A new class of highly-conducting polymer electrolyte membranes: Aromatic ABA triblock copolymers
Nanwen
Li
a,
So Young
Lee
ab,
Ying-Ling
Liu
cd,
Young Moo
Lee
*ab and
Michael D.
Guiver
*ae
aWCU Department of Energy Engineering, Hanyang University, Seoul, 133-791, Republic of Korea. E-mail: Michael.Guiver@nrc-cnrc.gc.ca; ymlee@hanyang.ac.kr
bSchool of Chemical Engineering, College of Engineering, Hanyang University, Seoul, 133-791, Republic of Korea
cDepartment of Chemical Engineering, National Tsing Hua University, Hsinchu, 30013, Taiwan
dR&D Center for Membrane Technology, Chung Yuan Christian University, Chungli, Taoyuan, 32023, Taiwan
eInstitute for Chemical Process and Environmental Technology, National Research Council, Ottawa, Ont KIA OR6, Canada
First published on 3rd November 2011
Abstract
Highly proton-conducting polymer electrolyte membrane (PEMs) materials are presented as alternatives to state-of-the-art perfluorinated polymers such as Nafion®. To achieve stable PEMs with efficient ionic nanochannels, novel fully aromatic ABA triblock copolymers (SP3O-b-PAES-b-SP3O) based on sulfonated poly(2,6-diphenyl-1,4-phenylene oxide)s (A, SP3O) and poly(arylene ether sulfone)s (B, PAES) were synthesized. This molecular design for a PEM was implemented to promote the nanophase separation between the hydrophobic polymer chain and hydrophilic ionic groups, and thus to form well-connected hydrophilic nanochannels that are responsible for the water uptake and proton conduction. Relative to other hydrocarbon-based PEMs, the triblock copolymer membranes showed a dramatic enhancement in proton conductivity under partially hydrated conditions, and superior thermal, oxidative and hydrolytic stabilities, suggesting that they have the potential to be utilized as alternative materials in applications operating under partly hydrated environments.
Broader contextAromatic triblock architecture leads to highly-conducting polymer electrolyte membranes (PEM) displaying nanophase-separated morphology containing well-connected conducting channels. The proton-conducting segments are based on sulfonated poly(2,6-diphenyl-1,4-phenylene oxide)s (SP3O). The unique polymer architecture results in nanophase separation between the conducting and non-conducting domains, which shows a wormlike and interconnected hydrophilic network of small ionic clusters of 5–10 nm in size, similar to Nafion. This results in high proton diffusion coefficients, and thus high proton conductivities, throughout a range of relative humidity conditions. Triblock membranes with low ion exchange capacity values (IEC = 0.97 meq./g) approaching that of Nafion (IEC = 0.90 meq./g) have higher proton conductivity values, which are maintained or comparable to Nafion 112, even at reduced relative humidity (30–50% RH). High proton conductivities, even at low IEC values, coupled with good conductivity at reduced RH and low in-plane dimensional swelling suggests the triblock architecture is a promising approach for the design of PEMs to be used in fuel cells for automotive applications, where an environment of elevated temperature and low humidity may be present. Elevated temperature operation allows a simplified cooling system and other advantages such as improved tolerance of the catalyst to carbon monoxide impurities in the hydrogen, and improved electrode reaction kinetics. |
Introduction
The search for new highly proton-conducting materials has been a subject of intense research because of their potential applications in fuel cells, sensors, and other areas.1,2 Two types of proton-conducting mechanisms are well-recognized: the vehicle mechanism and the Grøtthuss-type mechanism also called structure diffusion.3–6 The vehicle mechanism, which has been reported to occur in a variety of environments7 (aqueous acidic solutions,8 acidic polymers,9etc.), occurs by the formation of an ion adduct composed of a proton and a diffusible carrier molecule (e.g., H2O). The Grøtthuss-type mechanism is the transport of protons from site to site without a carrier molecule, and its activation energy depends on the hydrogen bond breaking energy and the distance between sites.10,11 In most cases, these two proton-conducting mechanisms cannot be entirely separated from one another and occur simultaneously to some degree. Early studies using pulsed field gradient spin echo 1H NMR indicated that the dominant mode of proton-conduction in the commercial state-of-the-art perfluorosulfonic acid PEMs (e.g.Nafion®, which shows good conductivity and stability) at low relative humidity (RH) is via the vehicle mechanism, while in the PEMs at high humidity, protons are rapidly exchanged between hydrated proton exchange sites via the Grøtthuss mechanism.7,12 Several perceived problems of commercial Nafion membranes including high cost, environmental incompatibility, and limited operation temperature (low glass-transition temperature, ca. 100 °C) have stimulated the development of alternative PEMs such as sulfonated hydrocarbon polymers.13–15 Nevertheless, proton conduction in the majority of hydrocarbon PEMs at low RH has been shown to be less effective than Nafion.13 To date, alternative PEMs that provide equal or better overall properties to the perfluorosulfonic acid class of materials at elevated temperatures and reduced RH is still one of the biggest challenges for new proton-conducting materials.14–16Proton conduction at low RH requires water to diffuse through the membrane, which can occur effectively through continuous hydrophilic pathways by the vehicle mechanism, such as those found in Nafion. Thus, current research has focused on the development of alternative PEM materials which, like Nafion, present nanophase separated morphology between the hydrophobic polymer main chain and the acidic moieties, leading to conducting nanochannels responsible for water uptake and proton transport.17 Various synthetic strategies have been explored to obtain proton-conducting materials containing efficient ionic nanochannels, that is, well-defined structures including multi-block or graft copolymers that have controlled segment lengths.18,19,26 Among them, the diblock or triblock copolymers prepared from controlled/living radical polymerization techniques combined with efficient coupling reactions have received significant attention.20,21 Their unique structures provide a template, whereby phase separation occurs on a nanometer scale due to the thermodynamic incompatibility between unlike blocks. As a result, they are capable of forming a variety of self-assembled morphologies including spheres arranged on a cubic lattice, hexagonally packed cylinders, interpenetrating gyroids, and alternating lamellae.22 Various kinds of diblock or triblock copolymers with fully or partly sulfonated blocks have been studied as PEMs.23–28 Self-organization of these block copolymers offers the opportunity for precise control of membrane morphology by manipulation of chemical compositions and relative volumes of the constituent blocks. Phase-separated morphology with hydrophilic nanochannels and enhanced proton conductivity were observed for these block copolymers. However, the synthesis of most di- and tri-block copolymers relies on styrene and vinyl block systems, which exhibit poor thermal and chemical stability, thus largely limiting their utilization as proton-conducting PEMs in fuel cell applications.29,30
To avoid the relative instability of aliphatic chains, fully aromatic di- or tri-block copolymers could be incorporated into the molecular design, to improve the chemical and thermal stability and mechanical strength of the PEMs. However, to our knowledge, they have hitherto been unreported. It is a difficult challenge to design this polymer architecture, since a mono-functional terminated aromatic block chain is required to construct the di- or tri-block copolymer. Poly(phenylene oxide) (PPO) derivatives, which are synthesized by catalyzed oxidative coupling of substituted phenols, are unique among aromatic polymers in having a mono-functional chain terminus.31–33 Thus, we identified mono-phenol-terminated PPO oligomers as ideal candidates for use as aromatic block chains, and our work builds upon that of other researchers34–36 who reported the synthesis of P3O derivatives with low molecular weights. Most recently, we successfully utilized mono-phenol-terminated poly(2,6-diphenyl-1,4-phenylene oxide) oligomers (the abbreviation P3O is commonly used for diphenyl-substituted PPO) to construct fully aromatic comb-shaped copolymer architecture having efficient ionic nanochannels to achieve highly proton-conducting PEMs.37
For the utilization of mono-phenol-terminated P3O oligomers, it is important to develop novel proton-conducting materials that are able to self-assemble to provide nanochannels leading to efficient proton transport. Herein, we report a new class of fully aromatic ABA triblock copolymers (SP3O-b-PAES-b-SP3O), which consists of two sulfonated end blocks of poly(2,6-diphenyl-1,4-phenylene oxide) (P3O) and a middle block of poly(arylene ether sulfone). The hydrophobic PAES chain is expected to be immiscible with the highly sulfonated P3O blocks, thus driving membranes to self-assemble and form nanoscale domains that contain enhanced local concentrations of sulfonic acid, which facilitate effective proton transport.
