The Baylis–Hillman reaction: a novel concept for creativity in chemistry
Deevi
Basavaiah
* and
Gorre
Veeraraghavaiah
School of Chemistry, University of Hyderabad, Hyderabad-500 046, India. E-mail: dbsc@uohyd.ernet.in; Fax: +91-40-23012460
First published on 6th September 2011
Abstract
This tutorial review highlights the way in which the Baylis–Hillman reaction has been increasingly attracting the attention of synthetic and medicinal chemists; it not only helps in originating new ideas to create novel methodologies and molecules but also offers intellectual challenges to understand and address the present day needs in the areas of organic and medicinal chemistry.
 Deevi Basavaiah | Deevi Basavaiah was born in Valiveru, a village near Tenali, India. He obtained PhD degree from the Banaras Hindu University, India, in 1979 under the supervision of Professor Gurbakhsh Singh. He then worked in the research group of Professor H. C. Brown, Purdue University, USA, as a post-doctoral fellow for three years. In 1984 he joined as a faculty member in the School of Chemistry, University of Hyderabad, India, where he is currently Professor. The main objective of his research is the development of the Baylis–Hillman reaction as a useful and powerful synthetic tool in organic synthesis and his research group has been working towards this direction for the last 27 years. Chiral catalysis is another important area of his research interest. |
 Gorre Veeraraghavaiah | Gorre Veeraraghavaiah was born in 1983 in Rajavolu, a village in Cherukupalle Mandal, Guntur (Dist.), Andhra Pradesh, India. After completion of graduation from the Acharya Nagarjuna University, Guntur, he joined as a post-graduate student in 2005 in School of Chemistry, University of Hyderabad, Hyderabad, and obtained his MSc (Chemistry) degree in 2007. Presently he is working towards PhD degree in the University of Hyderabad on the development of the Baylis–Hillman reaction and its synthetic applications under the supervision of Professor Basavaiah. |
1. Introduction
Creativity originates from thinking which, in fact, is the origin of every man-made event. Every idea (either good or bad) generates from the concept of our thinking. Perception of an object differs from mind to mind depending on the existing knowledge and familiarity levels of that particular mind. Very often the difference in the pattern of thinking is so much as zero to millions. Let us consider the simple example shown in Fig. 1. How do people think about this Fig. 1? A mathematician may treat that it is a rhombus while a chemist would probably say that it is a cyclobutane ring. A common man may assume that it is just a four-line boundary whereas a bridge player may think that it is a diamond, one of the four suits of the playing cards (which is treated as above clubs but below spades and hearts in playing cards language). Thus, even though the object is the same, the way of understanding differs from person to person depending on their familiarity, knowledge and interest levels. Similarly different perceptions prevail in the case of chemistry also.†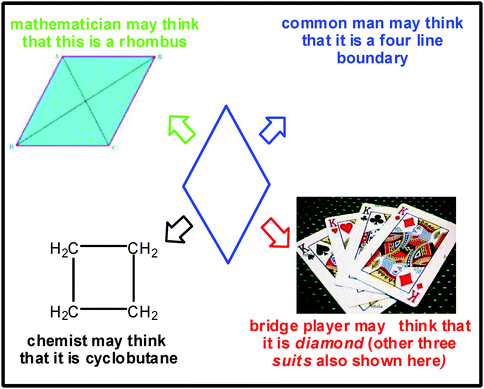 | ||
| Fig. 1 Variation in thinking and mindset about an object. | ||
2. Creativity and proximity
2.1. Molecules with one functional group
Bond forming and bond cleavage reactions essentially involving carbon are the most fundamental components in the ocean of organic chemistry. In these reactions, functional groups are the key players.1,2 The most important functional groups in organic chemistry are carbonyl (keto, aldehyde, acid, ester, amide etc.), alkene, alkyne, hydroxy, amino, cyano, and nitro groups. Each functional group in its own right plays a specific role in organic chemistry. Let us take the case of the carbonyl group: it can be reduced to alcohol, transformed into lactones, can undergo nucleophilic addition reactions, and can stabilize carbanion (Scheme 1A); also it can undergo a diverse range of other reactions. Similarly alkene can be reduced to alkane, converted into diol, epoxide, transformed into two carbonyl compounds (Scheme 1B) etc. Now consider the hydroxyl group: it can be oxidized to aldehyde (in the case of primary alcohol) or ketone (in the case of secondary alcohol), can be converted into alkene, alkane, or halide (Scheme 1C).1,2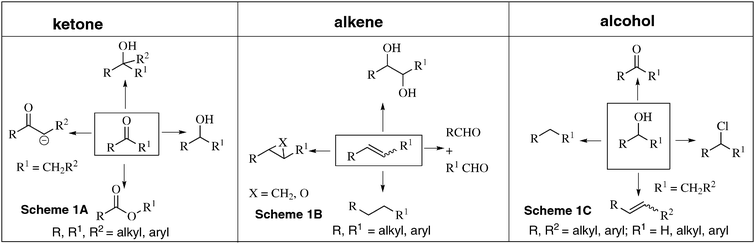 | ||
