Vapor phase pH induced fluorescence switching of a dimethylaminostyryl terpyridine derivative in thin films†
Srivathsa
Vaidya
* and
Russell H.
Schmehl
Department of Chemistry, Tulane University, New Orleans, LA 70118, USA. E-mail: sri_chemistry@yahoo.com
First published on 23rd November 2011
Abstract
The luminescence of thin film polymer matrices of polystyrene (PS) containing the sensing molecule dimethylaminostyryl terpyridine derivative (MNpvpt) is affected upon exposure to vapors of organic acids and bases. Fluorescence changes are due to protonation and deprotonation of MNpvpt and control of intramolecular charge transfer, leading to a molecular optical switch.
The manipulation of molecular excited-state luminescence is a particularly compelling approach to signal transduction in many chemical sensing schemes.1–5 In recent years considerable effort has been dedicated to the development of gas sensors for either medical or environmental health purposes.6–12
The development of compounds that reversibly interchange between different forms characterised by clearly detectable signals is of great interest in fundamental and applied research.13–18 There have been numerous investigations on luminescence based sensors for determining the pH of solutions,19–21 very often employing chromophores based on intramolecular charge transfer transitions (ICT).22–26 Fluorescence sensors have also been developed for detection of volatile organics.1,8,13,14,27 We have reported the photophysical properties of a dimethylaminostyryl terpyridine derivative (MNpvpt, Fig. 1) that shows large emission solvatochromism upon exposure to solvent vapors.28
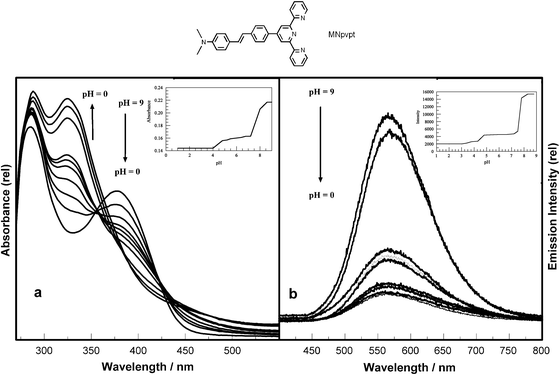 | ||
| Fig. 1 Effect of pH on the (a) absorption and (b) emission spectra of MNpvpt (λex @ 355 nm). Inset (a) shows the plot of absorbance at 380 nm versus pH 0–10 and (b) shows emission intensity at 562 nm versus pH. | ||
In this paper we show that MNpvpt exhibits switching behavior regulated by vapor phase acid–base chemical control of ICT fluorescence. The chromophore is embedded in polystyrene films and the success of the system is in large part due to the ability of gases to diffuse through the polymer matrix and interact with the intercalated sensing agent, inducing a measurable response very rapidly. The pH changes between the ground and excited states observed are consistent with changes anticipated for an intramolecular charge transfer excited state and the chromophore is able to distinguish between bases with pKa values in the 5–7 range from those that are considerably more basic due to the existence of two independent protonatable sites on the molecule that have unique fluorescence properties.
The chromophore was synthesized by Wittig coupling of a terpyridyl containing ylide with p-dimethylaminobenzaldehyde following a published procedure.28,29 The complex is strongly luminescent in most solvents and the luminescence is highly solvatochromic. The compound also has four basic nitrogen centers; the three pyridyl components of the terpyridyl have pKa values of 5 or lower for the protonated pyridines. The protonated dimethylanilino nitrogen has a much higher pKa value. Fig. 1 shows the effect of pH on the UV-visible absorption and emission spectra of MNpvpt in CHCl3. Two sequential isosbestic points are observed in absorption spectra at 350 nm and 430 nm indicating that two distinct protonation steps are observed. While there are four species that will coexist to varying degrees in solution below pH 5, the net result is only two spectrophotometrically unique species. On increasing the acidity of the solution from pH 8 to 1 (using triethylamine/acetic acid buffer for higher pH's and trifluoroacetic acid/morpholine for lower pH's) it was observed that the absorption maximum shifts from 380 nm to 330 nm and the emission maximum shifts from 562 nm to 552 nm.
