyclodextrins were described by Corradini and co-workers (Fig. 9).19 Addition of D- or L-amino acids to the Cu2+ complexes induced a ‘switch on’ of the fluorescence. The complex Cu–(S)-17 showed better discriminating properties than Cu–(R)-17. The best enantioselectivity was observed for Pro with both cyclodextrins, with reversed order (ΔFD/ΔFL = 3.89 for Cu–(S)-17 and ΔFD/ΔFL = 0.74 for Cu–(R)-17). Enantioselectivities with Cu–(S)-17 for Phe and Trp are ΔFD/ΔFL = 0.33 and ΔFD/ΔFL = 0.63, respectively. Cu–(S)-17 can be considered as an enantioselective fluorescence sensor for Pro in the concentration range analyzed (6 × 10−5–6 × 10−4 M), used for the determination of the optical purity of proline in dilute solutions (6 × 10−4 M, 0.1 mg total amount).
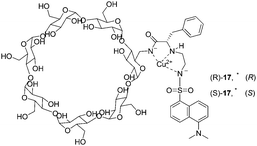 | ||
| Fig. 9 Schematic structures of the Cu2+ complexes of dansylated cyclodextrins (R)-17 and (S)-17. | ||
Fluorescent monofunctionalized β-cyclodextrins (18–23) bearing a Cu2+ binding side arm and a dansyl group were designed as enantioselective sensors for unmodified α-amino acids (Fig. 10).20 Enantioselectivity was evaluated by addition of Cu2+ complexes of D- or L-valine and D- or L-proline. The cyclodextrin 21 was found to be poorly enantioselective, as was similarly the case for 19, suggesting that the best design can be obtained by using L-amino acids. All L-amino acid containing cyclodextrins (18, 20, 22, 23) showed good enantioselectivities. With 18 and 20, enantiomers of α,α-disubstituted amino acids, N-methylamino acids, and amino acid amides were found to be discriminated.
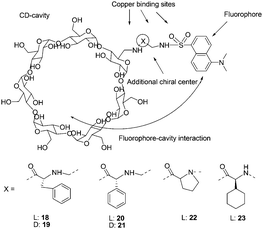 | ||
| Fig. 10 Structures of cyclodextrins 18–23, and structural elements considered in the design of enantioselective cyclodextrins. | ||
In 2004, Corradini's group continued their work and discovered significant insights into the role of the cavity in the recognition process (Fig. 11).21 Addition of D- or L-amino acids to the solution of 26 and 27 (0.1 M borate buffer pH = 7.3) induced increases in fluorescence intensity. With 26, good enantioselectivity and high significance were found only in the case of Pro; Val and Ser gave significant differences at low amino acid excess, while Leu gave a significant difference only at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 excess. When cyclodextrin 27 was used instead, the good enantioselectivities for Pro, Val, Leu, Ser, Phe, and PhGly at all ratios were obtained, while Lys and Tyr enantiomers were discriminated only at low molar excess values. The cyclodextrin 27 was more enantioselective than 26, suggesting that the self-inclusion in the cyclodextrin cavity strongly increases the chiral discrimination ability of the Cu2+ complex.
1 excess. When cyclodextrin 27 was used instead, the good enantioselectivities for Pro, Val, Leu, Ser, Phe, and PhGly at all ratios were obtained, while Lys and Tyr enantiomers were discriminated only at low molar excess values. The cyclodextrin 27 was more enantioselective than 26, suggesting that the self-inclusion in the cyclodextrin cavity strongly increases the chiral discrimination ability of the Cu2+ complex.
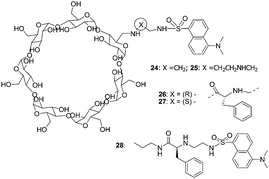 | ||
| Fig. 11 Structures of cyclodextrins 24–28, and structural elements considered in the design of enantioselective cyclodextrins. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; buffered with 10 mM HEPES at pH 7.4). Binding constants of 3.75 × 105 and 6.0 × 104 M−1 for 1
1; buffered with 10 mM HEPES at pH 7.4). Binding constants of 3.75 × 105 and 6.0 × 104 M−1 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of 29
1 binding of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 and 30
31 and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 were obtained, respectively. The addition of various amino acids to an ensemble of 29
31 were obtained, respectively. The addition of various amino acids to an ensemble of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 or 30
31 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 resulted in a color change from deep blue to yellow. The binding constants of 29 with hydrophobic amino acids such as glycine, valine, and phenylalanine were in the range of 1 × 104 M−1.
31 resulted in a color change from deep blue to yellow. The binding constants of 29 with hydrophobic amino acids such as glycine, valine, and phenylalanine were in the range of 1 × 104 M−1.
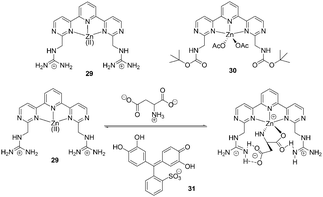 | ||
| Fig. 12 Structures of 29 and 30, and proposed binding mode of host 29, indicator with amino acids. | ||
A fluorescence macrocyclic receptor based on the Zn2+ complex of a C2-terpyridine and a crown ether has been developed by Kwong and Lee et al. for molecular recognition of zwitterionic amino acids in water/DMF solution (Fig. 13).23 Among the amino acids with a chelating group in the side-chain, L-aspartate (Asn) (K = 4.5 × 104 M−1, ΔG0 = 26.5 kJ mol−1) and L-cysteine (K = 2.5 × 104 M−1, ΔG0 = 25.1 kJ mol−1) showed the highest level of affinity towards receptor 32. Receptor 33 also exhibited 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding towards L-aspartate with K = 5 × 102 M−1, which is about 90 times smaller than the K value of receptor 32. This indicated that the Zn2+-tpy and the crown ether are both essential for binding towards zwitterionic L-amino acids in water/DMF solutions. In the enantiomeric recognition studies, receptor 32 showed enantioselectivity towards the D-amino acids with KD/KL = 3.0.
1 binding towards L-aspartate with K = 5 × 102 M−1, which is about 90 times smaller than the K value of receptor 32. This indicated that the Zn2+-tpy and the crown ether are both essential for binding towards zwitterionic L-amino acids in water/DMF solutions. In the enantiomeric recognition studies, receptor 32 showed enantioselectivity towards the D-amino acids with KD/KL = 3.0.
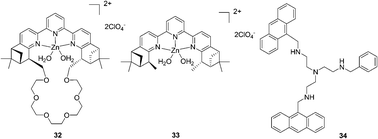 | ||
| Fig. 13 Structures of compounds 32–34. | ||
The tetra-amino tripodal ligand, 34, containing two anthracene subunits, has been prepared by Fabbrizzi and co-workers (Fig. 13).24 The zinc complex, [Zn2+(34)]2+, signals only the recognition of Trp through a drastic decrease of the fluorescent emission. The formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–amino acid adduct was proved, and the association constant log
1 receptor–amino acid adduct was proved, and the association constant log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K was 4.21 ± 0.02 for Trp and 4.48 ± 0.05 for Phe. A π-stacking interaction involving the aromatic moieties positioned on the complex and on the amino acid induced extra stability of adducts with Trp (7 kJ mol−1) and Phe (8 kJ mol−1).
K was 4.21 ± 0.02 for Trp and 4.48 ± 0.05 for Phe. A π-stacking interaction involving the aromatic moieties positioned on the complex and on the amino acid induced extra stability of adducts with Trp (7 kJ mol−1) and Phe (8 kJ mol−1).
Rao et al. reported a lower-rim naphthylidene conjugate of calix[4]arene, 35 (L). [ZnL] could recognize aspartic acid (Asp), Cys, His, and Glu from among the naturally occurring amino acids by exhibiting large fluorescence quenching (Fig. 14).25 The recognition features of [ZnL] toward amino acids seem to have bearing on protonation and chelating ability (His > Phe > Asp > Cys ≫ Glu), π–π interaction ability (Trp ≫ Tyr > His) of the side chain with the [ZnL], and the dechelation of [ZnL] (His > Cys > Tyr > Asp ∼ Trp). Extension of [ZnL] to proteins also resulted in dechelation of [ZnL] as well as aggregation of the protein with a trend: peanut agglutinin (PNA) < bovine serum albumin (BSA) < jacalin < human serum albumin (HSA).