| Scheme 1 Molecules with one functional group and their properties. | ||
2.2. Molecules with two functional groups: power of proximity
Proximity is yet another important component not only in real life but also in chemistry. If a molecule contains two functional groups several atoms apart they (functional groups) can only exhibit their individual properties. But when they are in proximity, while keeping their individual reactivities fairly intact they can also show additional and quite different properties. Let us consider a compound having ketone and alkene in close combination: it can undergo different reactions such as Diels–Alder reaction and Michael reaction in addition to their own individual reactions (Scheme 2A).1,2 In the case of a molecule containing alkene and alcohol in proximity, it can generate a carbonium ion more easily than alcohol or alkene and also can be an excellent substrate for Johnson–Claisen rearrangement (Scheme 2B).1,2 Thus proximity of two functional groups in a molecule plays a unique role since such compounds offer different reactions otherwise not shown by the individual groups.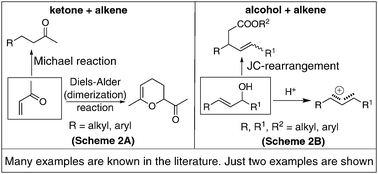 | ||
| Scheme 2 Influence of proximity of functional groups in molecules. | ||
2.3. Molecules with three functional groups
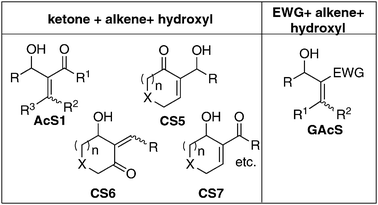
Let us now consider molecules containing three functional groups (ketone, alkene and hydroxyl) in proximity. In an acyclic system it appears that there is a possibility of four general structures (Fig. 2) [such as (1) alkene in the middle (here two possibilities) (2) keto group in the middle, (3) hydroxyl group in the middle]. In the case of a cyclic system one can notice that there are several possibilities. Some of the important structures (CS1–17) are presented in Fig. 2. If we generalize the structures AcS1 it will lead to the more general structures GAcS. Let us focus only on general structures GAcS and CS5–7—their synthesis, chemistry, applications and also challenges involved in these endeavors. A careful observation of these molecules indicates that these are nothing but the Baylis–Hillman adducts (Fig. 3).3–14 The major objective of writing this mini review is to summarize and discuss briefly the growth of the Baylis–Hillman (BH) reaction, influence of proximity of the functional groups in BH-adducts (GAcS and CS5–7) leading to unlimited synthetic applications and what makes this reaction to become an inspiring concept of creativity in chemistry.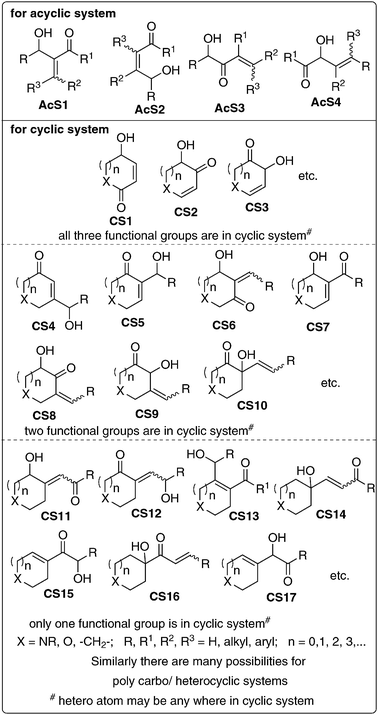 | ||
| Fig. 2 Possible structures of molecules with three (different) functional groups in proximity. | ||
 | ||
| Fig. 3 The Baylis–Hillman adducts. | ||
3. The Baylis–Hillman (BH) reaction3–14
3.1. Background
It is very appropriate to mention briefly the background history for this fascinating reaction. In the year 1963 Rauhut and Currier reported the dimerization of alkyl acrylates under the catalytic influence of trialkylphosphine to produce the corresponding dialkyl 2-methyleneglutarate derivatives.3a Morita and coworkers in the year 1968 described the reaction of various aldehydes with acrylates or acrylonitrile under the influence of tricyclohexylphosphine as a catalyst to provide 2-methylene-3-hydroxy alkanoates (or alkanenitriles) (Scheme 3).3b,c In the year 1972, Baylis and Hillman in their German patent reported an amine catalyzed coupling of various activated alkenes with aldehydes to produce a variety of densely functionalized molecules.3d,e This reaction is now referred to as the Baylis–Hillman reaction. This is essentially a three component reaction well equipped with large variations of parameters providing a huge reservoir of diverse classes of densely functionalized molecules (Scheme 4).3–14 This reaction is also known as the Morita–Baylis–Hillman (MBH) reaction. The main features of this fascinating reaction are given below.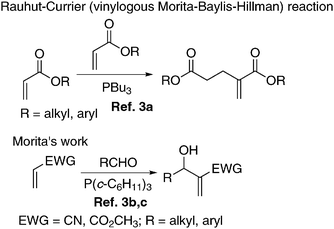 | ||