This increase in energy of the luminescence maximum results from protonation of the dimethylamino nitrogen, eliminating the ICT transition and leaving a higher energy styryl terpyridine localized π → π* transition. Protonation of the terpyridyl nitrogens occurs upon lowering the pH further as shown in Scheme 1. The changes in emission intensity are also explained by a decrease in donor capacity upon protonation of dimethylamino nitrogen, eliminating the ICT transition. Further protonation of the terpyridyl nitrogens leads to a further decrease in the emission intensity. The ground state pKa values were obtained from absorption data and excited state pKa's were calculated30,31 from luminescence spectral data and luminescence lifetimes (eqn (1)) and are shown in Table 1.
| pH = pKa* − log[τprotonated/τdeprotonated] | (1) |
To further examine the role of dimethylamino substitution on the absorption and emission behavior, the chromophore without the dimethylamino group, pvpt, was synthesized. Fig. 2 shows that upon lowering the pH from 8 to 1 the emission was slightly blue shifted with a decrease in emission intensity; the absorption spectrum was not much affected. Note that the maximum of the absorption of the MNpvpt decreases to around 330 nm following protonation of the dimethylanilino nitrogen, indicating that the π–π* transitions of pvpt and MNpvpt are electronically very similar and supporting the assignment of the longer wavelength absorption of MNpvpt as a dimethylamino to terpyridine charge transfer transition. Upon comparison of ground and excited state pKa's of MNpvpt with pvpt as shown in Table 1 it is evident that the pKa around 4.2 and 5.0 corresponds to the average ground state and excited state pKa's of the terpyridine nitrogens; these values match with the literature results.32,33
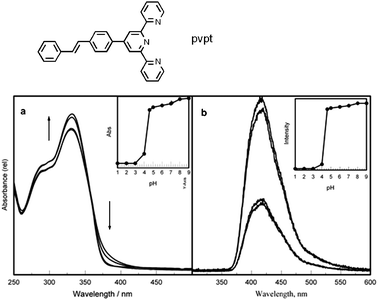 | ||
| Fig. 2 Effect of pH on the (a) absorption and (b) emission spectra of pvpt (λex @ 360 nm). Inset (a) shows the plot of absorbance at 330 nm versus pH and (b) shows the plot of emission intensity at 410 nm versus pH. | ||
| Property | MNpvpt | pvpt |
|---|---|---|
| pH = 8 | ||
| Abs λmax, nm | 280, 380 | 280, 330 |
| Emiss λmax, nm | 562 | 410 |
| Lifetime, ns | 1.76 | 0.26 |
| Excited state pKa | ||
| Pyridyl | 5.05 | 5.01 |
| –N(CH3)2 | 7.54 | — |
| Ground state pKa | ||
| Pyridyl | 4.5 | 4.25 |
| –N(CH3)2 | 7.8 | — |
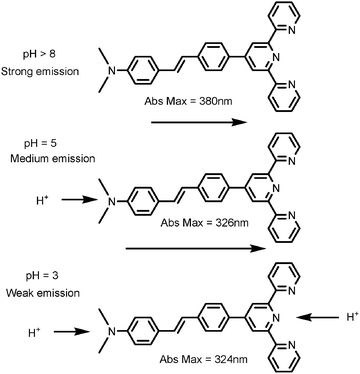 | ||
| Scheme 1 Successive protonation of MNpvpt. | ||
The data show that the excited state pKa* value for terpyridine nitrogens in both MNpvpt and pvpt is higher than the ground state pKa value, indicating some degree of charge transfer to the terpyridyl moiety in the excited state. In MNpvpt the excited state pKa* for the dimethylamino group is lower than the ground state pKa value, suggesting that in the excited state the electron density is lower on dimethylamino compared to its ground state, consistent with the excited state having dimethylamino to terpyridine charge transfer character. Based on the work reported earlier on fluorescence solvatochromism of MNpvpt, the difference between the ground and excited state dipoles is estimated to be 12.2 D from correlation of the emission maximum with the solvent optical and static dielectric constants.28
The chromophore MNpvpt was introduced as a guest in polystyrene (PS) host matrices and thin films of these polymer/chromophore complexes were coated on quartz. These films were placed in fluorescence cells saturated with trifluoroacetic acid vapor (TFAv) by simply injecting 20 μl of TFA into a small piece (2 mm diameter on average) of cotton gauze placed in the cell. When the quartz slide coated with the polymer-encapsulated chromophore is introduced into the cell saturated with TFA vapor the emission of the film is completely quenched in 1–2 min (Fig. 3), presumably because of protonation of the MNpvpt at both the dimethylamino and the terpyridyl nitrogen centers. The TFAv exposed film, upon exposure to vapors of NH3, showed a significant increase in emission. In the presence of vapors of a base with a higher pKa value (triethylamine), the emission regained in intensity similar to the original emission. The TFA vapor exposed film showed no increase in emission intensity when exposed to pyridine vapors. These observations, coupled with the pH studies in solution, clearly illustrate that upon exposure to vapors of TFA there is protonation of dimethylamino and terpyridine nitrogens. Further, upon exposure to a base like NH3 the terpyridine nitrogen is deprotonated and vapors of a strong base like TEA deprotonate the dimethylamino nitrogen. It is evident from Fig. 3 that the regained emission maximum is slightly red shifted compared to the original spectrum. It is important to note that this process is reversible at least over the span of 10 cycles.