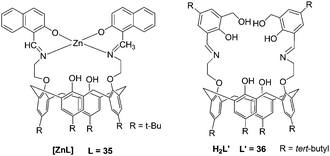 | ||
| Fig. 14 The structures of the complexes. | ||
A new 1,3-diderivative of calix[4]arene appended with hydroxymethyl salicylyl imine, 36 (L′), has been synthesized by Rao and co-workers (Fig. 14).26 The receptor H2L′ showed selectivity toward Zn2+ through switch-on fluorescence with a detection limit of 192 ppb. The complex [ZnL′] was titrated with 20 naturally occurring amino acids which resulted in fluorescence quenching to different extents, and it follows a trend: Cys > Asp ≫ His.
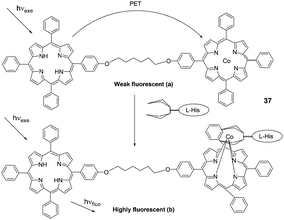 | ||
| Fig. 15 Basic photoinduced electron-transfer process in a porphyrin dimer system: (a) in a free state and (b) guest-species-bound. | ||
Xu et al. reported a cyclometalated palladium-azo complex, 38, as a differential chromogenic probe for amino acids in aqueous solution at physiological pH (Fig. 16).28 Amino acids with different side chain groups were clearly distinguishable in their UV-Vis absorption spectra. For most of the amino acids, such as Cys, Met and Ala, the characteristic absorptions increased linearly with the concentrations of the amino acids titrated with the 38 sensing solutions over a concentration range 2.0 × 10−6–10−5 M, without shifting the spectral shapes. However, in the case of Lys, λmax shifted from 570 to 609 nm at a relatively higher concentration. The efficiency of the colorimetric response was also susceptible to the presence of coexisting inorganic ions.
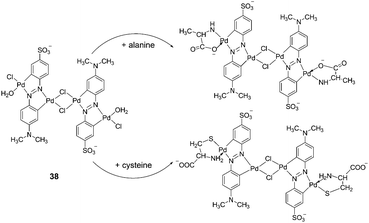 | ||
| Fig. 16 Reaction of 38 with different α-amino acids. | ||
Buryak and Severin reported a chemosensor array for the colorimetric identification of 20 natural amino acids (Fig. 17).29 Organometallic Cp*Rh complex 39 was employed as the receptor unit for these IDAs. As the indicators, the dyes gallocyanine, xylenol orange, and calcein blue were employed. A key component of the sensor array was the utilization of a variable pH to change the selectivity of the IDAs. The UV/vis response at 750 nm of an IDA composed of 39 and 40 was used to classify the amino acids into a high-affinity (consisting of His, Cys, Met, Asp, Asn, ΔA(750 nm) < 0.06) and a low-affinity (the remaining 15 amino acids (0.55 > ΔA(750 nm) > 0.06) group. Each member of the first group was analyzed by four IDAs (by two different dyes, 41 and 42, and at two different pH values), and each member of the second group was analyzed by five IDAs (by indicator 40, at five different pH values). The sole cyclic amino acid, proline, and the closely related analytes such as leucine and isoleucine, are clearly distinguishable.
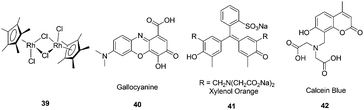 | ||
| Fig. 17 The structures of Cp*Rh complex 39 and the dyes gallocyanine (40), xylenol orange (41), calcein (42). | ||
2.2 Reaction based chemosensors
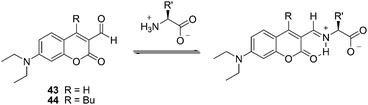 | ||
| Fig. 18 The reaction mechanism of 43, 44 with amino acids. | ||
In 2005, Glass et al. reported sensors for diamines prepared by dimerization of a quinolone aldehyde core (Fig. 19).31 The dimeric sensors bound the diamine guests by formation of a bis-iminium ion which produced large changes in the fluorescence of the quinolone core. In all cases, the diamines (lysine, ornithine, etc.) bound better than butylamine in 50% MeOH-buffered water (50 mM HEPES, 240 mM NaCl, pH = 7.4). The extent of the difference varied from 2.5 fold for sensor 45 to 160 fold for 51. The maximum fluorescence change for 48 was much larger for ornithine (Ka = 3300 M−1) than for lysine (Ka = 2100 M−1) which may indicate a better binding geometry for ornithine. With respect to 51, the bonds for lysine (Ka = 2800 M−1) and ornithine (Ka = 2400 M−1) are similar.
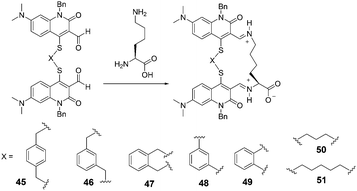 | ||
| Fig. 19 The reaction mechanism of 45–51 with lysine. | ||
Yoon and Lee et al. reported a selective colorimetric and fluorescent chemosensor based on pyrene for lysine (Fig. 20).32 A unique colorimetric change of 52 (CH3CN–HEPES buffer, 0.01 M, pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) from light yellow to pink takes place only in the presence of lysine. This induced color change can be easily observed by the naked eye. A dissociation constant for the complex formed between 52 and Lys was calculated to be Kd = 2.78 × 10−3 M from a linear plot of UV absorbance associated with 1
9, v/v) from light yellow to pink takes place only in the presence of lysine. This induced color change can be easily observed by the naked eye. A dissociation constant for the complex formed between 52 and Lys was calculated to be Kd = 2.78 × 10−3 M from a linear plot of UV absorbance associated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest binding. In addition, a selective fluorescence enhancement occurs when 52 reacts with lysine and not with the other tested amino acids. The calculations revealed that the observed colorimetric change is a consequence of formation of a stacked dimeric form of the products in which an intramolecular H-bond interaction exists between the C
1 host–guest binding. In addition, a selective fluorescence enhancement occurs when 52 reacts with lysine and not with the other tested amino acids. The calculations revealed that the observed colorimetric change is a consequence of formation of a stacked dimeric form of the products in which an intramolecular H-bond interaction exists between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and OH groups that is mediated by an H-bond water relay between the OH and lysine COOH groups.
N and OH groups that is mediated by an H-bond water relay between the OH and lysine COOH groups.
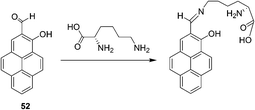 | ||
| Fig. 20 The reaction mechanism of 52 with lysine. | ||
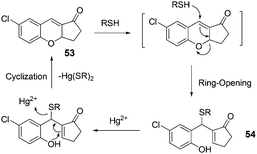 | ||
| Fig. 21 The mechanism for the reaction of 53. | ||
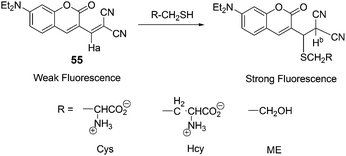
Kim and co-workers demonstrated a fluorescent probe 55 for biothiols (Cys, Hcy, GSH) (Fig. 22).3455 exhibits a sensitive and selective fluorescence enhancement toward the biothiols over other natural amino acids, which is attributable to the Michael addition of a thiol group to the α,β-unsaturated malonitrile unit of 55. In an aqueous solvent of 55 (DMSO/HEPES = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, 0.1 M HEPES, pH 7.4), Cys has shown a dramatic increase (F/Fo = 19) in the fluorescence intensity over other natural amino acids. Hcy and GSH enhance the fluorescence intensity of 1 (Fo) by F/Fo 12- and 5.6-fold, respectively. Probe 55 was further applied for the cellular expression and the detection of biothiols in HeLa cells.
2, v/v, 0.1 M HEPES, pH 7.4), Cys has shown a dramatic increase (F/Fo = 19) in the fluorescence intensity over other natural amino acids. Hcy and GSH enhance the fluorescence intensity of 1 (Fo) by F/Fo 12- and 5.6-fold, respectively. Probe 55 was further applied for the cellular expression and the detection of biothiols in HeLa cells.
Yoon and Shin et al. developed a new fluorescein-based fluorescent probe for the detection of thiol-containing molecules (Fig. 23).35 The probe 56 displayed fluorescence enhancements (λmax = 520 nm) and UV-Vis spectral changes, which were attributed to 1,4-addition of thiols to α,β-unsaturated ketone in the probe to form thioether, in the presence of thiol-containing analytes (Cys, homocysteine (Hcy) and glutathione (GSH)) in HEPES buffer (20 mM, pH 7.4, 1% CH3CN). 56 also showed high sensitivity towards thiols with a detection limit of 10−7–10−8 M, and the observed rate constants (kobs) at pH 7.4 were found to be 36.5, 8.0 and 11.5 min−1 for Cys, Hcy and GSH, respectively.