| Scheme 3 Earlier work of Rauhut–Currier and Morita. | ||
 | ||
| Scheme 4 The BH-reaction: a tool for the generation of a huge reservoir of densely functionalized molecules. | ||
3.2. Atom economy, organo-catalysis, and operational simplicity
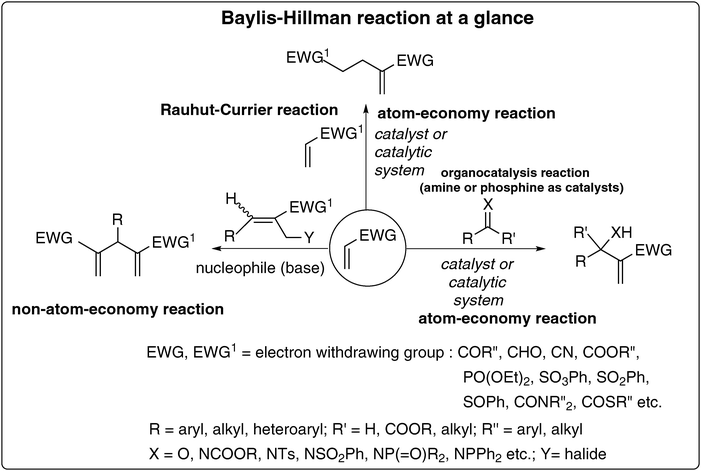
This is generally an atom economy carbon–carbon bond forming reaction involving the coupling of the α-position of activated alkenes with carbon electrophiles under the influence of a catalyst or a catalytic system. When the activated alkenes themselves act as electrophiles, Michael type dimers are produced in an atom economy process.15 This reaction is indeed one of the best and well known examples for organo-catalysis (and thus for green chemistry) when amines or phosphines are used as catalysts. BH-reactions involving allyl halides as electrophiles provide useful classes of functionalized 1,4-pentadienes.16 However, these reactions involve the loss of a halide group and thus do not fall in the category of atom-economy reactions. Most of the BH-reactions are operationally simple.3.3. Huge reservoir of densely functionalized molecules3–14
The starting materials i.e., activated alkenes and electrophiles are abundantly available or easily prepared. There is also a pool of catalysts. A variety of tertiary amines (such as DABCO, DBU, DMAP, TMG), phosphines (like tricyclohexyl, tributyl, triphenyl phosphines), and chalcogenides in combination with Lewis acids have been meticulously employed as catalysts. It is also interesting to note that some Lewis acids mediate a number of BH-reactions. In fact, a huge reservoir of diverse classes of densely functionalized molecules has been prepared using appropriate selection of essential components. It is indeed very attractive and challenging to discover or uncover new activated alke(y)nes and electrophiles as such efforts will produce various classes of new multi-functional molecules. A possible list of some of the most attractive and challenging activated alkenes and electrophiles that are not yet well explored is presented in Fig. 4 and Scheme 5 respectively. It will be fascinating to examine the appropriate combination of some of the catalysts and also discover various new catalysts to accelerate the rate of the reactions. Thus this reaction offers an unlimited scope for planning and designing different combinations of activated alkenes, electrophiles and catalysts to obtain many more classes of multi-functional molecules.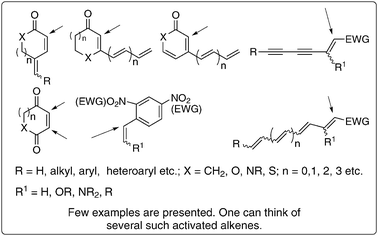 | ||
| Fig. 4 Potential activated alkenes: challenges ahead. | ||
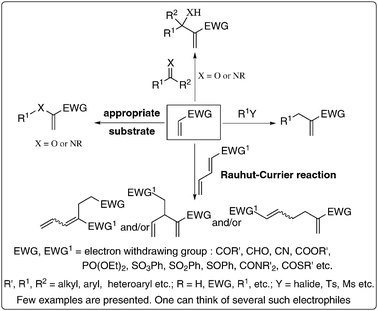 | ||
| Scheme 5 Potential electrophiles: future challenges. | ||
3.4. Opportunities in obtaining enantiomerically pure densely functionalized molecules3–14
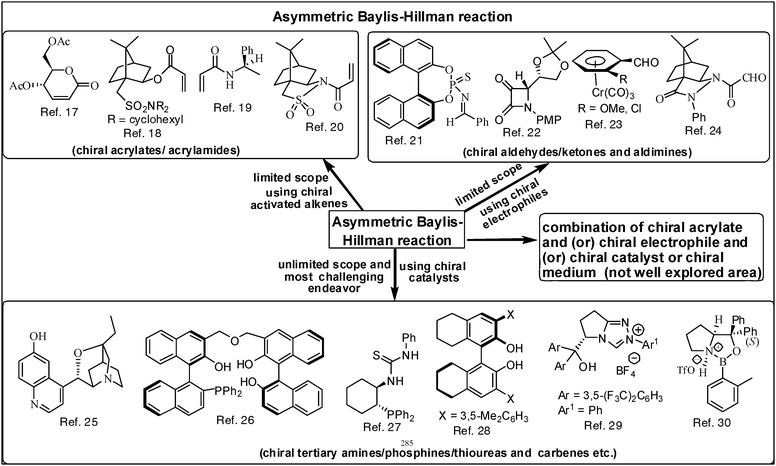 | ||
| Fig. 5 The asymmetric Baylis–Hillman reaction: a representative set of examples of chiral activated alkenes, electrophiles and catalysts that are well explored in the literature. | ||
Although considerable success has been achieved in the asymmetric version of the BH reaction using chiral catalysts we feel that this aspect is still at the initial stages as applications of these catalysts are limited to certain substrates only. Thus there is a serious need to develop new catalysts that would accommodate many more substrates in order to obtain several classes of enantiomerically pure molecules. In this connection we believe that chiral carbenes will offer a lot of promise as catalysts in addressing many unsolved problems and widening the scope of the asymmetric BH-reaction.29 It is therefore a challenge to design appropriate chiral carbenes and examine their potential as catalysts in BH reactions in the years to come.