Experimental section
Materials
Bis(4-fluorophenyl) sulfone (DFDPS) was obtained from Sigma-Aldrich and dried under vacuum at room temperature overnight. 4,4′-(Hexafluoroisopropylidene)diphenol (6F-BPA) was purchased from Sigma-Aldrich and recrystallized twice from toluene. Hexafluorobenzene (HFB) end-capped poly(2,6-diphenyl-1,4-phenylene oxide) oligomer (P3O-F) was prepared similarly to our previously reported procedure.37Synthesis of poly(arylene ether sulfone) (PAES) oligomers 1
A typical synthetic procedure for OH-terminated polymer, illustrated by the preparation of 1(X50) copolymers, is described as follows. Into a three-neck flask equipped with a mechanical stirrer, Dean–Stark trap, and an argon gas inlet were added 20 mmol of DFDPS, 24 mmol of 6F-BPA, and 30 mmol of K2CO3. Then, 30 mL of NMP and 15 mL of toluene were charged into the reaction flask under an argon atmosphere and the reaction mixture was heated to 145 °C. After dehydration and removal of toluene for several hours, the reaction temperature was increased to about 170 °C. When the increase of the solution viscosity became obvious, the mixture was cooled to room temperature and coagulated into a large excess of deionized water with vigorous stirring. The resulting fibrous copolymer was washed thoroughly with water or ethanol several times and dried under vacuum at 100 °C for 24 h. The copolymer was denoted 1(X50), where (X50) refers to the expected length of PAES chain. Yield: 95%.Synthesis of the triblock copolymers 2
The described copolymers are denoted as 2(Xx, where x refers to the expected length of polymer chain). 1(X100) (2.0 g, 0.07 mmol of OH group), P3O-F (0.37 g, 0.10 mmol), K2CO3 (0.01 g, 0.07 mmol), 15 mL of NMP, and 5 mL of toluene were added into an argon flushed reactor equipped with a Dean–Stark trap. The reaction mixture was heated to 105 °C for 12 h and then the reaction temperature was gradually increased over a period of 6 h to ∼160 °C, then maintained at this temperature for an additional 20 h. The mixture was coagulated into a large excess of dilute HCl (5 wt %) with vigorous stirring and the polymer washed with water. The resulting triblock copolymers 2(X100) were dried under vacuum at 100 °C for 24 h.Sulfonation of the triblock copolymer
To a round-bottomed flask equipped with a dropping funnel, 1.0 g of 2(X50) was charged. Then, dry dichloromethane (40 mL) was added into the flask, and the mixture was cooled to 5–7 °C by an ice bath. To the mixture was added dropwise a solution of chlorosulfonic acid (0.6 mL, 3 mmol) in dry dichloromethane (20 mL) at this temperature. The mixture was stirred vigorously at 5–7 °C for 30 min until a dark brown product precipitated out of the solution. The precipitate was filtered, washed with water several times, and dried overnight under vacuum at 80 °C for 10 h to give sulfonated triblock copolymers 3(X50).Preparation of membranes
A solution of the obtained sulfonated copolymer (1 g) in DMSO (10 mL) was filtered (10 μm filter) and then cast onto a flat glass plate with a doctor blade. The cast solution was dried at 80 °C overnight to give a transparent, tough film. The film was dried further in a vacuum oven at 100 °C for 20 h. The resulting film was treated with 2 M H2SO4 for 24 h, washed with water several times, and dried at room temperature.Measurements
1H NMR spectra were measured at 300 MHz on an AV 300 spectrometer using DMSO-d6 or CDCl3 as solvent. Ion exchange capacities (IEC) of the membranes were determined by back-titration and 1H NMR results. A piece of the membrane was equilibrated in a large excess of 0.5 M NaCl aqueous solution for 3 days. The released HCl by the ion exchange was titrated with standard 0.01 M NaOH solution. The reduced viscosities were determined on 0.5 g dL−1 concentration of polymer in NMP or DMSO with an Ubbelohde capillary viscometer at 30.0 ± 0.1 °C. Tensile measurements were performed with a mechanical tester Instron-1211 instrument at a speed of 1 mm/min. The thermogravimetric analyses (TGA) were obtained in nitrogen with a Perkin-Elmer TGA-2 thermogravimetric analyzer at a heating rate of 10 °C/min. The glass-transition temperature (Tg) was determined on a Seiko 220 DSC instrument at a heating rate of 20 °C/min under nitrogen protection. Tg is reported as the temperature at the middle of the thermal transition from the second heating scan. The molecular weights of polymers were determined by gel permeation chromatography (GPC) using a Waters 515 HPLC pump, coupled with a Waters 410 differential refractometer detector and a Waters 996 photodiode array detector. THF was used as the eluant and the μ-Styragel columns were calibrated by polystyrene standards.Membrane densities were determined from membrane dimensions and weights after drying at 100 °C for 8 h. Water uptake was measured after drying the membrane in acid form at 100 °C under vacuum overnight. The dried membrane was immersed in water and periodically weighed on an analytical balance until a constant weight was obtained, giving the weight-based (IEC) water uptake. The volume-based IEC (IECv) was obtained by multiplying the membrane density by the IEC values, which were estimated from the copolymer structure. This calculation resulted in IECv (dry) based on the dry membrane density. The IECv (wet) (meq./cm3) was then calculated based on membrane water uptake, using the following eqn (1):
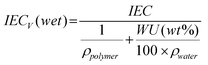 | (1) |
Proton conductivity (σ, Scm−1) of each membrane coupon (size: 1 cm × 4 cm) was obtained using σ = d/LsWsR (d is the distance between reference electrodes, and Ls and Ws are the thickness and width of the membrane, respectively). The resistance value (R) was measured over the frequency range from 100 mHz to 100 kHz by four-point probe alternating current (ac) impedance spectroscopy using an electrode system connected with an impedance/gain-phase analyzer (Solartron 1260) and an electrochemical interface (Solartron 1287, Farnborough Hampshire, ONR, UK). The membranes were sandwiched between two pairs of gold-plate electrodes. The conductivity measurements under fully hydrated conditions in the longitudinal direction were carried out with the cell immersed in liquid water. Proton conductivity under partially hydrated conditions was performed at 90 °C. Membranes were equilibrated at different relative humidity for 2 h in a humidity-temperature oven before each measurement.