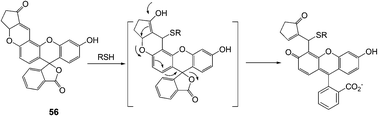 | ||
| Fig. 23 A plausible mechanism of 56 to thiols in aqueous solution. | ||
Kim, Lee and Kang et al. reported a new coumarin-based chemodosimeter 57, which could effectively and selectively recognize thiols based on a Michael type reaction, showing a preference for cysteine over other biological materials including homocysteine and glutathione (Fig. 24).36 In aqueous solution (PBS buffer, pH 7.4, 10% DMSO), the fluorescence intensity is proportional to the amount of Cys added at the submicromolar level, with a coefficient of R = 0.99557 and a detection limit of 30 nM. The pKa of Cys (8.30) is lower than that of Hcy (8.87) or GSH (9.20). The cysteine preference of 57 over homocysteine and glutathione was also demonstrated by LC-MS spectroscopic methods using the metabolite from the HepG2 cell line.
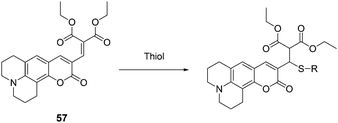 | ||
| Fig. 24 The mechanism for the reaction of 57. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the reaction of Hcy or Cys with probe 58 resulted in a significant fluorescence enhancement around 513 nm. The fluorescence quantum yields of 58 increased from 0.26 to 0.71 with Hcy, and from 0.26 to 0.63 with Cys. The binding constants for probe 58 with Hcy and Cys were determined to be 1300 M−1 and 741 M−1, respectively. Surprisingly, no significant changes were observed in the fluorescence emission spectra and absorption spectra of 59 in the presence of Hcy or Cys. Therefore, it was demonstrated that the location of the aldehyde group at the Bodipy core is important and has an effect on the cyclization with Hcy/Cys.
3), the reaction of Hcy or Cys with probe 58 resulted in a significant fluorescence enhancement around 513 nm. The fluorescence quantum yields of 58 increased from 0.26 to 0.71 with Hcy, and from 0.26 to 0.63 with Cys. The binding constants for probe 58 with Hcy and Cys were determined to be 1300 M−1 and 741 M−1, respectively. Surprisingly, no significant changes were observed in the fluorescence emission spectra and absorption spectra of 59 in the presence of Hcy or Cys. Therefore, it was demonstrated that the location of the aldehyde group at the Bodipy core is important and has an effect on the cyclization with Hcy/Cys.
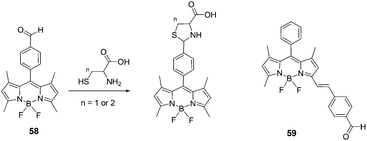 | ||
| Fig. 25 Structures of 58, 59 and the reaction of compound 58 with Hcy or Cys to form thiazinane (n = 2) or thiazolidine (n = 1). | ||
A unique ruthenium(II) complex, 60, has been reported by Yuan and Ye et al. as a luminescence probe for the recognition and detection of Cys and Hcy (Fig. 26).38 The nearly non-luminescent probe can rapidly react with Cys and Hcy to yield the corresponding thiazolidine and thiazinane derivatives, accompanied by the remarkable luminescence enhancement and a large blue-shift of the maximum emission wavelength from 720 to 635 nm. The dose-dependent luminescence enhancement of the probe shows a good linearity in the Cys/Hcy concentration range of 15 to 180 μM with detection limits of 1.41 μM and 1.19 μM for Cys and Hcy, respectively. The luminescence response of the probe is highly specific to Cys/Hcy even in the presence of various amino acids, protein, and DNA.
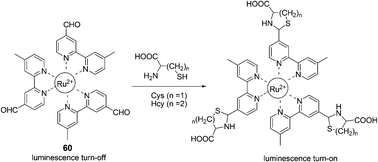 | ||
| Fig. 26 The mechanism for the reaction for 60 and Hcy/Cys. | ||
61, with efficient intramolecular charge transfer, was reported by Peng and Fan et al. as a fluorescent chemodosimeter for Cys and Hcy (Fig. 27).39 Upon addition of Cys/Hcy, 61 exhibited greatly enhanced fluorescence intensity in acetonitrile–HEPES buffer (20 mM, pH = 7.4) solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v). The fluorescence intensities of 61 at 560 nm showed a good linear relationship with the concentration of Cys, using physiological levels ranging from 0 to 500 mM. Its detection limit for Cys is 6.8 × 10 −7 M in acetonitrile–HEPES. A large absorption peak shift of 70 nm (from 430 nm to 500 nm) appeared with a distinct isosbestic point at 452 nm, corresponding to an apparent color change from orange to yellow.
7, v/v). The fluorescence intensities of 61 at 560 nm showed a good linear relationship with the concentration of Cys, using physiological levels ranging from 0 to 500 mM. Its detection limit for Cys is 6.8 × 10 −7 M in acetonitrile–HEPES. A large absorption peak shift of 70 nm (from 430 nm to 500 nm) appeared with a distinct isosbestic point at 452 nm, corresponding to an apparent color change from orange to yellow.
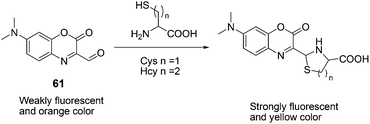 | ||
| Fig. 27 Mechanism for the reaction of 61. | ||
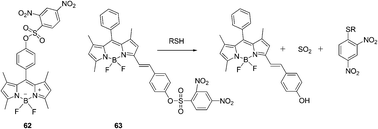 | ||
| Fig. 28 Structures of 62, 63 and the mechanism for the reaction of 63. | ||
A styryl boron-dipyrromethene (BODIPY)/2,4-dinitrobenzenesulfonyl (DNBS) dyad based red-emitting molecular probe for specific detection of cysteine among the biological thiols was reported by Zhao's group (Fig. 28).41 This probe showed intensive absorption at 556 nm and the probe was non-fluorescent. The DNBS moiety can be cleaved off by thiols, the red emission of the BODIPY fluorophore at 590 nm was switched on, with an emission enhancement of 46-fold in a mixed solvent of MeOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The probe showed good specificity toward cysteine over other biological molecules, and the detection limit of probe 63 for cysteine was determined to be 7.2 × 10−6 M. The emission of the probe is pH-independent in the physiological pH range. The probe was used for fluorescent imaging of cellular thiols in SGC-H446 cells.
1, v/v). The probe showed good specificity toward cysteine over other biological molecules, and the detection limit of probe 63 for cysteine was determined to be 7.2 × 10−6 M. The emission of the probe is pH-independent in the physiological pH range. The probe was used for fluorescent imaging of cellular thiols in SGC-H446 cells.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) solution containing phosphate buffered saline (PBS) (20 mM, pH 7.4), when GSH was added to the solution of 64, the maximum absorption peak showed an 85 nm red shift and the colour of the solution turned from colourless to jade-green. Thus it can serve as a “naked-eye” probe for thiols. The maximum emission peak undergoes a red-shift of 48 nm, and the ratio of fluorescence intensities (F533/F485) (λex = 400 nm) changes from 0.5 to 5.7 (R = 11.4-fold). A good linearity between the fluorescence intensity ratio, R (F533/F485) and the concentrations of GSH in the range of 0.5 to 10 mM with a detection limit of 28 μM was observed. The living cell image experiments further demonstrate the potential utility of 64 for the determination of thiols in living systems.
9, v/v) solution containing phosphate buffered saline (PBS) (20 mM, pH 7.4), when GSH was added to the solution of 64, the maximum absorption peak showed an 85 nm red shift and the colour of the solution turned from colourless to jade-green. Thus it can serve as a “naked-eye” probe for thiols. The maximum emission peak undergoes a red-shift of 48 nm, and the ratio of fluorescence intensities (F533/F485) (λex = 400 nm) changes from 0.5 to 5.7 (R = 11.4-fold). A good linearity between the fluorescence intensity ratio, R (F533/F485) and the concentrations of GSH in the range of 0.5 to 10 mM with a detection limit of 28 μM was observed. The living cell image experiments further demonstrate the potential utility of 64 for the determination of thiols in living systems.