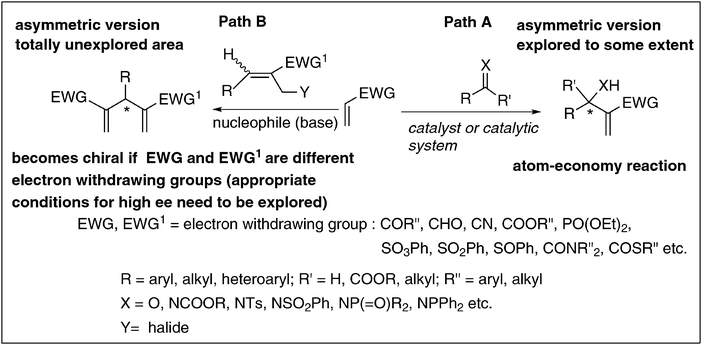 | ||
| Scheme 6 The asymmetric BH-reaction: enormous scope for expansion. | ||
3.5. Intramolecular Baylis–Hillman reaction and its asymmetric version5,10,11
If a substrate contains activated alkene and electrophilic components in appropriate positions there is a possibility of performing the intramolecular Baylis–Hillman reaction. Thus, this reaction offers opportunities to design various substrates for performing the intramolecular Baylis–Hillman reaction that would lead to the formation of a number of functionalized carbocyclic and heterocyclic molecules. Achieving its asymmetric version is yet another attractive endeavor that needs to be addressed. Some of the important examples that are reported in the literature are described in Scheme 7.31–35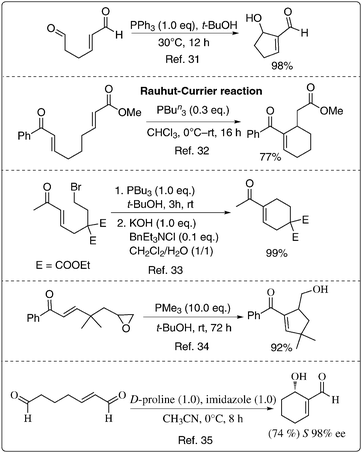 | ||
| Scheme 7 The intramolecular BH-reaction: known examples. | ||
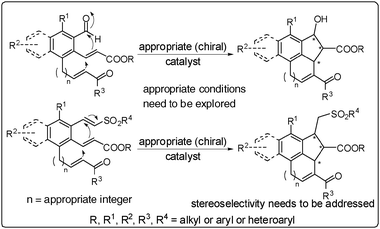 | ||
| Scheme 8 The intramolecular multi-BH-reactions: future projections. | ||
4. Single component Baylis–Hillman reaction
The literature records some examples that describe the BH-reaction of substrates containing two essential components.36,37 It is more appropriate to present here one such example, that is, pyridine-2-carboxaldehyde as a substrate in this category as it contains the electrophile and catalyst components leading to the formation of indolizine derivatives (Scheme 9).36 It will be intellectually exciting to discover BH-reactions with molecules containing all the three essential components, as these (single component) reactions not only provide the opportunity to design such substrates but also offer challenges in understanding the scope of this methodology. A possible example of a single component substrate which contains all the three essential components leading to the formation of tetracyclic molecules is presented in Scheme 10.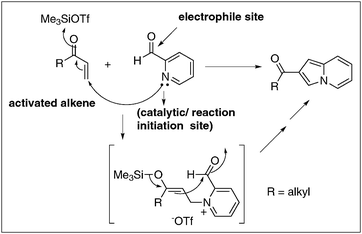 | ||
| Scheme 9 Electrophile induced BH-reaction: synthesis of indolizines. | ||
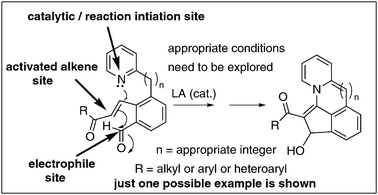 | ||
| Scheme 10 Single component BH-reactions: future challenges. | ||
5. Applications of Baylis–Hillman adducts5–14
This reaction produces densely functionalized molecules containing at least three functional groups, in proximity for appropriate use in various organic reactions and transformations. Different perspectives (looking at these molecules) about these densely functionalized molecules are pictorially presented in Fig. 6. Chemists have systematically explored the applications in all these directions and developed several methodologies and strategies for obtaining carbocyclic/heterocyclic/trisubstituted alkenes (Fig. 7).38–52 In fact several bioactive and natural products have been synthesized using the Baylis–Hillman adducts (Fig. 8).20,53–63 Although BH-adducts (acetates/bromides) have been extensively employed in a multitude of synthetic transformations, it looks to us that the power of proximity of functional groups in BH-adducts is not yet well understood/explored. Thus we believe that these adducts still offer enormous scope for developing and even discovering many new transformations and strategies in synthetic organic chemistry leading to the production of medicinally relevant compounds and even to certain important medicines.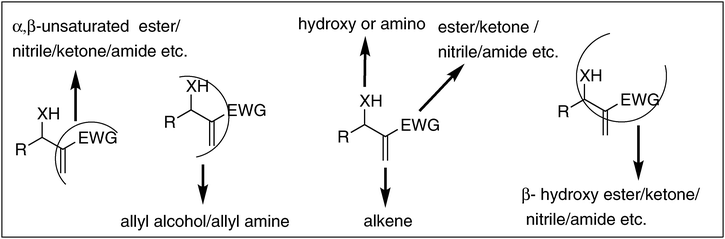 | ||