From the conductivity and density data, proton diffusion coefficients (Dσ) were calculated using the Nernst-Einstein eqn (2):
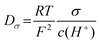 | (2) |
For transmission electron microscopy (TEM) observations, the membranes were stained with lead ions by ion exchange of the sulfonic acid groups in 0.5 M lead acetate aqueous solution, rinsed with deionized water, and dried in vacuum oven for 12 h. The stained membranes were embedded in epoxy resin, sectioned to 90 nm thickness with a Leica microtome Ultracut UCT, and placed on copper grids. Electron micrographs were taken with a Hitachi H7600 transmission electron microscope using an accelerating voltage of 80 k.
AFM micrographs were recorded with a bioatomic force microscopy (Bio-AFM). AFM tapping-mode height profiles were acquired with a JPK Instruments AG multimode NanoWizard (Germany). The instrument was equipped with a NanoWizard scanner. For tapping-mode AFM, a commercial Si cantilever (TESP tip) of about 320 kHz resonant frequency from JPK was used. The sample was kept fully hydrated during measurement.
Small angle X-ray scattering (SAXS, MXP3, Mac Science) was measured for 3membranes at 50% RH and room temperature. The membranes were enveloped in a Mylar bag and irradiated by X-ray (CuKα, λi = 1.54 Å) with 40 kV. The range of scattering vectors explored (q = 4πsin2θ/λi) was from 0.085 to 3.0 nm−1, where λi and 2θ are the incident wavelength and total scattering angle, respectively.
Results and discussion
ABA triblock copolymer synthesis
To obtain the aromatic ABA triblock copolymers, mono-phenoxide terminated poly(2,6-diphenyl-1,4-phenylene oxide) (P3O) oligomers with Mn of 3500 g/mol were synthesized viaCu(I)-catalyzed oxidative coupling reaction, according to our previous work.37 The graft chain repeat units of oligomers were determined experimental as approximately 12.3 from the 1H NMR spectra, which are similar to the values determined from GPC results (Y = 13.6). Subsequently, the P3O-OH oligomers were converted to reactive fluorine-terminated oligomers (P3O-F) by end-capping with hexafluorobenzene (HFB), since the OH-terminated P3O oligomers are reported to be capable of chain cleavage of the poly(arylene ether sulfone) (PAES) blocks under certain conditions.38The PAES polymers 1 bearing –OH end groups were synthesized, as shown in Scheme 1. The monomer composition was set so that the expected degree of polymerization would be 50, 70 and 100. The reaction proceeded in N-methylpyrrolidone (NMP) under typical nucleophilic substitution conditions using potassium carbonate as the base. The polymers 1 were obtained as white fiber and characterized by viscosity measurements and GPC analyses (Table 1). Molecular weight distributions were in the range of 1.4 to 1.7, typical of polycondensation reactions. The experimental x values calculated from Mn were 43, 65 and 94 for x = 50, 70 and 100, respectively. These values were approximately consistent with the ones expected from the comonomer feed ratios.
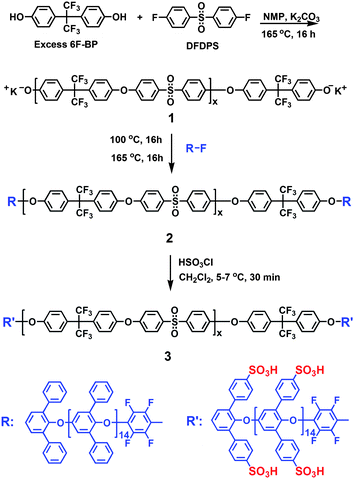 | ||
| Scheme 1 Synthesis of aromatic ABA triblock copolymers 3. | ||
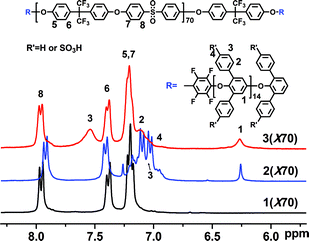
Polymer 1 was reacted with the activated fluorine atom of P3O-F by nucleophilic substitution to obtain the ABA triblock copolymers, as shown in Scheme 1. The coupling reactions proceeded smoothly, and no cross-linking or chain cleavage were evident when the temperature and reaction time were well controlled by an oil bath, which was confirmed by viscosity measurements (Table 1). The copolymers 2 were obtained as white fibers, which were soluble in chloroform, CH2Cl2 and NMP, but insoluble in dimethylformamide (DMF), dimethylacetamide (DMAc) and dimethyl sulfoxide (DMSO). Comparison of the 1H NMR spectra of copolymers 2 with those of the parent OH-terminated polymers 1, showed that the P3O protons appeared at 6.28 and 7.10 ppm (Fig. 1) indicating the formation of triblock copolymers.