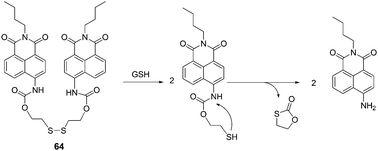 | ||
| Fig. 29 Reaction mechanism of 64 and GSH. | ||
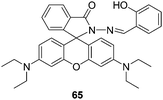 | ||
| Fig. 30 Structure of compound 65. | ||
The combination of a new squaraine 66 and varied concentrations of Hg2+ was utilized to probe thiol-containing amino acids in an absorption or fluorescence turn-on manner (Fig. 31).44 The formed complex between 66 and Hg2+ was consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode, and the association constant was calculated to be 5.7 × 104 M−1. Cysteine, homocysteine, glutathione, and histidine can completely restore the visible absorption and emission of 66 during the titration of acetonitrile/water (2
1 binding mode, and the association constant was calculated to be 5.7 × 104 M−1. Cysteine, homocysteine, glutathione, and histidine can completely restore the visible absorption and emission of 66 during the titration of acetonitrile/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions of 66 (1 mM) and Hg2+ (20 mM). The absorption change from colorless to green was easily discernible by the naked eye.
1) solutions of 66 (1 mM) and Hg2+ (20 mM). The absorption change from colorless to green was easily discernible by the naked eye.
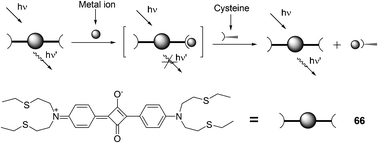 | ||
| Fig. 31 Chemical structure of 66 and pictorial demonstration of its sensing of thiol-containing amino acids. | ||
Jiang and co-workers found that Hg2+ highly selectively bound to 67 that bears an imide group similar to that of thymine and specifically induced aggregation of 67 that led to a dramatic quenching of 67 fluorescence at 532 nm, thereby allowing for a sensitive and selective Hg2+ sensing and affording a non-fluorescent chemosensing ensemble “67–Hg” for thiol-containing species (Fig. 32).45 Addition of thiol-containing amino acids to the Hg2+–67 ensemble solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF–H2O (v/v)) induced dissociation of the aggregates and recovery of the fluorescence. For Cys, a detection limit of 9.6 nM was obtained with a linear response over [Cys] of 0.05–0.3 μM.
1 DMF–H2O (v/v)) induced dissociation of the aggregates and recovery of the fluorescence. For Cys, a detection limit of 9.6 nM was obtained with a linear response over [Cys] of 0.05–0.3 μM.
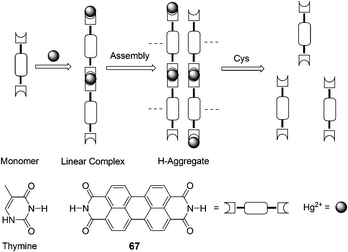 | ||
| Fig. 32 Scheme of Hg2+ induced aggregation of 67 and aggregate dissociation in the presence of cysteine. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The corresponding binding constant in H2O (MeOH 1%) is Ka = 6.65 × 103 M−1 and the detection of Cys is possible at the 100 nM level. The colorless to red color change (log
1. The corresponding binding constant in H2O (MeOH 1%) is Ka = 6.65 × 103 M−1 and the detection of Cys is possible at the 100 nM level. The colorless to red color change (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε530 nm = 4.512 M−1 cm−1) associated with the binding of 68–Au+ with Cys was detected visually. Other amino acids exhibited no color changes with 68–Au+ under the same conditions.
ε530 nm = 4.512 M−1 cm−1) associated with the binding of 68–Au+ with Cys was detected visually. Other amino acids exhibited no color changes with 68–Au+ under the same conditions.
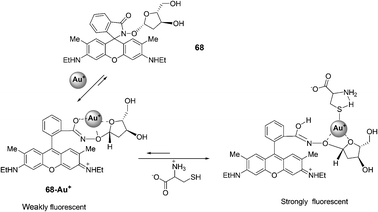 | ||
| Fig. 33 Reaction of compound 68 with Cys. | ||
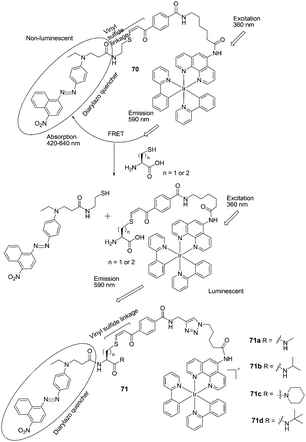 | ||
| Fig. 35 Structures of 70, 71a–71d and the mechanism for the reaction for 70 and Hcy/Cys. | ||
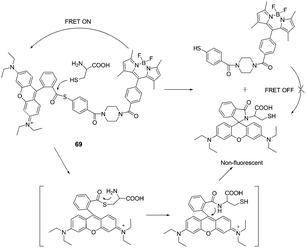
Lin et al. designed a native chemical ligation (NCL)-based ratiometric fluorescent thiol probe (69) for ratiometric imaging of thiols in living cells (Fig. 34).47 The intramolecular energy transfer efficiency from the Bodipy donor to the rhodamine acceptor in 69 was calculated to be 98.4%. When 69 was titrated with increasing concentrations of Cys in potassium phosphate buffer (pH 7.4, containing 45% CH3CN as a co-solvent), the intensity at 590 nm gradually decreased with the concomitant growth of a new emission peak at 510 nm, indicating that the FRET is off. The fluorescent intensity ratios at 510 and 590 nm (I510/I590) exhibited a drastic change from 0.09 to 24.82 with a 275-fold enhancement in the emission ratios. The detection limit of the probe was determined to be 8.2 × 10−8 M. 69 was then applied for thiol detection in real biological samples of serum, and for ratiometric imaging in living HeLa cells.
Che and Wong et al. developed FRET-based iridium(III) complexes for the detection of Hcy and Cys on the basis of conjugate addition of thiols to the vinyl sulfide linkage followed by elimination (Fig. 35).48 In pH = 8.1 PBS buffer/CH3CN (1/3), the probes feature high selectivity for Hcy and Cys over other amino acids and GSH under physiological conditions. For 70, the ratio of emission intensity enhancements upon the addition of Hcy and Cys is 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The iridium(III) probe 71d is able to differentiate Hcy from Cys in a ratio of 5
1. The iridium(III) probe 71d is able to differentiate Hcy from Cys in a ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, 71d could be used for the detection of Hcy and Cys in human blood plasma.
1. In addition, 71d could be used for the detection of Hcy and Cys in human blood plasma.
2.3 Chemosensors utilizing hydrogen-bonds and π–π interaction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.01 M HEPES buffer, pH = 7.4) by Chan's group (Fig. 36).49 When treated with L-aspartate, glutamate, N-acetyl-L-aspartate and N-acetyl-L-glutamate, the emission spectrum of 72 can be quenched by ca. 20%. On the basis of the change of fluorescent intensity, the complex association constants (Ka) were calculated using nonlinear least-squares curve fitting. It revealed that the preorganization of 72 permits three points binding with the guests leading to a more selective complexation with acidic amino acids than their corresponding dicarboxylates, and 72 strongly binds glutamate via multiple hydrogen bonds with a binding constant of (5.57 ± 0.88) × 106 M−1.
1, 0.01 M HEPES buffer, pH = 7.4) by Chan's group (Fig. 36).49 When treated with L-aspartate, glutamate, N-acetyl-L-aspartate and N-acetyl-L-glutamate, the emission spectrum of 72 can be quenched by ca. 20%. On the basis of the change of fluorescent intensity, the complex association constants (Ka) were calculated using nonlinear least-squares curve fitting. It revealed that the preorganization of 72 permits three points binding with the guests leading to a more selective complexation with acidic amino acids than their corresponding dicarboxylates, and 72 strongly binds glutamate via multiple hydrogen bonds with a binding constant of (5.57 ± 0.88) × 106 M−1.