| Fig. 6 BH-adducts: different perceptions. | ||
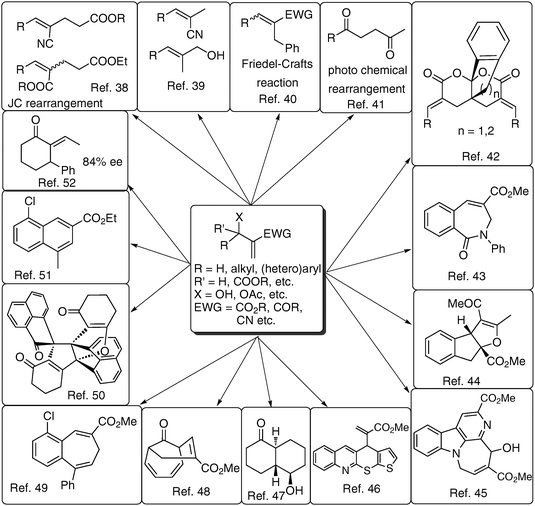 | ||
| Fig. 7 Applications of BH-adducts: various synthetic transformations. | ||
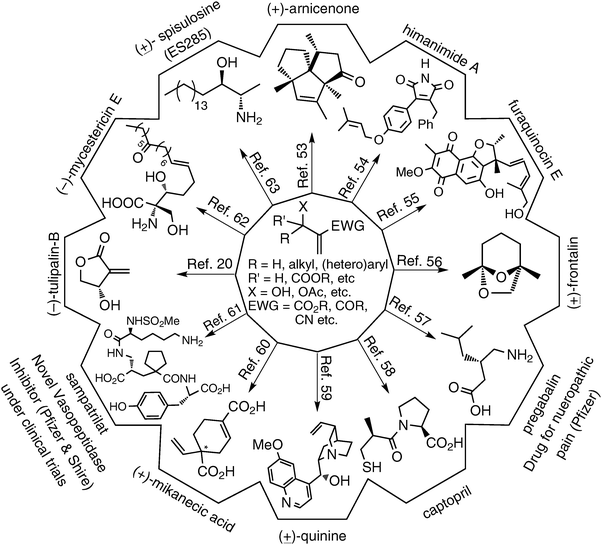 | ||
| Fig. 8 Applications of Baylis–Hillman (BH) adducts: synthesis of natural products and bio-active molecules. | ||
6. Mechanism5,9a,10,64
Understanding the mechanism of the Baylis–Hillman reaction is indeed an intellectually exciting area by itself. This is because of large variations of parameters such as diversity in the selection of activated alkenes, electrophiles and catalysts. Another important factor is the reaction conditions such as solvent or additives that influence the rate of the reaction. There are in fact several elegant publications on the mechanistic studies of the reaction.64 But so far there is no clear understanding about the rate determining step, and also transition states (either C–C bond formation or proton migration or release of catalyst or the presence of any new intermediate). Based on the available reports and evidence it is believed that in most of the BH-reactions the first step involves the Michael addition of catalyst (amine, phosphine, chalcogene, Lewis acid) to activated alkene to provide the zwitterionic enolate which will then react with a carbonyl compound (such as aldehyde, ketone, α-keto ester etc.) constructing a C–C bond in aldol fashion (proof provided by mass spectral studies by Coelho and coworkers in the case of DABCO as a catalyst and methyl acrylate as an activated alkene)64g producing multi functional molecules in an atom-economy process (Scheme 11).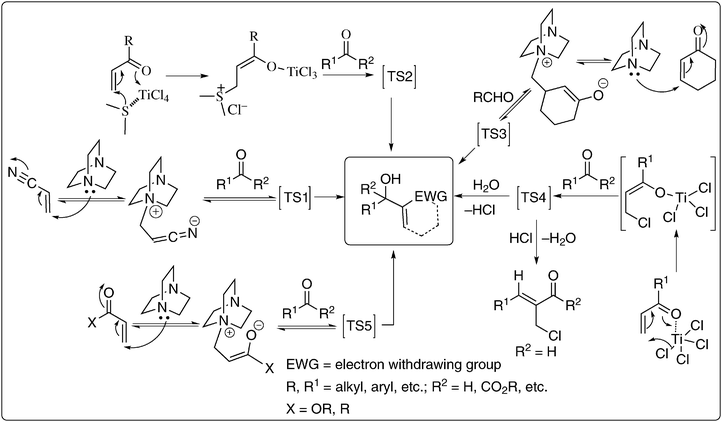 | ||
| Scheme 11 Various parameters that made the BH-reaction mechanism interesting.5,10,13 | ||
In the case of allyl halides as electrophiles, the first step might involve the formation of a quaternary salt and then the zwitterionic enolate A generated from activated alkene might react with this salt to provide the required pentadienes (Scheme 12).16 When the activated alkenes act as electrophiles, the zwitterion A adds on to the activated alkene in 1,4-fashion producing the Michael type dimers.5,11,15 It is interesting and desirable to understand the transition states and rate determining steps in these reactions (Scheme 12). Considering the fact that there are variations of parameters in performing the BH-reaction it is quite clear that a number of mechanistic pathways are possible depending on the substrates and conditions. It is indeed a challenge to understand and propose appropriate mechanistic pathway(s) for a given system of the BH-reaction.