Sulfonation of ABA triblock copolymers 2
The triblock copolymers 2 were sulfonated with chlorosulfonic acid in dichloromethane solution. Based on the number of pendent phenyl rings obtained, a five molar excess of chlorosulfonic acid was applied for the sulfonation reaction of 2. The sulfonation reaction was originally conducted at room temperature (about 25 °C). Although fully sulfonated triblock copolymer could be achieved in a short time (about 30 min), the reaction was not always reliable, since insoluble gel sometimes resulted. Therefore, sulfonation at reduced reaction temperatures (5–7 °C) was investigated to suppress crosslinking. Using reduced temperature conditions, sulfonated copolymers typically precipitated out of solution within 15 min. The reaction was continued for an additional 15 min to ensure completion of the sulfonation reaction. Even under these milder conditions, it was important to avoid extended sulfonation reaction time (>40 min), due to the possibility of crosslinking. It has been reported that strong sulfonation reagents such as chlorosulfonic acid have a tendency to cause side reactions, including crosslinking and polymer chain degradation.39 In the present work, there was no evidence of chain degradation occurring under these conditions, as indicated by viscosity measurements (Table 1) and the mechanical properties of the resulting sulfonated copolymer membranes. The sulfonated products 3 were isolated as white powders, which were fully soluble only in DMSO and showed partial solubility in common polar aprotic solvents (DMF, DMAc and NMP), resulting in opaque solutions.
Fig. 1 shows the 1H NMR spectrum of 3(X70) in the proton form. Comparison of sulfonated 3(X70) with the parent non-sulfonated copolymer 2(X70) reveals that the signals assigned to the non-sulfonated pendent phenyl groups of P3O (H3, H4) disappeared, while the other aromatic protons (H5, H6, H7, H8) remained after the sulfonation reaction. A new signal assigned to the P3O sulfonated pendent phenyl groups appeared at 7.66 ppm. The absence of the H4 signal is indicative of complete sulfonation (100% of degree of sulfonation - DS) of the P3O pendent phenyl groups. The integration ratio of H1 to either H2 or H3 in P3O is very close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, suggesting that substitution occurred at the para position of the pendent phenyl substituents, which has been similarly observed in sulfonated poly(arlyene ether sulfone)s.40,41 Further evidence for pendent phenyl sulfonationvs. main-chain sulfonation is provided by the H1 signal at ∼6.28 ppm. The chemical shift is very similar for the non-sulfonated and sulfonated P3O, as would be expected for pendent phenyl sulfonation. Main-chain sulfonation would result in a noticeable shift of the H1 signal,40 which was not observed. This pendent-phenyl sulfonated P3O is different from a previous sulfonated P3O,42,43 in which sulfonation was reported to occur exclusively on the main-chain of P3O, unless the P3O was first brominated. A possible explanation for this is the mild sulfonation conditions employed in the present work and the unique triblock architecture of 3; an additional detailed study would be needed to confirm this. The 1
1, suggesting that substitution occurred at the para position of the pendent phenyl substituents, which has been similarly observed in sulfonated poly(arlyene ether sulfone)s.40,41 Further evidence for pendent phenyl sulfonationvs. main-chain sulfonation is provided by the H1 signal at ∼6.28 ppm. The chemical shift is very similar for the non-sulfonated and sulfonated P3O, as would be expected for pendent phenyl sulfonation. Main-chain sulfonation would result in a noticeable shift of the H1 signal,40 which was not observed. This pendent-phenyl sulfonated P3O is different from a previous sulfonated P3O,42,43 in which sulfonation was reported to occur exclusively on the main-chain of P3O, unless the P3O was first brominated. A possible explanation for this is the mild sulfonation conditions employed in the present work and the unique triblock architecture of 3; an additional detailed study would be needed to confirm this. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 integration ratio of H6 to H8 indicates the absence of sulfonation in the non-P3O mid-blocks, because of the electron withdrawing effect of –CF3 and –SO2– groups.41 Moreover, these results further confirm the formation of triblock copolymers rather than the blend, since the sulfonated P3O oligomers could be dissolved readily in water. The IEC values of 3 were readily calculated by comparing the integration ratios of the isolated signals H1 and H8. As shown in Table 1, the IEC of 3 was in the range of 0.91 to 1.86 meq./g according to the 1H NMR results, which were consistent with the titration values. Tough and flexible membranes were cast from DMSO solutions of 3 in the sulfonic acid form.
1 integration ratio of H6 to H8 indicates the absence of sulfonation in the non-P3O mid-blocks, because of the electron withdrawing effect of –CF3 and –SO2– groups.41 Moreover, these results further confirm the formation of triblock copolymers rather than the blend, since the sulfonated P3O oligomers could be dissolved readily in water. The IEC values of 3 were readily calculated by comparing the integration ratios of the isolated signals H1 and H8. As shown in Table 1, the IEC of 3 was in the range of 0.91 to 1.86 meq./g according to the 1H NMR results, which were consistent with the titration values. Tough and flexible membranes were cast from DMSO solutions of 3 in the sulfonic acid form.
Morphological structures
The hydrophilic-hydrophobic nanophase separation morphology is particularly important for PEM materials because it affects the water uptake and the proton transport pathway in the ionomer membranes. As an example, the morphology of triblock copolymer 3(X70) was investigated by tapping mode atomic force microscopy (AFM) and transmission electron microscopy (TEM). It has been shown that water adsorbed on the surface of a sample increases adhesive forces between the tip and sample.44 This causes energy dissipation which results in a phase lag between the cantilever's oscillation and the initial oscillation imparted by the piezoelectric actuator. In our case, the sulfonic acid groups on the 3(X70) membrane with an IEC value of 1.28 meq./g adsorb water, resulting in an increased phase lag. Consequently, the ionic domains of the films appear darker in the AFM phase images while the nonionic domains appear brighter. As can be seen in Fig. 2a, the phase image exhibits a clear hydrophilic/hydrophobic phase separation with the hydrophilic nanochannels in the size range of 10–15 nm.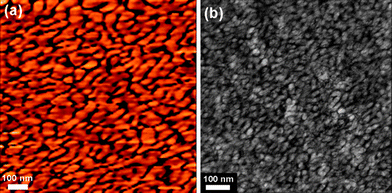 | ||
| Fig. 2 (a) AFM tapping phase image of surface, and (b) TEM image of cross-section for 3(X70) with IEC of 1.28 meq./g. | ||
A similar behavior was also observed by investigating cross-sectional morphology of 3(X70) membranes stained with lead ions. As shown in Fig. 2b, the dark areas of TEM images correspond to the hydrophilic P3O end-capping chains while the bright domains represent the hydrophobic PAES blocks. A wormlike and interconnected hydrophilic network of small ionic clusters of 5–10 nm in size were observed, similar to Nafion®, which has a ‘cluster-network’ morphology composed of ∼5 to 10 nm ionic clusters interconnected by narrow ionic nanochannels.45,46 Of considerable significance is that there is little evidence for dead end channels or larger spheroidal clusters. Moreover, small-angle X-ray scattering (SAXS) was applied to analyze the hydrophilic clusters of 3(X70) triblock copolymer membrane, as shown in Fig. 3. In general, the characteristic separation lengths between the ion-rich domains in the hydrophobic polymer-rich domains in ionomers can be observed in terms of the values of q corresponding to the so-called ionomer peak. Triblock copolymer 3(X70) showed a distinct peak at 0.14 nm−1, and a less distinct peak at ∼0.28 nm−1, suggesting longer-distance order and the lamellar microphase separation structure, as shown in Fig. 3. The value of d for 3(X70) membrane, calculated from d = 2π/q, was 45 nm, which is in good agreement with the TEM results, but much larger than that of Nafion.47 This large d and unique phase-separated structure likely originate from the triblock copolymer structure, which facilitates phase separation between hydrophilic and hydrophobic aggregates to form nanochannels, and is expected to provide a nanochannel pathway for efficient proton-transport. The morphological considerations will be further discussed below with water uptake and proton conductivity properties.