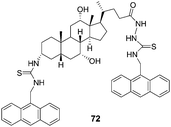 | ||
| Fig. 36 Structure of 72. | ||
Chan and co-workers further reported that the chiral fluorescent probes 73 and 74 demonstrated differential binding toward trifunctional amino moieties (Fig. 37).50 The emission spectrum of 73 was quenched by as much as 80% with the addition of 10 equivalents of D-serine, and the binding interaction of 73 to serine (D-serine Ka = (2.26 ± 0.17) × 105 M−1) is slightly stronger than that of 73 to threonine (D-threonine Ka = (1.82 ± 0.14) × 105 M−1), lysine (D-lysine Ka = (1.37 ± 0.11) × 105 M−1) and tyrosine (D-tyrosine Ka = (1.94 ± 0.09) × 105 M−1). Among the four pairs of enantiomeric amino acids, 73 showed a consistent bias for the D-amino acids with high KD/KL values from 6.7 to 8.9. In contrast to host 73, opposite enantioselectivity toward serine, threonine, lysine and tyrosine was displayed by host 74 with high KL/KD values from 6.2 to 8.1. This implies that the chiral discrimination power of hosts 73 and 74 may be due to the 1,2-diaminocyclohexane moiety.
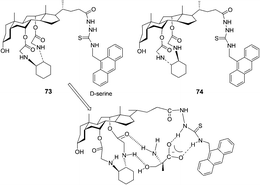 | ||
| Fig. 37 Structures of fluorescent sensors 73, 74 and the proposed binding mode of host 73 with D-serine. | ||
Two new anthracene thiourea derivatives, 75 and 76, were investigated as fluorescent chemosensors for the chiral recognition of amino acids (Fig. 38).51 The association constants of 75 with D- and L-t-Boc alanine were calculated as 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 and 2160 M−1, respectively, and KD/KL was found to be 5.5. The association constants of 76 with D and L-t-Boc alanine were calculated as 2300 and 23
800 and 2160 M−1, respectively, and KD/KL was found to be 5.5. The association constants of 76 with D and L-t-Boc alanine were calculated as 2300 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1, respectively, and KL/KD was found to be 10.4. These intriguing opposite D/L binding affinities for 75 and 76 were obtained without (or with) H–π interaction between the anthracene moiety and the methyl groups, which was explained by extensive high-level theoretical investigations taking into account the dispersion energy.
900 M−1, respectively, and KL/KD was found to be 10.4. These intriguing opposite D/L binding affinities for 75 and 76 were obtained without (or with) H–π interaction between the anthracene moiety and the methyl groups, which was explained by extensive high-level theoretical investigations taking into account the dispersion energy.
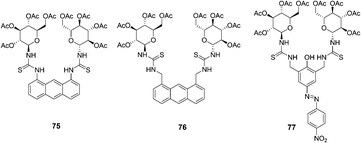 | ||
| Fig. 38 Structures of 75, 76 and 77. | ||
A new colorimetric anion sensor 77 was reported for chiral recognition (Fig. 38).52 According to the linear Benesi–Hilderand expression, the measured absorption [77/(A − A0)] at 523 nm varied as a function of amino acids in linear relationship (R ≈ 0.9995), indicating the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between amino acids and hosts. The association constants of 77 with L- and D-t-Boc threonine were calculated as 68
1 stoichiometry between amino acids and hosts. The association constants of 77 with L- and D-t-Boc threonine were calculated as 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 22
900 and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, respectively, and KL/KD was found to be 3.13. The D/L selectivity for alanine of host 77 was observed as high as 3.6, which was attributed to the glucopyranosyl unit (chiral barrier) of the host.
000 M−1, respectively, and KL/KD was found to be 3.13. The D/L selectivity for alanine of host 77 was observed as high as 3.6, which was attributed to the glucopyranosyl unit (chiral barrier) of the host.
The anthracene based chiral fluorescent receptors 78 and 79 containing thiourea and amide groups were synthesized by He and co-workers (Fig. 39).53 When the concentration of anions was gradually increased in DMSO, the fluorescence emission intensities of 78 at 429 nm decreased, while a new peak gradually enhanced at 538 nm. The solution color of 78 changed from light yellow to orange-red, which could be easily observed by the naked eye. Receptors 78 and 79 exhibited good enantioselective recognition towards the L-enantiomer of the chiral guests (L/D-malate, L/D-aspartate, and L/D-glutamate) with the Kass(L)/Kass(D) values from 2.18 to 9.65.
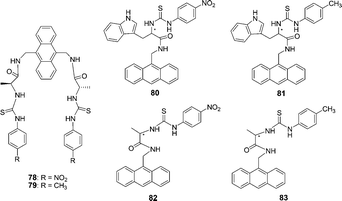 | ||
| Fig. 39 Structures of 78–83. | ||
The charge neutral chiral optical sensors 80–83 containing thiourea and amide groups were synthesized by He and co-workers (Fig. 39).54 The different increasing efficiencies (ΔIL/ΔID = 1.8) indicated that receptor 80 has a good enantioselective recognition ability between L- and D-α-Phe. In addition, the association constant (Kass) of 80 with L-Phe is (2.96 ± 0.16) × 104 M−1, while that of 80 with D-Phe is (5.26 ± 0.25) × 103 M−1, and the enantioselectivity (Kass(L)/Kass(D)) is 5.63. On gradual increase of the concentration of L- and D-Phe, the intensity of the absorption band at 370 nm decreased, and a new absorption band appeared with a maximum absorption at 475 nm.
In 2011, Chan and co-workers continue their interest in this research direction and report two new sensing probes 84 and 85 (Fig. 40).55 Operating on the PET mechanism, the fluorescent emission band of the host at 413 nm was quenched gradually upon the addition of the guest. As revealed by the respective association constants, sensor 84 showed the highest affinity to isophthalate (Ka = (6.25 ± 0.61) × 104 M−1) in acetonitrile. The preorganization of sensor 85 permits three sites binding with the guests leading to enhanced complexation with aspartate and glutamate. The chiral recognition ability of the sensors is however, only moderate.
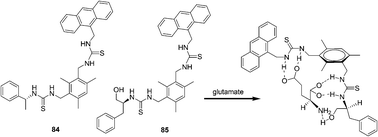 | ||
| Fig. 40 Structures of fluorescent sensors 84, 85 and the proposed binding mode of host 85 with glutamate. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex for Leu, His, and Pro and a 2
1 complex for Leu, His, and Pro and a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex for Glu. The titration of 86 with Ala, Cys, or Lys resulted in quenching of the fluorescence intensity. Both the changes of 86 in the fluorescence and absorbance followed an order: Lys (Ka = 3162 ± 300) > Cys (Ka = 1778 ± 250) > Ala.
1 complex for Glu. The titration of 86 with Ala, Cys, or Lys resulted in quenching of the fluorescence intensity. Both the changes of 86 in the fluorescence and absorbance followed an order: Lys (Ka = 3162 ± 300) > Cys (Ka = 1778 ± 250) > Ala.
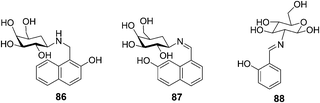 | ||
| Fig. 41 Structures of 86, 87 and 88. | ||
A glucose based C2-glyco-conjugate (88) has been reported by Rao's group (Fig. 41).57 Titration of 88 with all the 20 naturally occurring amino acids resulted in a large fluorescence intensity enhancement only in the case of aromatic amino acids, that is, Phe > Trp > His > Tyr. The association constants (Ka) were found to be 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 600 for the aromatic amino acids. Two other non-aromatic amino acids, specifically alanine and arginine, exhibited Ka values of 3360 ± 225 and 1930 ± 150, respectively. Thus the aromatic amino acids combine with 88 by a factor of 5–10 times higher in affinity as compared to the non-aromatic ones. Therefore, 88 could recognize aromatic amino acids down to 1.5–3 ppm through switch-on fluorescence behavior.
400 ± 600 for the aromatic amino acids. Two other non-aromatic amino acids, specifically alanine and arginine, exhibited Ka values of 3360 ± 225 and 1930 ± 150, respectively. Thus the aromatic amino acids combine with 88 by a factor of 5–10 times higher in affinity as compared to the non-aromatic ones. Therefore, 88 could recognize aromatic amino acids down to 1.5–3 ppm through switch-on fluorescence behavior.
N′-Substituted guanidiniocarbonyl pyrroles 89–94 were synthesized by Schmuck and Bickert as the efficient receptors for the complexation of amino acid even in water (bis-tris-buffer, 3 mM at pH = 6.1) (Fig. 42).58 The addition of amino acid to a solution of 94 was tested using UV-titration studies. The absorbance of the pyrrole moiety at 290 nm decreased upon 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association model complex formation. 94 strongly bound amino acid carboxylates in aqueous buffer solution with association constants of Kass ≥ 103 M−1. Furthermore, the complex stability seems to depend on the nature of the amino acid side chain. Valine is bound nearly two times better than alanine (Kass = 1750 and 1000 M−1, respectively).