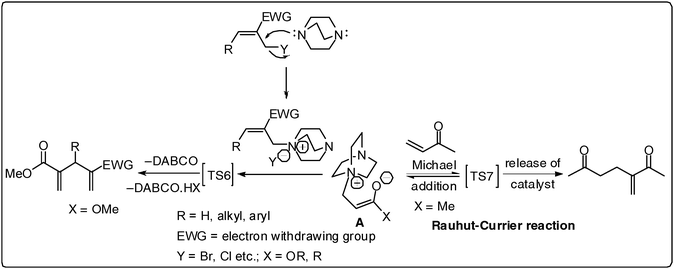 | ||
| Scheme 12 Parameters that influence the mechanism of the BH-reaction. | ||
7. The factors that decide growth of a reaction: standing of the Baylis–Hillman reaction
In science the most important thing is the identification of research area that should involve creativity and intellectual challenges finally leading to the applications to human well-being. In the case of synthetic chemistry discovery of new and truly useful carbon–carbon bond forming reactions undoubtedly represents one such area of research. It is equally important to recognize any C–C bond forming reaction which is known, but buried in the literature and to bring the same from an unknown status to the limelight of high applicability. In the case of C–C bond forming reactions there are certain factors that influence the growth of the reaction. Some of these factors might be: (1) abundance and easy accessibility of starting materials in general and scope for creativity in designing new substrates for growth and diversification of the reaction, (2) operational simplicity–organocatalysis–atom-economy–green chemistry, (3) functional group attraction for developing carbon assemblies, (4) stereochemical and mechanistic challenges, (5) provision for intramolecular version, and (6) the products with unlimited source of applications and opportunities for creativity and diversity in planning, design and synthesis. The Baylis–Hillman reaction is one such reaction that is well equipped with all these requirements (Fig. 9) grown from an unknown patent level to high popularity and applicability. Another elegant aspect in science is the art of creativity that can address the present day needs. From this brief review it is quite evident that the Baylis–Hillman reaction has already attained the status as a novel concept for creativity in chemistry.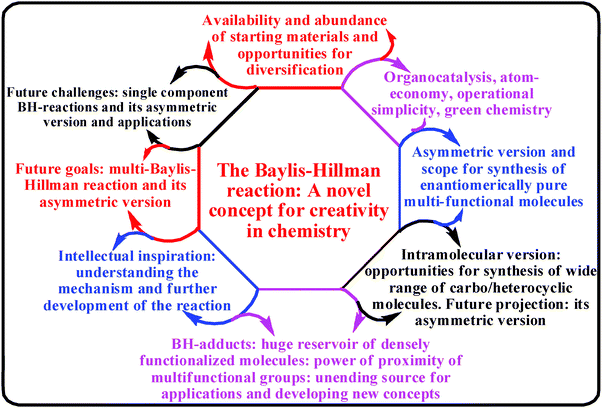 | ||
| Fig. 9 Important features: standing of the BH reaction. | ||
8. Conclusions
Considering the way in which the Baylis–Hillman reaction has been progressing we envision that this reaction will continue to grow to further heights. In conclusion, we have briefly presented the highlights of the BH reaction in this review and also predict many more brilliant and important contributions from this reaction to chemistry/medicinal chemistry leading towards human well being in the years to come.Abbreviations
| AcS | acyclic system |
| CS | cyclic system |
| DABCO | 1,4-diazabicyclo(2.2.2)octane |
| DBU | 1,8-diazabicyclo(5.4.0)undec-7-ene |
| DMAP | 4-(dimethylamino)pyridine |
| EWG & EWG1 | electron withdrawing group |
| GAcS | general acyclic system |
| JC | Johnson–Claisen |
| LA | Lewis acid |
| Ms | methanesulfonyl |
| MVK | methyl vinyl ketone |
| NMI | N-methylimidazole |
| PMP | p-methoxyphenyl |
| Tf | trifluoromethanesulfonyl |
| TMG | tetramethylguanidine |
| TS | transition state |
| Ts | p-toluenesulfonyl |
Acknowledgements
We thank DST (New Delhi) for financial support. We thank UGC (New Delhi) for recognizing the School of Chemistry as a “Center for Advanced Studies in Chemistry” and providing some instrumental facilities. GV thanks CSIR (New Delhi) for his research fellowship.Notes and references
-
(a)
M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th edn, Wiley, New York, 2007 Search PubMed
; (b) F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry, Parts A & B, 5th edn, Springer, New York, 2007 Search PubMed
.
-
(a)
R. C. Larock, Comprehensive Organic Transformations: a guide to functional group transformations, VCH, New York, 1989 Search PubMed
; (b) Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, New York, 1991, vol. 1–9 Search PubMed
.
-
(a)
M. M. Rauhut and H. Currier, American Cyanamide Co. U. S. Patent, 1963, 3074999. Chem. Abstr., 1963, 58, 11224a Search PubMed
; (b) K. Morita, Z. Suzuki and H. Hirose, Bull. Chem. Soc. Jpn., 1968, 41, 2815 CrossRef CAS
; (c) K. Morita and T. Kobayashi, Bull. Chem. Soc. Jpn., 1969, 42, 2732 CrossRef CAS
; (d) A. B. Baylis and M. E. D. Hillman, German patent 2155113, 1972, Chem. Abstr., 1972, 77, 34174q Search PubMed
; (e) M. E. D. Hillman and A. B. Baylis, U. S. Patent, 3,743,669, 1973 Search PubMed
.
-
M. Shi, F. Wang, M.-X. Zhao and Y. Wei, Chemistry of the Morita–Baylis–Hillman Reaction, RSC Catalysis Series, 2011 Search PubMed
.
- D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS
.
- V. Declerck, J. Martinez and F. Lamaty, Chem. Rev., 2009, 109, 1 CrossRef CAS
.
- V. Singh and S. Batra, Tetrahedron, 2008, 64, 4511 CrossRef CAS
.
- Y.-L. Shi and M. Shi, Org. Biomol. Chem., 2007, 5, 1499 CAS
.
-
(a) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS
; (b) G. Masson, C. Housseman and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 4614 CrossRef CAS
; (c) P. Langer, Angew. Chem., Int. Ed., 2000, 39, 3049 CrossRef CAS
.
- D. Basavaiah, K. V. Rao and R. J. Reddy, Chem. Soc. Rev., 2007, 36, 1581 RSC
.