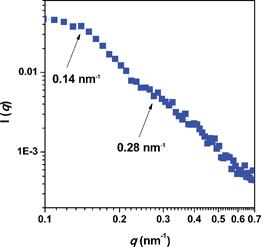 | ||
| Fig. 3 Small angle X-ray scattering (SAXS) of 3(X70) membrane. | ||
Water behavior of ABA triblock copolymer membranes
Table 2 compares the density, IEC, and water uptake (weight and volume based) of 3 and Nafion membranes. As expected, higher IEC membranes absorbed more water due to the increased hydrophilicity. The water uptake of the 3membranes with IEC values in the range of 0.97 to 1.83 meq./g was 47.4–91.2% at 20 °C in water. However, similar values of λ (the number of H2O molecules per sulfonic acid group) were obtained for all the membranes (Table 2). Each sulfonic acid group was solvated by approximately 26 water molecules, a value much higher than that of Nafion 112 (λ = 12.0). The higher water uptake could be expected to be beneficial for proton transport.| samples | density (g/cm3) | IEC (meq./g) | IECv (meq./cm3) | water uptake | conductivity | ||||
|---|---|---|---|---|---|---|---|---|---|
| dry | wet | wt % | λ | vol % | mS/cm | D σ (cm2/S) | |||
| 3(X100) | 1.39 | 0.97 | 1.35 | 0.81 | 47.4 | 27.1 | 65.9 | 130 | 4.2 × 10−5 |
| 3(X70) | 1.43 | 1.28 | 1.83 | 0.97 | 61.5 | 26.7 | 87.9 | 170 | 4.6 × 10−5 |
| 3(X50) | 1.50 | 1.83 | 2.74 | 1.16 | 91.2 | 27.7 | 136.8 | 190 | 4.3 × 10−5 |
| Nafion 112 | 1.98 | 0.90 | 1.78 | 1.29 | 19.3 | 12.0 | 37.6 | 90 | 1.8 × 10−5 |
Dimensional stability of 3membranes was also evaluated by the water swelling ratio, which is defined as increased length or thickness of swollen membranes divided by the dimension of dry membranes. The 3membranes showed strongly anisotropic swelling behavior, with larger dimensional change in the through-plane (thickness) direction than in the in-plane direction (Fig. 4). This is significant in terms of fabricating a membrane electrode assembly (MEA) from a PEM, since it is important that the in-plane swelling is restricted to prevent delamination of the catalyst layer occurring from a dimensional mismatch between the two systems. For example, 3(X100) membrane showed 32% swelling ratio in the through-plane direction, in contrast to only 5% in the in-plane direction. Other samples showed a similar tendency, which was in accordance with the behavior reported for multiblock sulfonated copolymers.48–51 However, unlike the previously reported random or multiblock copolymers,48–52 in which higher temperature induced excessive swelling, the temperature had less of an influence on the water uptake and dimensional swelling of such ABA triblock copolymer membranes, as shown in Fig. 5. Using the 3(X50) membrane (IEC = 1.83 meq./g) as an example, the water uptake of 125% and swelling ratio of 24% at 100 °C was not excessively higher than the corresponding values at 20 °C (91.2% water uptake and 16% swelling ratio), especially when compared with other copolymer systems at this temperature difference.52 Although each sulfonic acid group was solvated by about 26 water molecules, at reduced relative humidity and elevated temperature, a similar but higher water uptake tendency compared with Nafion 112 was observed (Fig. 6a). These overall results demonstrate that triblock copolymer structures, while containing a high amount of water, were effective in preventing excessive water swelling, even at elevated temperatures (>80 °C). The morphological structure with well-connected hydrophilic nanochannels is believed to be responsible for the lower swelling ratio: the formation of small nanochannels allows for a more continuous and cohesive hydrophobic matrix that opposes the increasing osmotic pressure induced by increasing temperature.
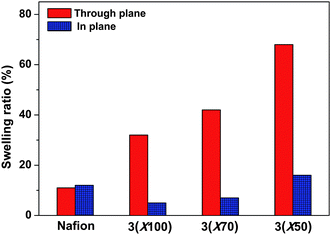 | ||
| Fig. 4 Comparison of dimensional swelling data for 3 and Nafion membranes at room temperature in water. | ||
![(a) The water uptake and (b) swelling ratio in-plane direction dependence of temperature in water. (The data of S2-70 from ref. [52]).](/image/article/2012/EE/c1ee02556b/c1ee02556b-f5.gif) | ||
| Fig. 5 (a) The water uptake and (b) swelling ratio in-plane direction dependence of temperature in water. (The data of S2-70 from ref. [52]). | ||
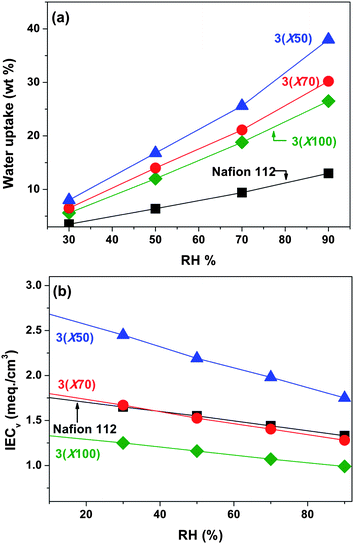 | ||
| Fig. 6 (a) Water uptake and (b) volumetric IECv for 3membranes and Nafion 112 as a function of relative humidity at 90 °C. | ||
For a more realistic comparison of the water uptake among the membranes, volumetric IEC (IECv, meq./cm3) that is defined as molar concentration of sulfonic acid groups per unit volume containing absorbed water, was calculated. The IECv (wet) reflects the concentration of ions within the polymer matrix under hydrated conditions. The IECv (wet) of 3membranes, measured at 20 °C, increased from 0.81 to 1.16 meq./cm3, corresponding to IEC values increasing from 0.97 to 1.83 meq./g. All the values were lower than that of Nafion 112 under the same testing conditions (IECv(wet)=1.29 meq./cm3). The increased sulfonic acid group concentration of the dry 3membrane was retained after equilibration with water, and thus lower IECv (wet) values of 3membranes in water. In contrast, the IECv (wet) of 3(X50) membrane at reduced relative humidity is higher than that of Nafion 112 at all RHs investigated, in spite of their higher water uptake, which is the result of their relatively higher gravimetric IECw. As shown in Fig. 6b, the IECv values became lower with increasing humidity due to increased water volume within the polymer matrix. Nafion 112 and 3(X70) membrane showed approximately the same IECv values throughout the measured range of relative humidity, since the differences in their gravimetric IEC were counterbalanced by the differences in their density of 1.98 g/cm3 for Nafion and 1.43 g/cm3 for 3(X70).