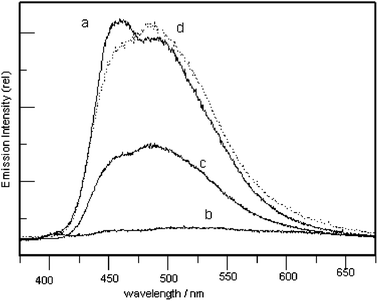 | ||
| Fig. 3 Luminescence of MNpvpt films upon exposure to vapors of (a) air only, (b) TFA, (c) NH3, and (d) N(Et)3. | ||
Upon warming the film to 50 °C the original spectrum was restored. This may reflect the fact that, following addition of base, the film contains ammonium trifluoroacetate salt. Apparently, complexation of the terpyridine could perturb the overall electron-donating ability of the amino group. Such a perturbation on the electron donor is sufficient to effect the energy of the ICT state. Hence, the red shifted maximum could be attributed to a planar intramolecular charge transfer state. Upon warming it is possible that ammonium trifluoroacetate salt releases the amine and acid, restoring the original composition of the film.
In summary we have demonstrated how MNpvpt as a chromophore encapsulated in polystyrene films can be used to sense vapors of volatile organic bases and can distinguish between intermediate (ammonia) and stronger (alkyl amines) bases. The success of the system is mainly due to the ability of gases to diffuse through the polymer matrix and interact with the intercalated sensing agent, inducing a measurable response very rapidly. It can be said that this system translates the change in pH as an optical output because of the initiation of a photophysical process. The fluorescence changes observed in our system are due to protonation and deprotonation of MNpvpt and control of intramolecular charge transfer, leading to a molecular optical switch. This fluorescence system could serve as a potential gas/vapor sensing system if the sensitivity of the polymer encapsulated dye can be improved.
Experimental
All absorption spectra were recorded using a Hewlett Packard 8452 Diode Array Spectrophotometer and the emission spectra were obtained using a Spex Fluorolog Fluorimeter equipped with a CCD detector.Thin film fabrication
Thin films are prepared from CH3Cl solution containing MNpvpt (10−6 M) and PS in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (w/w). Quartz slides (1 cm × 4 cm) were mounted on a stepping motor driven dipper and the solution was coated on 1.5 cm of the length of the slide in a single dip cycle. It yielded uniform films and the thickness measured using profilometry. The surface morphology was examined using AFM (Atomic Force Microscopy). Profilometry and AFM profiles are reported in our previous work.28
25 (w/w). Quartz slides (1 cm × 4 cm) were mounted on a stepping motor driven dipper and the solution was coated on 1.5 cm of the length of the slide in a single dip cycle. It yielded uniform films and the thickness measured using profilometry. The surface morphology was examined using AFM (Atomic Force Microscopy). Profilometry and AFM profiles are reported in our previous work.28
Films prepared by this method were typically 420 ± 10 nm thick in the mid-portion of the film as assessed by profilometry. The dip coating procedure is highly reproducible, and while it also results in formation of a thick region of the film at the bottom of the glass slide, this region is small and well away from the area subjected to luminescence interrogation.
The films were placed in fluorescence cells saturated with acid or base vapor by simply injecting 20 μl of acid or base into a small piece (2 mm diameter on average) of cotton gauze placed in the cell. The quartz slide coated with the polymer-encapsulated chromophore is introduced into the cell saturated with acid or base vapor and the emission of the film is recorded.
Acknowledgements
The authors wish to thank the administrators of the Petroleum Research Fund (PRF# 39792-AC3) of the American Chemical Society for support of this work.References
- L. J. Grove, J. M. Rennekamp, H. Jude and W. B. Connick, J. Am. Chem. Soc., 2004, 126, 1594 CrossRef CAS.
- A. P. de Silva, H. Q. Gunaratne, T. Gunnlaugsson, A. J. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS.
- P. J. Anzenbacher, D. S. Tyson, K. Jursikova and F. N. Castellano, J. Am. Chem. Soc., 2002, 124, 6232 CrossRef CAS.
- M. H. Keefe, K. D. Benkstein and J. T. Hupp, Coord. Chem. Rev., 2000, 205, 201 CrossRef CAS.
- D. T. McQuade, A. E. Pullen and T. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS.
- F. N. Castellano and J. R. Lakowicz, Photochem. Photobiol., 1998, 67, 179 CrossRef CAS.