- D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS
.
-
E. Ciganek, in Organic Reactions, ed. L. A. Paquette, Wiley, New York, 1997, vol. 51, p. 201 Search PubMed
.
- D. Basavaiah, P. D. Rao and R. S. Hyma, Tetrahedron, 1996, 52, 8001 CrossRef CAS
.
- S. E. Drewes and G. H. P. Roos, Tetrahedron, 1988, 44, 4653 CrossRef CAS
.
- D. Basavaiah, V. V. L. Gowriswari and T. K. Bharathi, Tetrahedron Lett., 1987, 28, 4591 CrossRef CAS
.
-
(a) D. Basavaiah, N. Kumaragurubaran and D. S. Sharada, Tetrahedron Lett., 2001, 42, 85 CrossRef CAS
; (b) D. Basavaiah, D. S. Sharada, N. Kumaragurubaran and R. M. Reddy, J. Org. Chem., 2002, 67, 7135 CrossRef CAS
.
- P. R. Krishna, P. S. Reddy, M. Narsingam, B. Sateesh and G. N. Sastry, Synlett, 2006, 595 CrossRef CAS
.
- D. Basavaiah, V. V. L. Gowriswari, P. K. S. Sarma and P. D. Rao, Tetrahedron Lett., 1990, 31, 1621 CrossRef CAS
.
- K. He, Z. Zhou, G. Zhao and C. Tang, Heteroat. Chem., 2006, 17, 317 CrossRef CAS
.
- L. J. Brzezinski, S. Rafel and J. W. Leahy, J. Am. Chem. Soc., 1997, 119, 4317 CrossRef CAS
.
- A. Lu, X. Xu, P. Gao, Z. Zhou, H. Song and C. Tang, Tetrahedron: Asymmetry, 2008, 19, 1886 CrossRef CAS
.
- B. Alcaide, P. Almendros, C. Aragoncillo and R. Rodriguez-Acebes, J. Org. Chem., 2004, 69, 826 CrossRef CAS
.
- E. P. Kundig, L. H. Xu, P. Romanens and G. Bernardinelli, Tetrahedron Lett., 1993, 34, 7049 CrossRef
.
- J.-F. Pan and K. Chen, Tetrahedron Lett., 2004, 45, 2541 CrossRef CAS
.
-
(a) Y. Iwabuchi, M. Nakatani, N. Yokoyama and S. Hatakeyama, J. Am. Chem. Soc., 1999, 121, 10219 CrossRef CAS
; (b) For the recent application of this catalyst see: X.-Y. Guan, Y. Wei and M. Shi, Chem.–Eur. J., 2010, 16, 13617 CrossRef CAS
.
- Y.-H. Liu, L.-H. Chen and M. Shi, Adv. Synth. Catal., 2006, 348, 973 CrossRef CAS
.
- K. Yuan, L. Zhang, H.-L. Song, Y. Hu and X.-Y. Wu, Tetrahedron Lett., 2008, 49, 6262 CrossRef CAS
.
- N. T. McDougal and S. E. Schaus, J. Am. Chem. Soc., 2003, 125, 12094 CrossRef CAS
.
- L. He, Y.-R. Zhang, X.-L. Huang and S. Ye, Synthesis, 2008, 2825 CAS
.
- B. K. Senapati, G.-S. Hwang, S. Lee and D. H. Ryu, Angew. Chem., Int. Ed., 2009, 48, 4398 CrossRef CAS
.
- J. E. Yeo, X. Yang, H. J. Kim and S. Koo, Chem. Commun., 2004, 236 RSC
.
- P. M. Brown, N. Kappel, P. J. Murphy, S. J. Coles and M. B. Hursthouse, Tetrahedron, 2007, 63, 1100 CrossRef CAS
.
- M. E. Krafft, K. A. Seibert, T. F. N. Haxell and C. Hirosawa, Chem. Commun., 2005, 5772 RSC
.
- M. E. Krafft and J. A. Wright, Chem. Commun., 2006, 2977 RSC
.
- S.-H. Chen, B.-C. Hong, C.-F. Su and S. Sarshar, Tetrahedron Lett., 2005, 46, 8899 CrossRef CAS
.
- D. Basavaiah and A. J. Rao, Chem. Commun., 2003, 604 RSC
.
- H. Kinoshita, T. Osamura, S. Kinoshita, T. Iwamura, S.-i. Watanabe, T. Kataoka, G. Tanabe and O. Muraoka, J. Org. Chem., 2003, 68, 7532 CrossRef CAS
.
-
(a) S. E. Drewes, N. D. Emslie, N. Karodia and G. Loizou, Synth. Commun., 1990, 20, 1437 CrossRef CAS
; (b) D. Basavaiah and S. Pandiaraju, Tetrahedron Lett., 1995, 36, 757 CrossRef CAS
; (c) D. Basavaiah, S. Pandiaraju and M. Krishnamacharyulu, Synlett, 1996, 747 CrossRef CAS
.
- D. Basavaiah and P. K. S. Sarma, J. Chem. Soc., Chem. Commun., 1992, 955 RSC
.
- D. Basavaiah, M. Krishnamacharyulu, R. S. Hyma and S. Pandiaraju, Tetrahedron Lett., 1997, 38, 2141 CrossRef CAS
.
- S. Matsumoto, Y. Okubo and K. Mikami, J. Am. Chem. Soc., 1998, 120, 4015 CrossRef CAS
.
- D. Basavaiah and T. Satyanarayana, Org. Lett., 2001, 3, 3619 CrossRef CAS
.