Proton transport in ABA triblock copolymer membranes
Proton conductivity for 3membranes in liquid water at 20 °C was determined and the values are listed in Table 2. Compared to Nafion, all of the 3membranes displayed higher proton conductivities. With increasing IEC values from 0.97 to 1.83 meq./g, proton conductivities of 3membranes increased from 0.13 to 0.19 S/cm at 20 °C in water. These values are much higher than the Nafion 112 membrane (0.09 S/cm, at 20 °C). To further elucidate the proton-conducting properties of the triblock copolymer membranes, the proton diffusion coefficients (Dσ) through the membranes were estimated from the proton conductivity and the IECv(wet). As shown in Table 2, the proton diffusion coefficients (Dσ) through the triblock 3membranes are two times higher than that of Nafion 112, in spite of their lower IECv(wet) values. It is assumed that the triblock polymer structure and highly sulfonated pendent sulfonic acid groups influence the size and shape of the hydrophilic ionic domains through which proton transport occurs. The morphological transition occurring over the triblock-shaped regime decreased the morphological barrier, thereby resulting in the formation of effective nanochannels for proton transport, as observed by AFM and TEM above, which results in the high Dσ values, and thus higher proton conductivities of 3membranes. The proton conductivity over the 20–100 °C range in water was studied and the conductivity values are reported in Fig. 7. The 3membranes show qualitatively Arrhenius-type increases in conductivity with temperature. Higher temperatures increase the conductivity due to the enhanced charge transport. The experimental evidence revealed that the proton conductivity displays a remarkably stable behaviour, with values above 2 × 10−1 S/cm even at 100 °C – the temperature at which water evaporation dramatically affects the hydration of Nafion membrane.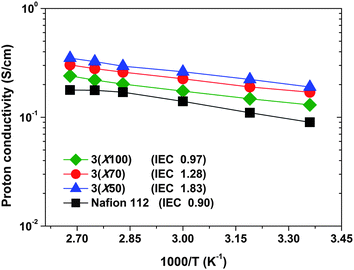 | ||
| Fig. 7 Proton conductivity of 3membranes under fully hydrated state (in water) as a function of temperature. | ||
Furthermore, the humidity dependence of proton conductivity was measured for 3 and Nafion 112 membranes at 90 °C. Surprisingly, we observed high conductivity values (∼10−2 to >10−1 S/cm) for 3membranes over a large range of 30–90% relative humidity (Fig. 8a); values were higher or similar in comparison to that of Nafion, even at the lowest IEC values. 3(X100) with the IEC value of only 0.97 meq./g had a proton-conductivity value of 0.9 × 10−2 S/cm at 30% relative humidity. If the density of 3(X100) (1.39 g/cm3) and Nafion (1.98 g/cm3) are taken into account, the volumetric IEC value of 1.35 meq./cm3 for 3(X100) is much lower than that of Nafion (1.78 meq./cm3). Thus, the well-connected hydrophilic proton conducting nanochannel morphology in the triblock copolymers contributes strongly to the high proton conductivity. To further explore the behaviour of the triblock copolymers, we monitored the proton-conductivity values at 30% relative humidity over a period of 24 h. Fig. 8b shows that the proton conductivity is nearly constant over the monitoring period.
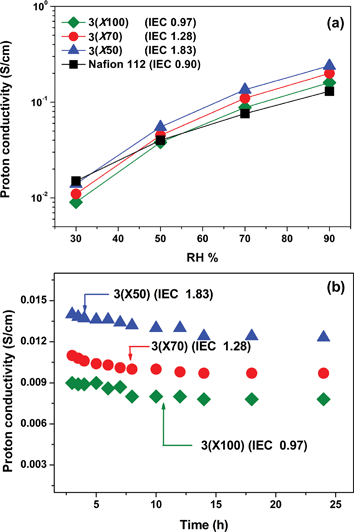 | ||
| Fig. 8 (a) Proton conductivity of 3membranes (a) as a function of RH at 90 °C, (b) as a function of test time at 30% RH and 90 °C. | ||
Fig. 9 compares the proton diffusion coefficients (Dσ) as a function of IECv at reduced relative humidity. The Nafion region for Dσ is from 2.67 × 10−6 to 2.62 × 10−5 cm2/s, which is slightly narrower than that of the triblock copolymer membranes. The wider range of Dσ for the triblock 3membranes implies that they are more dependent on relative humidity, probably because of their weaker acidity and different morphological structures, in common with most aromatic ionomers but to a much lesser extent. At 90% RH, which corresponds to the uppermost data point for each membrane sample, the Dσ values were higher than that of Nafion 112. Therefore, the 3membranes have higher proton conductivity than Nafion at high RH values. Even the 3(X100) membrane showed a higher Dσ value at 90% RH, in spite of having a similar IECv(dry) to Nafion. In addition, the triblock 3membranes still displayed relatively good Dσ values of about 2.0 × 10−6 cm2/s even at 30% RH, which was comparable to that of Nafion 112 (2.67 × 10−6 cm2/s) and much higher than those of previously reported segmented or multiblock copolymer membranes.53 The results are congruent with the above-mentioned morphological data and validate our strategy of fully aromatic triblock copolymers with highly sulfonated blocks having pendent sulfonic acid groups for highly proton conductive ionomer membranes.