1 association model complex formation. 94 strongly bound amino acid carboxylates in aqueous buffer solution with association constants of Kass ≥ 103 M−1. Furthermore, the complex stability seems to depend on the nature of the amino acid side chain. Valine is bound nearly two times better than alanine (Kass = 1750 and 1000 M−1, respectively).
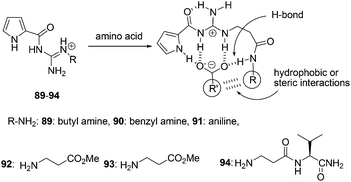 | ||
| Fig. 42 Structures of sensors 89–94, and the hydrophobic or steric interactions in their binding modes. | ||
Suzuki and co-workers reported receptors based on triaza-18-crown-6 ether combined with two guanidinium groups, which could bind amino acids in aqueous methanol solution (pH = 9.5) and showed fluorescence enhancement response by a PET (photoinduced electron transfer) mechanism (Fig. 43).59 The absorption spectra of receptors 95–97 showed no changes upon the addition of guests or upon pH changes. Fluorescence spectra of receptors 95 and 96 showed little or no response towards amino acids except for lysine, which has a higher pKa value (10.53) due to its alkyl ammonium terminal. The order of fluorescence enhancement factor (FE) values of 95 and 96 is n-BuNH3+ > lysine > GABA. In contrast, the order of FE values of receptor 97 is GABA > lysine > n-BuNH3+ > glycine.
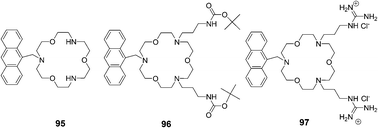 | ||
| Fig. 43 Structures of sensors 95–97. | ||
The interaction and colorimetric sensing properties of the calix[4]pyrrole–TCBQ charge-transfer complex with amino acids and amines in CHCl3/EtOH/H2O were investigated by Shao et al. (Fig. 44).60 Upon the addition of lysine and arginine, the blue complex solution instantly turned orange-yellow. The CT absorption band at 612 nm nearly vanished, while a new absorption band appeared in the region of 400–500 nm. A similar, but less dramatic color change from blue to yellow-green was observed for histidine. The addition of aromatic amine and pyridine caused less significant changes in color and the CT absorption band at 612 nm, with the trend of aliphatic amines > pyridine > aromatic amine. Changes in color and absorption spectra of complex 98·99 in the presence of basic amino acids and aliphatic amines resulted from the disaggregation of the calix[4]pyrrole–TCBQ complex and the formation of a tetrachlorosemiquinone anion radical (TCBQ˙−).
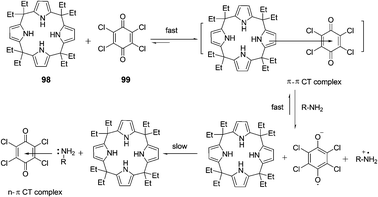 | ||
| Fig. 44 The interaction mode of 98–99. | ||
2.4 Chemosensors based on polymers
Compared to small organic compounds, polymer based optical sensors display several important advantages. Conjugated polymer chains with multiple recognition elements can increase both the binding efficiency and recognition selectivity for specific analytes. In addition, incorporating several different recognition units into a functional polymer can produce a valuable conclusion based on the combination of different outputs.61In 2009, Li's group reported a disubstituted polyacetylene (100) which was utilized to sense α-amino acids over β- and γ-amino acids based on a ‘turn-on’ model (Fig. 45).62 The strong blue fluorescence of 100 was quenched by trace copper ions efficiently, then the addition of α-amino acids to the 100/Cu2+ complex in ethanol could recover the strong fluorescence of 100 to report the presence of α-amino acids. Among α-amino acids, the detection of His demonstrated even higher sensitivity with the detection limit lower than 1.3 × 10−5 M (2.1 ppm), and the fluorescent intensity of 100 was able to recover to about 88% at the concentration as low as 1.1 × 10−4 M. With the aid of a normal UV lamp, histidine could be visually differentiated from other α-amino acids by the observation of its strong blue fluorescence, at concentrations as low as 4.0 × 10−5 M.
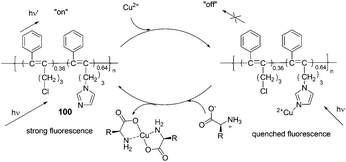 | ||
| Fig. 45 The speculated conversion cycle of 100 in the presence of Cu2+ and α-amino acids. | ||
A conjugated fluorescent polymer was utilized to probe α-amino acids as shown by Li et al. (Fig. 46).63 The strong fluorescence of the prepared polyfluorene (101) was quenched by trace copper ions. As Gly was added to the complex of 101 and copper ions, the quenched fluorescence of 101 turned on even at a concentration of Gly as low as 0.33 × 10−5 M (0.24 ppm). All of the α-amino acids could be detected sensitively, among them the detection of histidine (His) demonstrated highest sensitivity. The fluorescent intensity of 101 recovered to about 96% at a His concentration as low as 3.0 × 10−5 M. The reason that the detection sensitivity of His was higher than those of other α-amino acids was because the imidazole moieties in it may help His take the copper ions from the imidazole moieties in 101.
 | ||
| Fig. 46 The structure of 101. | ||
Jiang's group developed a spectral signaling ensemble that is highly selective for and sensitive to Cys, which operates in a “mix-and-measure” mode. The formation of the coordination polymers is shown to be facilitated by both the Ag+–Ag+ interaction and the interaction between the side chains in the polymeric backbone. It shows that the electrostatic interaction among the Cys side chains plays an important role in maintaining the Ag+–SR polymeric structure, and extended sensing systems can be constructed by manipulating the interactions among the –SR chains (Fig. 47).64 With preliminary data on the corresponding Cu+–Cys and Au+–Cys systems that exhibited similar spectral signals, the M+–SR coordination polymers (M = Au, Ag, or Cu) are assumed to be able to serve as spectral sensing platforms.
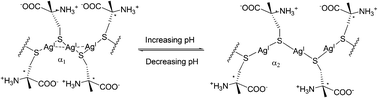 | ||
| Fig. 47 Switching argentophilic attraction by the pH-modulated electrostatic interaction between adjacent ligands along the Ag(I)-Cys polymeric backbone. α1 and α2 are the Ag–S–Ag angles at low and high pH, respectively, α1 < α2. | ||
2.5 Chemosensors based on gold/silver nanoparticles
Patel and Menon reported p-sulfonatocalix[4]arene thiol modified gold nanoparticles as a colorimetric sensor to probe lysine, arginine and histidine in water (Fig. 48).65 When 11 different aqueous solutions of amino acids in PBS buffer were added to calyx-capped gold nanoparticles, after half an hour, the solutions containing Lys, Arg or His had changed the color from red to purple with a red shift (524 nm to 550 nm region) and a broadening of the surface plasmon band which was accompanied by aggregation of the nanoparticles. However after 24 h, the color disappeared very slowly due to sedimentation. A linear correlation existed between Aλmax and the logarithm of the Lys, Arg and His concentration C over the range of 10−6–10−4 M, 4 × 10−6–10−4 M and 2 × 10−6–10−4 M with the linear detection limits of 1 μM, 4 μM and 2 μM, respectively.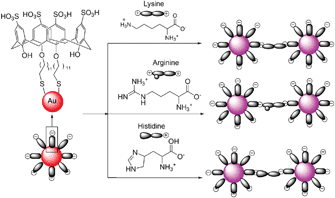 | ||
| Fig. 48 A schematic representation of the amino acid induced aggregation of calix-capped gold nanoparticles. | ||
Joo et al. reported that hydrogen bonding-induced redispersion of the aggregated Au nanoparticles upon N-hydroxysuccinimide ester bioconjugations could work as an optical sensor to detect a trace amount of amino acids as low as 10−6 M in an aqueous solution (Fig. 49).66 The color of initially red pristine AuNPs became blue after modification with the DPAN group. The color returned to red upon conjugation with tyrosine in the concentration range of 10−6–10−2 M within 2 h. Glycine, histidine, or tyrosine exhibited improved recovery behaviours than asparagines, glutamine, leucine, lysine, serine, and tryptophan under the present experimental conditions.