- C. P. Chang, C. Y. Chao, J. H. Huang, A. K. Li, C. S. Hsu, M. S. Lin, B. R. Hsieh and A. C. Shu, Synth. Met., 2004, 144, 297 CrossRef CAS.
- T. J. Wadas, Q. M. Wang, Y. J. Kim, C. Flaschenreim, T. N. Blanton and R. Eisenberg, J. Am. Chem. Soc., 2004, 126, 16841 CrossRef CAS.
- X. Chen, L. Lin, P. Li, Y. Dai and X. Wang, Anal. Chim. Acta, 2004, 506, 9 CrossRef CAS.
- E. A. Baldauffa and J. M. Buriak, Chem. Commun., 2004, 2028 RSC.
- A. Satsuma, K. I. Shimizu, T. Hattori, H. Nishiyama, S. Kakimoto, S. Sugaya and H. Yokoi, Sens. Actuators, B, 2007, 123, 757 CrossRef.
- G. J. Mohr, S. Draxel, K. Trznadel, F. Lehmann and M. E. Lippitsch, Anal. Chim. Acta, 1998, 360, 119 CrossRef CAS.
- C. A. Daws, C. L. Exstrom, J. R. Sowa and K. R. Mann, Chem. Mater., 1997, 363, 9 Search PubMed.
- M. C. Janzen, J. B. Ponder, D. P. Bailey, C. K. Ingison and K. S. Suslick, Anal. Chem., 2006, 78, 3591 CrossRef CAS.
- J. Tolosa, E. D. Barra, P. S. Verdu and J. R. Lopez, Tetrahedron Lett., 2006, 47, 4647 CrossRef CAS.
- C. Hubert, D. Fichou, P. Valat and F. Garnier, Polymer, 1995, 36, 2663 CrossRef CAS.
- Q. Chang, J. Sipior, J. R. Lakowicz and G. Rao, Anal. Biochem., 1995, 232, 92 CrossRef CAS.
- (a) F. Galindo, J. C. Lima, S. V. Luis, M. J. Melo, A. J. Parola and F. Pina, J. Mater. Chem., 2005, 15, 2840 RSC; (b) E. C. Constable, C. E. Housecroft, A. C. Thompson, P. Passaniti, S. Silvi and M. Maestri, Inorg. Chim. Acta, 2007, 360, 1102 CrossRef CAS.
- K. M. Wong, W. S. Tang, X. X. Lu, N. Zhu and V. W. Yam, Inorg. Chem., 2005, 44, 1492 CrossRef CAS.
- M. Duati, S. Tasca, F. C. Lynch, H. Bohlen and J. G. Vos, Inorg. Chem., 2003, 42, 8377 CrossRef CAS.
- A. P. de Silva and R. D. Rupasinghe, Chem. Commun., 1985, 1670 Search PubMed.
- S. M. Cheung and W. H. Chan, Tetrahedron Lett., 2006, 62, 8379 CAS.
- L. H. Liu, H. Zhang, A. F. Li, J. W. Xie and Y. B. Jiang, Tetrahedron, 2006, 62, 10441 CrossRef CAS.
- J. S. Yang, Y. D. Lin, Y. H. Chang and S. S. Wang, J. Org. Chem., 2005, 70, 6066 CrossRef CAS.
- A. Caballero, R. Martinez, V. Lloveras, I. Ratera, J. V. Gancedo, K. Wurst, A. Tarraga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666 CrossRef CAS.
- Y. Zhao, V. Khodorkovsky, J. Cohen and Z. Priel, J. Photochem. Photobiol., A, 1996, 99, 23 CrossRef CAS.
- J. Chen and Y. Cao, Sens. Actuators, B, 2006, 114, 65 CrossRef.
- S. Vaidya, C. Johnson, X. Y. Wang and R. H. Schmehl, J. Photochem. Photobiol., A, 2007, 187, 258 CrossRef CAS.
- B. Wang and M. R. Wasielewski, J. Am. Chem. Soc., 1997, 119, 12 CrossRef CAS.
- P. J. Giordano, C. R. Bock, M. S. Wrighton, L. V. Interrante and R. F. Williams, J. Am. Chem. Soc., 1977, 3187, 3187 CrossRef.
- P. J. Giordano, C. R. Bock and M. S. Wrighton, J. Am. Chem. Soc., 1978, 100(22), 6960 CrossRef CAS.
- Md. K. Nazeeruddin, S. M. Zakeeruddin, R. H. Baker, T. A. Kaden and M. Gratzel, Inorg. Chem., 2000, 39, 4542 CrossRef CAS.
- K. Y. Kim and G. H. Nancollas, J. Phys. Chem., 1977, 81, 948 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1nj20754g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2012 |