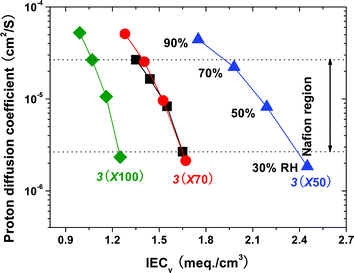 | ||
| Fig. 9 Proton diffusion coefficients of 3 and Nafion 112 membranes as a function of volumetric IECv at 90 °C. | ||
Stability and mechanical properties
As shown in Table 3, the 3membranes showed good thermal, oxidative and hydrolytic stabilities, The 5% weight loss temperature of 3membranes was approximately 300 °C according to the TGA results, which was much higher than block copolymers containing polystyrene sulfonic acid (about 200 °C).16 The Fenton test at 80 °C for 1 h did not dissolve all of 3membranes. As expected, the 3membranes showed a higher residual weight, which increased with decreasing IEC. The inherent viscosity of the residues evaluated was more than 50% of the original inherent viscosity. The slower degradation for lower IEC membranes is reasonable due to the lower water uptake and accordingly lower diffusion of peroxide and the derived radical species into the material. Compared to previously reported multiblock aromatic copolymers, which suffered significant weight loss in the Fenton test,48,53 the triblock 3membranes were more stable to oxidation. Although sulfonated P3O has previously been reported to exhibit instability in extended fuel cell tests of 500 h,43 the structure of those tested SP3O was found to be main-chain sulfonated42,43,54 rather than the present pendent-phenyl sulfonated, possibly due to different reaction conditions being used. The present triblock copolymers based on SP3O appear to be at least as stable as other aromatic PEMs, probably because pendent-phenyl sulfonated aromatic polymers have been demonstrated to have a more stable structure than main-chain sulfonated PEMs.40| samples | IEC (meq./g) | oxidative stability a (% decrease) | water stability b (% decrease) | T d 5%(°C) | ||
|---|---|---|---|---|---|---|
| weight | ηinh | weight | ηinh | |||
| a In Fenton's reagent (3% H2O2 containing 2 ppm FeSO4) at 80 °C for 1 h. b Accelerated hydrolytic test in 140 °C water for 24 h. c Decrease in molecular weight by GPC. | ||||||
| 3(X100) | 0.97 | 16 | 27 | 0 | 0 | 332 |
| 3(X70) | 1.28 | 28 | 43 | 0 | 0 | 328 |
| 3(X50) | 1.83 | 46 | 53 | 25 | 0 | 310 |
| SPAESK 19 | 1.62 | 60 | -- | 0 | -- | -- |
| SPAESK 53 | 1.74 | 81 | 79c | 0 | 0c | -- |
In the hydrolytic stability test, the 3(X100) and 3(X70) membranes with lower IEC values did not show any losses in weight and inherent viscosity after the accelerated hydrolysis testing at 140 °C for 24 h. Although the 3(X50) partially dissolved in water at 140 °C, the residue retained its original inherent viscosity, indicating no observable hydrolytic degradation. The 3membranes do not degrade under these severe hydrolytic conditions, similar to previous multiblock48,53 and comb-shaped copolymers.37
The mechanical property stress vs. strain curves were affected by the length of poly(arylene ether sulfone) block because of the differences in molecular weight (Fig. 10). The 3membranes at ambient conditions had tensile stress in the range of 19.7–34.4 MPa and elongation at break values of 18.7–46.2%, with the higher IEC values having lower tensile stress. The mechanical properties did not match those of some of our previously reported ionomers.52 We attribute this to the hydrophilic water-containing domains formed by the proton-conducting blocks that segregate the hydrophobic main chain, resulting in weaker physical interactions between hydrophobic blocks and lower mechanical strength of the membranes. In addition, the triblock membranes did not show any peaks after initial elongation, which is often observed in the random or other block copolymer membranes.53 Since this behavior is regarded as the onset of disentanglement of bundles in the hydrophobic components, the results support the idea that the hydrophobic interaction is less strong in the triblock copolymer membranes.
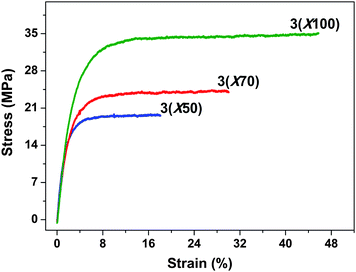 | ||
| Fig. 10 Stress vs. strain curves of triblock copolymer 3membranes at room temperature and 50% RH. | ||
Conclusions
A novel class of highly proton-conducting fully aromatic ABA triblock copolymers was synthesized for the first time by exploiting mono-phenoxide-terminated P3O oligomer end-capped onto poly(arylene ether sulfone). The resulting copolymers were subsequently post-treated to obtain sulfonated triblock copolymers containing highly sulfonated blocks, and the degree of sulfonation of the P3O phenyl substituents was almost 100%. Although one of the copolymer membranes 3(X100) had a low IEC value (IEC = 0.97 meq./g), it maintained good proton conductivity comparable to Nafion 112, even at low relative humidity values (30–50% RH). It appears that the unique polymer architecture brought about by nanophase separation between the extreme opposing hydrophobic and hydrophilic domains is responsible for the high proton diffusion coefficients, and thus high proton conductivities, throughout a wide range of relative humidity conditions. The membranes also exhibit strongly anisotropic swelling behaviour, with low in-plane swelling. The results suggest that careful consideration of polymer architecture and nanoscale morphology is a key element in the design of efficient PEMs. The combination of comparatively good thermal and oxidative stabilities, mechanical properties and excellent proton conductivity makes aromatic triblock copolymer membranes attractive as PEM materials for further study in fuel cell applications.Additionally, the PPO end-capped platform is versatile because it can be prepared to contain either methyl or aryl groups, which may be further modified for various applications. For example, PPO methyl groups can be modified by bromination, followed by quaternary ammonium or ATRP for water treatment or anion exchange membrane applications. Further investigations on this class of copolymers are ongoing in our laboratory.
Acknowledgements
This research was supported by the WCU (World Class University) program, National Research Foundation (NRF) of the Korean Ministry of Science and Technology (No.R31-2008-000-10092-0), which we gratefully acknowledge. NRCC publication number 53004.References
- M. Z. Jacobson, W. G. Colella and D. M. Golden, Science, 2005, 308, 1901 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345 CrossRef CAS.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637 CrossRef CAS.
- K. D. Kreuer, Chem. Mater., 1996, 8, 610 CrossRef CAS.
- K. D. Kreuer, A. Rabenau and W. Weppner, Angew. Chem., Int. Ed. Engl., 1982, 21, 208 CrossRef.
- N. Agmon, Chem. Phys. Lett., 1995, 244, 456 CrossRef CAS.
- K. D. Kreuer, Solid State Ionics, 2000, 136–137, 149 CrossRef CAS.