- H. Cao, T. O. Vieira and H. Alper, Org. Lett., 2011, 13, 11 CrossRef CAS
.
- E. S. Kim, K. H. Kim, S. Park and J. N. Kim, Tetrahedron Lett., 2010, 51, 4648 CrossRef CAS
.
- S. Hutait and S. Batra, Tetrahedron Lett., 2010, 51, 5781 CrossRef CAS
.
- W. Zhong, W. Ma and Y. Liu, Tetrahedron, 2011, 67, 3509 CrossRef CAS
.
- P. Wasnaire, M. Wiaux, R. Touillaux and I. E. Marko, Tetrahedron Lett., 2006, 47, 985 CrossRef CAS
.
- Y. Du, J. Feng and X. Lu, Org. Lett., 2005, 7, 1987 CrossRef CAS
.
- S. Gowrisankar, K. Y. Lee, C. G. Lee and J. N. Kim, Tetrahedron Lett., 2004, 45, 6141 CrossRef CAS
.
- D. Basavaiah, S. Roy and U. Das, Tetrahedron, 2010, 66, 5612 CrossRef CAS
.
- J. N. Kim, Y. J. Im, J. H. Gong and K. Y. Lee, Tetrahedron Lett., 2001, 42, 4195 CrossRef CAS
.
- T. Gendrineau, J.-P. Genet and S. Darses, Org. Lett., 2010, 12, 308 CrossRef CAS
.
- Y. Iura, T. Sugahara and K. Ogasawara, Org. Lett., 2001, 3, 291 CrossRef CAS
.
- D. Basavaiah, B. Devendar, K. Aravindu and A. Veerendhar, Chem.–Eur. J., 2010, 16, 2031 CrossRef CAS
.
- B. M. Trost, O. R. Thiel and H.-C. Tsui, J. Am. Chem. Soc., 2002, 124, 11616 CrossRef CAS
.
- A. Weichert and H. M. R. Hoffmann, J. Org. Chem., 1991, 56, 4098 CrossRef CAS
.
- (a) “Pregabalin” Pfizer, Drugs of Future 2002, 27, 426; (b) Pregabalin is presently an anticonvulsant drug used for neuropathic pain (Pfizer)—From Wikipedia, the free encyclopedia (http://en.wikipedia.org/wiki/Pregabalin).
- M. P. Feltrin and W. P. Almeida, Synth. Commun., 2003, 33, 1141 CrossRef CAS
.
- P. Webber and M. J. Krische, J. Org. Chem., 2008, 73, 9379 CrossRef CAS
.
- D. Basavaiah, P. K. S. Sarma and S. Pandiaraju, Tetrahedron Lett., 1994, 35, 4227 CrossRef CAS
.
- (a) P. J. Dunn, Abstracts of papers, 223rd ACS National Meeting, Orlando, FL, United States, April 7–11, 2002; (b) P. J. Dunn, Pfizer Global Research and Development, Sandwich, Kent, UK, personal communication; (c) Sampatrilat is a dual inhibitor of neutral endopeptidase (NEP) and angiotensin converting enzyme (ACE) (under development by Pfizer and Shire for possible treatment of hypertension).
- Y. Iwabuchi, M. Furukawa, T. Esumi and S. Hatakeyama, Chem. Commun., 2001, 2030 RSC
.
- G. W. Amarante, M. Cavallaro and F. Coelho, Tetrahedron Lett., 2010, 51, 2597 CrossRef CAS
.
-
(a) T. Regiani, V. G. Santos, M. N. Godoi, B. G. Vaz, M. N. Eberlin and F. Coelho, Chem. Commun., 2011, 47, 6593 RSC
; (b) C. Patel and R. B. Sunoj, J. Org. Chem., 2010, 75, 359 CrossRef CAS
; (c) D. Roy and R. B. Sunoj, Org. Lett., 2007, 9, 4873 CrossRef CAS
; (d) R. Robiette, V. K. Aggarwal and J. N. Harvey, J. Am. Chem. Soc., 2007, 129, 15513 CrossRef CAS
; (e) K. E. Price, S. J. Broadwater, B. J. Walker and D. T. McQuade, J. Org. Chem., 2005, 70, 3980 CrossRef CAS
; (f) V. K. Aggarwal, S. Y. Fulford and G. C. Lloyd-Jones, Angew. Chem., Int. Ed., 2005, 44, 1706 CrossRef CAS
; (g) L. S. Santos, C. H. Pavam, W. P. Almeida, F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 4330 CrossRef CAS
; (h) G. Li, J. Gao, H.-X. Wei and M. Enright, Org. Lett., 2000, 2, 617 CrossRef CAS
; (i) T. Kataoka, H. Kinoshita, T. Iwama, S.-i. Tsujiyama, T. Iwamura, S.-i. Watanabe, O. Muraoka and G. Tanabe, Tetrahedron, 2000, 56, 4725 CrossRef CAS
; (j) Y. Fort, M. C. Berthe and P. Caubere, Tetrahedron, 1992, 48, 6371 CrossRef CAS
; (k) M. L. Bode and P. T. Kaye, Tetrahedron Lett., 1991, 32, 5611 CrossRef CAS
; (l) J. S. Hill and N. S. Isaacs, J. Phys. Org. Chem., 1990, 3, 285 CrossRef CAS
.
Footnote |
| † Since there is a limit on the number of references, only essential references have been selected and several leading references could not be cited. The references, which appeared in earlier reviews4–14 (except the very relevant ones), are also not cited |
| This journal is © The Royal Society of Chemistry 2012 |