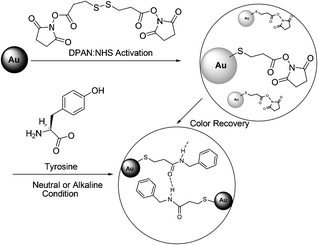 | ||
| Fig. 49 Experimental scheme of NHS coupling of tyrosine and 3,3′-dithiopropionic acid di(NHS ester) (DPAN) adsorbed on Au nanoparticles. | ||
Yan et al. reported a resonance light scattering (RLS) bioassay for detecting Hcy in biological fluids based on Hcy-involved assembly of polyethyleneimine (PEI)-capped Ag-nanoclusters (Fig. 50).67 It revealed that the increased RLS signal did not originate from the aggregation of the Ag-nanoclusters, rather from Hcy-involved assembling of PEI-capped Ag-nanoclusters. ΔRLS linearly increased with CHcy from 0.2 to 3.5 μM with a calibration function of ΔRLS = 2346CHcy − 66 (CHcy in μM, R2 = 0.9982). The detection limit (S/N = 3) is 42 nM, and the precision for eleven replicate detections of Hcy at 1 μM is 0.6%, showing excellent reproducibility of the present bioassay. The bioassay allows discrimination of Hcy from Cys, GSH and other amino acids, and permits detecting trace Hcy in biological fluids (human serum samples).
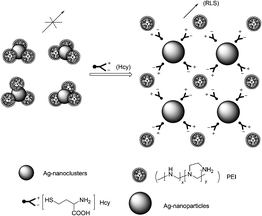 | ||
| Fig. 50 Schematic illustration of Hcy-involved assembling of PEI-capped Ag-nanoclusters. | ||
2.6 Chemosensor arrays based on cucurbiturils supramolecular assembly
With the fluorescent dye Dapoxyl and the macrocyclic host cucurbit[7]uril (CB7, composed of 7 glycoluril units), a multiparameter sensor array has been designed by Nau et al. (Fig. 51).68 It showed the highly selective sensing of biomolecular analytes, either an amino acid (histidine, arginine, lysine, and tyrosine) or its corresponding decarboxylase. The detection can be transferred to a microtiter plate format with a limiting sensitivity of 2.4 nM per well. The extension of the high selectivity and μM sensitivity of the tandem assay principle has also allowed for the accurate measurement of D-lysine enantiomeric excesses of up to 99.98%.![Structures of cucurbi[7]uril and dapoxyl.](/image/article/2012/CS/c1cs15159b/c1cs15159b-f51.gif) | ||
| Fig. 51 Structures of cucurbi[7]uril and dapoxyl. | ||
A sensor array made by combining four fluorescent tricyclic basic dyes with cucurbiturils was reported by Baumes and Garcia et al. (Fig. 52).69 This array was able to identify and discriminate 18 α-amino acids up to 10−4 M without the need of enzyme activation. The chemosensor array achieved a perfect cross-selectivity for AA, with a discrimination event (color change in fluorescence images), which was easily detected by the naked eye. It appeared that the discrimination between AA and the corresponding carboxylic acid is easier for PF and AO, while discrimination between a given AA and its corresponding amine is clearer for the OX dye. Pyronine (PYY) discriminates all three (AA, amine, and carboxylic acid derivative) in one shot.
2.7 Chemosensors utilizing DNA
Ren's group reported a selective fluorescence turn-on assay for detection of cysteine and histidine using a DNA/ligand/ion ensemble (Fig. 53).70 The high sensitivity and selectivity for cysteine and histidine were achieved by changing the metal ions (Hg+ and Cu2+). Upon the addition of cysteine to the solution of the TO/DNA/Hg2+ ensemble, the quenched fluorescence of TO/DNA turned on immediately. The enhanced fluorescence intensity is proportional to the cysteine concentration in the range from 2.5 × 10−9 to 1.1 × 10−7 M, and the detection limit of cysteine is 5.1 × 10−9 M. In the TO/DNA/Cu2+ system, both histidine and cysteine dislodged Cu2+ and restored the fluorescence of TO/DNA. The detection of histidine demonstrated even greater sensitivity than cysteine in this system. The detection limit was 1.0 × 10−8 M for histidine, and the linear range was from 0 to 4.4 × 10−6 M. Additionally, the detection and discrimination process can be detected with the naked eye under a hand-held UV lamp and may be easily adapted to automated high-throughput screening.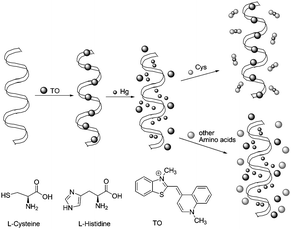 | ||
| Fig. 53 Illustration of the TO/DNA/Metal ion-based amino acid sensor. | ||
3. Concluding remarks
In this tutorial review we focused on the recent noteworthy reports regarding amino acid sensing since 2000. Depending on the significant characteristic properties of various amino acids thus far, the optical probes utilizing indicator-displacement assays,11–18,22,29 metal complexes coordination,19–21,23–29,48 specific reactions between probes and amino acids30–50 and other ways have been widely studied. For the reaction based probes, we further classified them into imine formation, Michael addition, thiazinane or thiazolidine formation, cleavage of a sulfonate ester, cleavage of disulfide, metal complexes-displace coordination and others. Due to the similarity in structure and reactivity of some amino acids, it is still challenging to develop sensors that can selectively and sensitively discriminate amino acids from one another, such as the three biological thiols Cys, Hcy and GSH.35–39,46–48 This is not an easy task but the rapid progress documented so far has been encouraging and indicates that more successful probes differentiating various amino acids can be developed with fine tuning and careful design, especially intelligent combinations of basic organic reactions and communicating methods. Furthermore, sensing of various amino acids can be even possible by using receptor arrays composed of different chemosensors.71 Finally, it is our hope that this tutorial review will inspire more chemists and biologists to explore novel and unconventional chemical scaffolds for the challenge of amino acid recognition and their applications in complex biological systems.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (2010-0018895) and by the Converging Research Center Program through the Ministry of Education, Science and Technology (2010K001202). The paper (YZ) was also supported by RP-grant 2010 of Ewha Womans University. YZ also acknowledges the Natural Science Foundation for the Youth (of China).Notes and references
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS.
- H. Yoshida, Y. Nakano, K. Koiso, H. Nohta, J. Ishida and M. Yamaguchi, Anal. Sci., 2001, 17, 107 CrossRef CAS.
- G. N. Chen, X. P. Wu, J. P. Duan and H. Q. Chen, Talanta, 1999, 49, 319 CrossRef.
- G. M. Mackay, C. M. Forrest, N. Stoy, J. Christofides, M. Egerton, T. W. Stone and L. G. Darlington, Eur. J. Neurol., 2006, 13, 30 CrossRef CAS.
- H. Refsum, P. M. Ueland, O. Nygård and S. E. Vollset, Annu. Rev. Med., 1989, 49, 31 CrossRef.
- C. S. Lee, P. F. Teng, W. L. Wong, H. L. Kwong and A. S. C. Chan, Tetrahedron, 2005, 61, 7924 CrossRef CAS.
- J. Wang, M. Chtrathi and B. Tian, Anal. Chem., 2000, 72, 5774 CrossRef CAS.
- D. Vardanega and C. Giradet, Chem. Phys. Lett., 2009, 469, 172 CrossRef CAS.
- Y. Zhou, Z. Xu and J. Yoon, Chem. Soc. Rev., 2011, 40, 2222 RSC.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC.
- G. Klein and J.-L. Reymond, Angew. Chem., 2001, 113, 1821 ( Angew. Chem., Int. Ed. , 2001 , 40 , 1768 ) CrossRef.
- K. E. S. Dean, G. Klein, O. Renaudet and J.-L. Reymond, Bioorg. Med. Chem. Lett., 2003, 13, 1653 CrossRef CAS.
- M. A. Hortalá, L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, J. Am. Chem. Soc., 2003, 125, 20 CrossRef.
- M. Bonizzoni, L. Fabbrizzi, G. Piovani and A. Taglietti, Tetrahedron, 2004, 60, 11159 CrossRef CAS.
- J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, Chem.–Eur. J., 2005, 11, 5319 CrossRef CAS.
- J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2005, 127, 7986 CrossRef CAS.
- J. F. Folmer-Andersen, M. Kitamura and E. V. Anslyn, J. Am. Chem. Soc., 2006, 128, 5652 CrossRef CAS.
- D. Leung, J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2008, 130, 12318 CrossRef CAS.
- S. Pagliari, R. Corradini, G. Galaverna, S. Sforza, A. Dossena and R. Marchelli, Tetrahedron Lett., 2000, 41, 3691 CrossRef CAS.
- R. Corradini, C. Paganuzzi, R. Marchelli, S. Pagliari, S. Sforza, A. Dossena, G. Galaverna and A. Duchateau, Chirality, 2003, 15, 30 CrossRef.
- S. Pagliari, R. Corradini, G. Galaverna, S. Sforza, A. Dossena, M. Montalti, L. Prodi, N. Zaccheroni and R. Marchelli, Chem.–Eur. J., 2004, 10, 2749 CrossRef CAS.
- H. Aït-Haddou, S. L. Wiskur, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2001, 123, 11296 CrossRef.
- H.-L. Kwong, W.-L. Wong, C.-S. Lee, C.-T. Yeung and P.-F. Teng, Inorg. Chem. Commun., 2009, 12, 815 CrossRef CAS.
- L. Fabbrizzi, M. Licchelli, A. Perotti, A. Poggi, G. Rabaioli, D. Sacchi and A. Taglietti, J. Chem. Soc., Perkin Trans. 2, 2001, 2108 RSC.
- P. C. Jugun, A. Acharya, A. Kumar and C. P. Rao, J. Phys. Chem. B, 2009, 113, 12075 CrossRef.
- R. Joseph, J. P. Chinta and C. P. Rao, J. Org. Chem., 2010, 75, 3387 CrossRef CAS.
- R. Yang, K. Wang, L. Long, D. Xiao, X. Yang and W. Tan, Anal. Chem., 2002, 74, 1088 CrossRef CAS.
- S.-H. Li, C.-W. Yu and J.-G. Xu, Chem. Commun., 2005, 450 RSC.
- A. Buryak and K. Severin, J. Am. Chem. Soc., 2005, 127, 3700 CrossRef CAS.
- E. K. Feuster and T. E. Glass, J. Am. Chem. Soc., 2003, 125, 16174 CrossRef CAS.
- K. Secor, J. Plante, C. Avetta and T. Glass, J. Mater. Chem., 2005, 15, 4073 RSC.
- Y. Zhou, J. Won, J. Y. Lee and J. Yoon, Chem. Commun., 2011, 47, 1997 RSC.
- F.-J. Huo, Y.-Q. Sun, J. Su, Y.-T. Yang, C.-X. Yin and J.-B. Chao, Org. Lett., 2010, 12, 4756 CrossRef CAS.
- H. Kwon, K. Lee and H.-J. Kim, Chem. Commun., 2011, 47, 1773 RSC.
- X. Chen, S.-K. Ko, M. J. Kim, I. Shin and J. Yoon, Chem. Commun., 2010, 46, 2751 RSC.
- H. S. Jung, K. C. Ko, G.-H. Kim, A.-R. Lee, Y.-C. Na, C. Kang, J. Y. Lee and J. S. Kim, Org. Lett., 2011, 13, 1498 CrossRef CAS.
- Y. Yue, Y. Guo, J. Xu and S. Shao, New J. Chem., 2011, 35, 61 RSC.
- R. Zhang, X. Yu, Z. Ye, G. Wang, W. Zhang and J. Yuan, Inorg. Chem., 2010, 49, 7898 CrossRef CAS.
- M. Hu, J. Fan, H. Li, K. Song, S. Wang, G. Cheng and X. Peng, Org. Biomol. Chem., 2011, 9, 980 CAS.
- J. Lu, C. Sun, W. Chen, H. Ma, W. Shi and X. Li, Talanta, 2011, 83, 1050 CrossRef CAS.
- J. Shao, H. Guo, S. Ji and J. Zhao, Biosens. Bioelectron., 2011, 26, 3012 CrossRef CAS.
- B. Zhu, X. Zhang, Y. Li, P. Wang, H. Zhang and X. Zhuang, Chem. Commun., 2010, 46, 5710 RSC.
- X. Lou, L. Zhang, J. Qin and Z. Li, Langmuir, 2010, 26, 1566 CrossRef CAS.
- C. Luo, Q. Zhou, B. Zhang and X. Wang, New J. Chem., 2011, 35, 45 RSC.
- Y.-B. Ruan, A.-F. Li, J.-S. Zhao, J.-S. Shen and Y.-B. Jiang, Chem. Commun., 2010, 46, 4938 RSC.
- Y.-K. Yang, S. Shim and J. Tae, Chem. Commun., 2010, 46, 7766 RSC.
- L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893 RSC.
- H.-Y. Shiu, M.-K. Wong and C.-M. Che, Chem. Commun., 2011, 47, 4367 RSC.
- S.-Y. Liu, L. Fang, Y.-B. He, W.-H. Chan, K.-T. Yeung, Y.-K. Cheng and R.-H. Yang, Org. Lett., 2005, 7, 5825 CrossRef CAS.
- H. Wang, W.-H. Chan and A. W. M. Lee, Org. Biomol. Chem., 2008, 6, 929 CAS.
- Y. K. Kim, H. N. Lee, N. J. Singh, H. J. Choi, J. Y. Xue, K. S. Kim, J. Yoon and M. H. Hyun, J. Org. Chem., 2008, 73, 301 CrossRef CAS.
- M. K. Choi, H. N. Kim, H. J. Choi, J. Yoon and M. H. Hyun, Tetrahedron Lett., 2008, 49, 4522 CrossRef CAS.
- X.-H. Huang, Y.-B. He, Z.-H. Chen, C.-G. Hu and G.-Y. Qing, Can. J. Chem., 2008, 86, 170 CrossRef CAS.
- X.-H. Huang, Y.-B. He, C.-G. Hu and Z.-H. Chen, J. Fluoresc., 2009, 19, 97 CrossRef CAS.
- X. Zhou, Y. Yip, W. Chan and A. W. M. Lee, Beilstein J. Org. Chem., 2011, 7, 75 CrossRef CAS.
- R. Ahuja, N. K. Singhal, B. Ramanujam, M. Ravikumar and C. P. Rao, J. Org. Chem., 2007, 72, 3430 CrossRef CAS.
- A. Mitra, J. P. Chinta and C. P. Rao, Tetrahedron Lett., 2010, 51, 139 CrossRef CAS.
- C. Schmuck and V. Bickert, Org. Lett., 2003, 5, 4579 CrossRef CAS.
- S. Sasaki, A. Hashizume, D. Citterio, E. Fujii and K. Suzuki, Tetrahedron Lett., 2002, 43, 7243 CrossRef CAS.
- K. Liu, L. He, X. He, Y. Guo, S. Shao and S. Jiang, Tetrahedron Lett., 2007, 48, 4275 CrossRef CAS.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79 RSC.
- Q. Zeng, L. Zhang, Z. Li, J. Qin and B. Z. Tang, Polymer, 2009, 50, 434 CrossRef CAS.
- Z. Li, X. Lou, Z. Li and J. Qin, ACS Appl. Mater. Interfaces, 2009, 1, 232 CAS.
- J. Shen, D. Li, M. Zhang, J. Zhou, H. Zhang and Y. Jiang, Langmuir, 2011, 27, 481 CrossRef CAS.
- G. Patel and S. Menon, Chem. Commun., 2009, 3563 RSC.
- J.-H. Park, E. O. Ganbold, D. Uuriintuya, K. Lee and S.-W. Joo, Chem. Commun., 2009, 7354 RSC.
- S.-K. Sun, H.-F. Wang and X.-P. Yan, Chem. Commun., 2011, 47, 3817 RSC.
- D. M. Bailey, A. Hennig, V. D. Uzunova and W. M. Nau, Chem.–Eur. J., 2008, 14, 6069 CrossRef CAS.
- L. A. Baumes, M. Buaki, J. Jolly, A. Corma and H. Garcia, Tetrahedron Lett., 2011, 52, 1418 CrossRef CAS.
- F. Pu, Z. Huang, J. Ren and X. Qu, Anal. Chem., 2010, 82, 8211 CrossRef CAS.
- A. T. Wright and E. V. Anslyn, Chem. Soc. Rev., 2006, 35, 14 RSC.
| This journal is © The Royal Society of Chemistry 2012 |

![Structures of cucurbi[7]uril, oxonine, pyronine, acridine orange and proflavine.](/image/article/2012/CS/c1cs15159b/c1cs15159b-f52.gif)