- T. Dippel and K. D. Kreuer, Solid State Ionics, 1991, 46, 3 CrossRef CAS.
- Y. Sone, P. Ekdunge and D. Simonsson, J. Electrochem. Soc., 1996, 143, 1254 CrossRef CAS.
- M. Schuster, T. Rager, A. Noda, K. D. Kreuer and J. Maier, Fuel Cells, 2005, 5, 355 CrossRef CAS.
- H. Steininger, M. Schuster, K. D. Kreuer, A. Kaltbeitzel, B. Bingöl, W. H. Meyer, S. Schauff, G. Brunklaus, J. Maier and H. W. Spiess, Phys. Chem. Chem. Phys., 2007, 9, 1764 RSC.
- T. A. Zawodzinski, M. Neeman, L. O. Sillerud and S. Gottesfeld, J. Phys. Chem., 1991, 95, 6040 CrossRef CAS.
- C. H. Park, C. H. Lee, M. D. Guiver and Y. M. Lee, Prog. Polym. Sci., 2011, 36, 1443 CrossRef CAS.
- R. Devanathan, Energy Environ. Sci., 2008, 1, 101 CAS.
- J. M. Spurgeon, M. G. Walter, J. Zhou, P. A. Kohl and N. S. Lewis, Energy Environ. Sci., 2011, 4, 1772 CAS.
- R. Subbaraman, H. Ghassemi and T. A. Zawodzinski, J. Am. Chem. Soc., 2007, 129, 2238 CrossRef CAS.
- Y. Yang and S. Holdcroft, Fuel Cells, 2005, 5, 171 CrossRef CAS.
- T. J. Peckham and S. Holdcroft, Adv. Mater., 2010, 22, 4667 CrossRef CAS.
- A. E. Yossef and A. H. Michael, Macromolecules, 2011, 44, 1 CrossRef.
- C. J. Hawker, J. Am. Chem. Soc., 1994, 116, 11185 CrossRef CAS.
- V. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro and S. Sahoo, J. Am. Chem. Soc., 2006, 128, 14156 CrossRef CAS.
- I. W. Hamley, The Physics of Block Copolymers; Oxford University Press: New York, 1998 Search PubMed.
- T. A. Kim and W. H. Jo, Chem. Mater., 2010, 22, 3646 CrossRef CAS.
- K. Xu, K. Li, P. Khanchaitit and Q. Wang, Chem. Mater., 2007, 19, 5937 CrossRef CAS.
- J. Gao, Y. Yang, D. Lee, S. Holdcroft and B. J. Frisken, Macromolecules, 2006, 39, 8060 CrossRef CAS.
- G. Dorenbos and K. Morohoshi, Energy Environ. Sci., 2010, 3, 1326 CAS.
- M. J. Park, K. H. Downing, A. Jackson, E. D. Gomez, A. M. Minor, D. Cookson, A. Z. Weber and N. P. Balsara, Nano Lett., 2007, 7, 3547 CrossRef CAS.
- T. Saito, H. D. Moore and M. A. Hickner, Macromolecules, 2010, 43, 599 CrossRef CAS.
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K. I. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904 CrossRef CAS.
- D. S. Kim, Y. S. Kim, M. D. Guiver, J. Ding and B. S. Pivovar, J. Power Sources, 2008, 182, 100 CrossRef CAS.
- A. S. Hay, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 505 CrossRef CAS.
- T. Xu, D. Wu and L. Wu, Prog. Polym. Sci., 2008, 33, 894 CrossRef CAS.
- K. P. Chan, D. S. Argyropoulos, D. M. White, G. W. Yeagers and A. S. Hay, Macromolecules, 1994, 27, 6371 CrossRef CAS.
- A. S. Hay, Macromolecules, 1969, 2, 107 CrossRef CAS.
- K. Mühlbach and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 1987, 25, 2605 CrossRef.
- K. Saito, T. Tago, T. Masuyama and H. Nishide, Angew. Chem., Int. Ed., 2004, 43, 730 CrossRef CAS.
- N. Li, C. Wang, S. Y. Lee, C. H. Park, Y. M. Lee and M. D. Guiver, Angew. Chem., Int. Ed., 2011, 50, 9158 CrossRef CAS.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft and A. S. Hay, Macromolecules, 2008, 41, 281 CrossRef CAS.
- A. Linkous, H. R. Anderson, R. W. Kopitzke and G. L. Nelson, Int. J. Hydrogen Energy, 1998, 23, 525 CrossRef.
- B. Liu, G. P. Robertson, D. S. Kim, X. Sun, Z. Jiang and M. D. Guiver, Polymer, 2010, 51, 403 CrossRef CAS.
- K. Matsumoto, T. Higashihara and M. Ueda, Macromolecules, 2009, 42, 1161 CrossRef CAS.
- R. B. Hodgdon, S. Hamilton, A. S. Hay, US patent: 3528858, 1967.
- A. E. Steck, C. Stone, in 2nd Int. Symp. New Materials for Fuel Cell Systems ed. O. Savadogo, P. R. Roberge, T. N. Veziroglu, pp. 792–807, 1997. Montreal: Ecole Polytechnique Search PubMed.
- P. J. James, M. Antognozzi, J. Tamayo, T. J. McMaster, J. M. Newton and M. J. Miles, Langmuir, 2001, 17, 349 CrossRef CAS.
- W. Y. Hsu and T. D. Gierke, Macromolecules, 1982, 15, 101 CrossRef CAS.
- L. Rubatat, A. L. Rollet, G. Gebel and O. Diat, Macromolecules, 2002, 35, 4050 CrossRef CAS.
- G. Gebel, Polymer, 2000, 41, 5829 CrossRef CAS.
- B. Bae, T. Yoda, K. Miyatake, H. Uchida and M. Watanabe, Angew. Chem., Int. Ed., 2010, 49, 317 CrossRef CAS.
- A. S. Badami, A. Roy, H. S. Lee, Y. Li and J. E. McGrath, J. Membr. Sci., 2009, 328, 156 CrossRef CAS.
- H. S. Lee, A. Roy, O. Lane, S. Dunn and J. E. McGrath, Polymer, 2008, 49, 715 CrossRef CAS.
- K. Nakabayashi, T. Higashihara and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2757 CrossRef CAS.
- N. Li, D. W. Shin, D. S. Hwang, Y. M. Lee and M. D. Guiver, Macromolecules, 2010, 43, 9810 CrossRef CAS.
- B. Bae, K. Miyatake and M. Watanabe, Macromolecules, 2010, 43, 2684 CrossRef CAS.
- A. S. Hay, Prog. Polym. Sci., 1999, 24, 45 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |