Seeding approach to noble metal decorated conducting polymer nanofiber network†
Zhen
Liu
,
Selcuk
Poyraz
,
Yang
Liu
and
Xinyu
Zhang
*
Department of Polymer and Fiber Engineering, Auburn University, Auburn, AL 36849, USA. E-mail: xzz0004@auburn.edu; Fax: +1-334-844-4068; Tel: +1-334-844-5439
First published on 11th November 2011
Abstract
Metal displacement reactions between conducting polymers—“synthetic metals”—and noble metals (Pt, Au and Ag) have been demonstrated using a seeding polymerization technique, to produce a synthetic metal nanofiber network decorated with noble metal nanoparticles, in one-step.
We describe a novel metal displacement reaction occurring between “synthetic metals” (conducting polymers) and noble metals, namely gold (Au) and platinum (Pt), using a “nanofiber seeding”1 approach to synthesize, in one step, bulk quantities of the Au or Pt nanoparticles decorated conducting polymer nanofiber network. Pyrrole was seeded by vanadium pentoxide (V2O5) sol–gel nanofibers2 and polymerized by adding noble metal salt aqueous solutions, such as AuCl, HAuCl4·3H2O, PtCl2, and PtCl4. The implementation of V2O5 nanofiber seeds in the metal salt solutions can afford to drastically change the morphology of the as-produced polypyrrole (PPy) from irregular shaped granules to almost exclusively nanofibers. The formation of these PPy Nanofiber–Noble Metal Nanoparticle (PNNMN) composites can be attributed to the seeding effect of V2O5 which could promote the formation of PNNMN. The polymerization induction stage, monitored by an open circuit potential, plays a critical role in determining the morphology of the as-precipitated polymers.3 Due to their seeding and catalytic dual functions, V2O5 nanofibers can be defined as catalytic seeds, which are not only involved in orchestrating the nanofiber formation of polymer nanostructure as a seeding agent, but are also able to exhibit the catalytic capability by raising the overall oxidative potential in metal salts with relatively low oxidation potentials, such as Pt(II)Cl2 and Pt(IV)Cl4.
Organic/inorganic hybrid nanomaterials span a broadband of research and applications, ranging from novel synthesis, such as polymer and metal nanofiber composites,4,5 ultrathin free-standing films of metal oxide–polymer composite,6 unique functional nanodevices, e.g., mesoporous separation membranes of polymer-coated copper hydroxide nanostrands,7 to highly advanced biological sensors6 and chemical sensors.8,9 Among those, dispersed nanostructured noble metals, e.g., both Au and Pt exhibit excellent properties in magnetism,10 plasma resonance,11 and catalysis.12,13 On the other hand, nanostructured conducting polymers such as PPy nanofibers,1nanotubes14 and nanoclips15 possess both superior electronic and mechanical properties,16 which make them ideal candidates for the fabrication of next-generation nano-electronic devices.
There are a number of endeavours to immobilize the nanostructured noble metal nanoparticles onto the conducting polymers matrix, i.e., the utilization of ionic liquid for PPy/Au and PPy/Ag nanocomposites;17 the use of poly(vinyl pyrrolidone) (PVP) to achieve the PPy/Au core–shell nanostructure by interfacial polymerization.18 Furthermore, conducting polymer precursors, such as polyaniline,19polypyrrole14 and polythiophene nanotubes20 were utilized as templates to form coaxial nanocables with encapsulated Co, Ag and Au nanostructures. Porous aluminium oxide was reported as a hard template to assist the formation of an Au-capped, protein-modified polypyrrole nanowire through electrochemical polymerization.21 However the simultaneous control of morphologies of both conducting polymers and noble metals remains a challenge. Our previous discovery of the seeding polymerization technique, among the myriad of others, can offer a facile, one-step approach to achieve concurrent control of morphologies of both conducting polymer and noble metals, without the assistance of any other templates or capping agents. In this seeding approach, a catalytic amount of nanofibrillar V2O5 (1–2 mg) serves as the “seed”, which could not only direct the formation of PPy nanofibers, but also promote the polymerization process by oxidizing the pyrrole monomer into its nanofibril oligomer form.22 As the reactive seeds, the V2O5 nanofibers will react with pyrrole monomers as the noble metal ions do.1 And the nanofibrillar oligomers, formed due to the reaction between pyrrole monomers and V2O5 nanofibers, will further react with the noble metal ions and reduce them to noble metal nanocrystals. This type of reaction can be considered as a novel metal displacement reaction, since the redox reaction has taken place between the synthetic metal (PPy) and its oligomers, and the noble metal ions, which possess a high oxidation potential.
In a typical synthesis, 1–2 mg of V2O5 nanofibers were introduced into 50 mL of aqueous noble metal salt solutions, i.e., AuCl, HAuCl4·3H2O, PtCl2, and PtCl4. Pyrrole monomers were then added into the mixture to initiate the polymerization reaction, after 15 min magnetic stirring. Polymerization reaction will last for 24 h and then followed by filtration and purification steps. Potential–time profiling is monitored and reported in Fig. 2, providing real time monitoring for the oxidative potential change of the reaction mixture, throughout the polymerization process. Since the pyrrole monomer can be polymerized by the noble metal salt ions solely, and as an example, granular PPy was obtained from the oxidation of pyrrole monomer using PtCl2 without V2O5 nanofibers (Fig. 1A). While with the addition of catalytic amount of V2O5 nanofibers as reactive seeds, the as-produced PNNMN composite powders exhibit a homogeneous nanofibrillar network, on which noble metal nanoparticles were deposited. The diameter of the PPy nanofibers in these PNNMN composites is in the range of 15–30 nm (Fig. 1B and C), and the size of nanoparticles of the Au or Pt is around 80–100 nm. Spectroscopically, the PPy nanofibers in the composites are similar to the previously published work.15Elemental analysis of these composites was carried out by Energy-dispersive X-ray (EDX) spectroscopy, which indicates the loading ratio of noble metals is in the range of 35–55 wt% (Table S1†), depending on different types of metals and the initial concentrations of their cations. These EDX data are also consistent with our Thermogravimetric Analysis (TGA) (Fig. S7†) tests of the nanocomposites. It is to be noted that the PPy nanofibers were shortened in the TEM image (Fig. 1D), which is probably due to the ultrasonication step during the sample preparation process (ESI†).
 | ||
| Fig. 1 SEM images: (A) PPy granules oxidized by PtCl2 without V2O5 seeds; (B) PPy/Au nanofiber composites from the V2O5/pyrrole/AuCl system; (C) PPy/Pt nanofiber composites from the V2O5/pyrrole/PtCl4 system; and (D) TEM image of PPy/Pt nanofiber composites from V2O5/pyrrole/PtCl4, scale bar: 500 nm. | ||
It is found that the initial oxidation potentials of both AuCl and HAuCl4·3H2O solutions are around 0.9 V, which are ready to polymerize the pyrrole monomer to PPy granules. With the addition of V2O5 nanofibers, there is not much enhancement in the existing potentials of Au cations, but the morphology of PPy is changed from irregular granules to homogeneous nanofibers. As a result, Au cations were reduced to corresponding metal nanoparticles. Regarding PtCl2 and PtCl4 systems, the situation is different. The initial potentials of PtCl2 and PtCl4 are around 0.3 V and 0.6 V, respectively. The addition of V2O5 will enhance both the oxidation potentials (Fig. 2C and D) by about 0.1 V for the PtCl2 system and 0.005 V for the PtCl4 system, which could assist the polymerization process. Thus it could be concluded that V2O5 nanofibers could direct the formation of the nanofibrillar morphology, possibly due to their oxidizing ability of pyrrole to the formation of nanofibrillar oligomers.1 As a comparison, the sequence of adding reagents can be exchanged and further prove that the addition of V2O5 nanofibers at the early state not only provides a sufficient oxidation potential but also orchestrates the nano-fibrillar growth of polypyrrole.
 | ||
| Fig. 2 Potential–time profiles of pyrrole polymerizationvia different systems between the seeding (red) and conventional (black) approach: (a) V2O5/pyrrole/AuCl; (b) V2O5/pyrrole/HAuCl4; (c) V2O5/pyrrole/PtCl2; and (d) V2O5/pyrrole/PtCl4. | ||
These PNNMN composites can be applied as precursors for microwave-assisted nanocarbonization, resulting in nanocarbon–noble metal composites.23 Aiming to initiate nano-carbonization, the PPy/noble metal nanocomposites were processed by a standardized microwave heating procedure: 50 mg of the as-synthesized nanocomposite material were placed in a glass vial and then irradiated in a conventional microwave oven under ambient conditions for 5–6 min. Sparks were observed on the surfaces of the nanocomposites, which became red hot as an indication of very high temperature.23 As a result, the PPy nanofiber network will lose the heteroatoms of the polymer backbones and will be converted to the carbon nanofiber network.23 It is also worth noting that variation of oxidation potentials offered by different metal ions will affect final products' conductivity,24 which is directly associated with its microwave activity. For example, the nanocarbon–Pt composite made from the PPy/Pt precursor using microwave heating did not show a significant difference in the FTIR spectra (Fig. S8C†) compared to the original precursor. The low oxidative potential of the PtCl2 system may yield PPy with lower conductivity, which results in incomplete nanocarbonization of PPy. This is consistent with the FTIR spectra.
We believe this discovery of the novel metal displacement reactions and the consequent process with microwave will play an important role in the development of nano-structured polymer/carbon–noble metal nanoparticle composites, which can be used as next-generation catalyst materials and energy storage devices, such as fuel cell membranes. Nanoparticles of Au and Pt exhibit high specific area of active surfaces, and therefore will improve the catalytic efficiency, which in general, offers promising insights to make low-loading catalyst systems.25 In return, these systems will be attractive for fuel cell applications due to lowering of the cost.25 Moreover, the nanofibrillar network composed of conducting PPy will make an efficient supporting layer for the noble metal catalysts, largely due to the ease of the electron transfer between the noble metals and the “synthetic metals”, which would enhance the catalytic properties and stability of the nanocomposites by reducing the conduction energy loss.25 Upon microwave treatment, PNNMN composites will be converted into nanocarbon–noble metal composites, which possess better thermal and electrochemical stability,23 and this conversion may enhance the overall catalytic performance of the noble metal nanoparticles.
To test our hypothesis, methanol (MeOH) sensing was examined using these as-synthesized nanocomposites through a cyclic voltammetry (CV) technique. A graphite rod was used as the bare working electrode (BWE); PPy (control), PNNMN composites and nanocarbon–noble metal nanocomposites (1–2 mg) were uniformly decorated on the tip of the graphite rod electrode with carbon colloids paste to fabricate three types of working electrodes, respectively. The CV measurements were conducted in 1 M aq. KOH solution, with 5 vol% of MeOH. Another bare graphite rod was used as the counter electrode and Saturated Calomel Electrode (SCE) was used as the reference electrode. As shown in Fig. 3, the BWE and PPy decorated BWE have no oxidative peaks of MeOH, while the PPy–Pt nanocomposites decorated BWE have characteristic peaks for MeOH oxidation, e.g., the peak at the forward cycle (−0.082 V) represents the oxidation of MeOH, and the second oxidation peak at the reverse cycle (−0.301 V) indicates the oxidation of CO. The positions of these oxidation peaks are consistent with the previous reports.26 Interestingly, after the conversion of PPy–Pt to nanocarbons–Pt using microwave, the potentials for the oxidation of MeOH and CO shifted to more negative positions: −0.241 V and −0.411 V, respectively. The reduced onset potentials could be caused by the enhanced electron donor–acceptor interactions between the noble metal nanoparticles and the microwave-produced nanocarbon supports, which could facilitate easier transfer of the charges within the electrodes and reduce the resistance. The lowered resistance in the electrode will result in a reduced onset potential.27 This lowered resistance or improved conductivity of the nanocarbon–noble metal composite could also be evidenced by its improved discharge capacity (Fig. S9†), which substantially indicates better electron transfer efficiency of the hybrid materials, compared to the PPy nanofiber–noble metal composite.28 In addition, the specific surface area has been increased upon microwave heating,23 which may improve the contact between the nanocarbons supported Pt catalyst and the MeOH–KOH electrolyte solution. Improved electronic interaction of the nanocarbon support to the Pt catalyst could be another reason for the reduced onset oxidative potential for MeOH, which may assist in the enhancement of the fuel cell efficiency.29,30
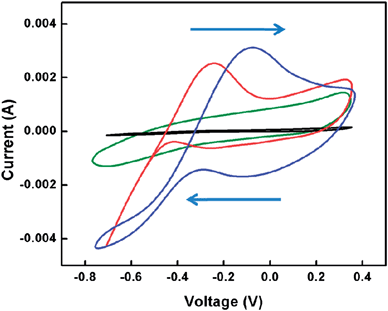 | ||
| Fig. 3 Cyclic voltammograms: black, bare graphite electrode; green, PPy nanofiber on the graphite electrode; blue, PPy nanofiber–Pt nanoparticles on the graphite electrode; red, microwave heated PPy nanofiber–Pt nanoparticles on the graphite electrode. | ||
In summary, we have demonstrated for the first time (i) a novel concept of synthetic metal–noble metal displacement reaction; (ii) a facile, one-step approach to PPy/noble metal nanocomposites starting from the monomer and noble metal salts; (iii) rapid microwave conversion of the PPy–Pt catalyst to the nanocarbon–Pt catalyst; and (iv) the “nanofiber seeding” method can be extended to make, in one step, the conducting polymer nanofiber network deposited with noble metal nanoparticles. This discovery is not just limited to the Pt and Au systems, it can be extended to the Ag system as well, to produce an Ag nanocrystal decorated PPy nanofiber network (Fig. S4†). Hence, this novel metal displacement reaction is not just a new concept, but also a general methodology to produce noble metal nanoparticles decorated conducting polymer nanofiber networks.
We gratefully acknowledge financial support from the Department of Commerce, National Science Foundation Award CMMI-1000491, and Auburn University.
Notes and references
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 12714 CrossRef CAS.
- J. K. Bailey, G. A. Pozarnsky and M. L. Mecartney, J. Mater. Res., 1992, 7, 2530 CrossRef CAS.
- X. Zhang, H. S. Kolla, X. Wang, K. Raja and S. K. Manohar, Adv. Funct. Mater., 2006, 16, 1145–1152 CrossRef CAS.
- D. Li, J. Huang and R. B. Kaner, Acc. Chem. Res., 2009, 42, 135 CrossRef CAS.
- J. Yuan and A. H. E. Muller, Polymer, 2010, 51, 4015 CrossRef CAS.
- X. Peng, J. Jin, E. M. Ericsson and I. Ichinose, J. Am. Chem. Soc., 2007, 129, 8625 CrossRef CAS.
- X. Peng, J. Jin and I. Ichinose, Adv. Funct. Mater., 2007, 17, 1849 CrossRef CAS.
- H. Huang, X. Feng and J. Zhu, Nanotechnology, 2008, 19, 7 Search PubMed.
- J. Janata and M. Josowicz, Nat. Mater., 2003, 2, 19 CrossRef CAS.
- J. de la Venta, A. Pucci, E. F. Pinel, M. A. Garcia, C. D. J. Fernandez, P. Crespo, P. Mazzoldi, G. Ruggeri and A. Hernando, Adv. Mater., 2007, 19, 875 CrossRef CAS.
- J. Yang, J. Wu, Y. Wu, J. Wang and C. Chen, Chem. Phys. Lett., 2005, 416, 215 CrossRef CAS.
- M. Duca, M. O. Cucarella, P. Rodriguez and M. T. M. Koper, J. Am. Chem. Soc., 2010, 132, 18042 CrossRef CAS.
- M. Min, C. Kim and H. Lee, J. Mol. Catal. A: Chem., 2010, 333, 6–10 CrossRef CAS.
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2005, 127, 14156 CrossRef CAS.
- Z. Liu, X. Zhang, S. Poyraz, S. P. Surwade and S. K. Manohar, J. Am. Chem. Soc., 2010, 132, 13158 CrossRef CAS.
- L. Wang, X. Li and Y. Yang, React. Funct. Polym., 2001, 47, 125 CrossRef CAS.
- J. M. Pringle, O. Winther-Jensen, C. Lynam, G. G. Wallace, M. Forsyth and D. R. MacFarlane, Adv. Funct. Mater., 2008, 18, 2031 CrossRef CAS.
- X. Feng, H. Huang, Q. Ye, J. Zhu and W. Hou, J. Phys. Chem. C, 2007, 111, 8463 CAS.
- H. Cao, Z. Xu, H. Sang, D. Sheng and C. Tie, Adv. Mater., 2001, 13, 121 CrossRef CAS.
- J. Zhang, G. Shi, C. Liu, L. Qu, M. Fu and F. Chen, J. Mater. Sci., 2003, 38, 2423 CrossRef CAS.
- R. M. Hernandez, L. Richter, S. Semancik, S. Stranick and T. E. Mallouk, Chem. Mater., 2004, 16, 3431 CrossRef CAS.
- Z. Liu, Y. Liu, S. Poyraz and X. Zhang, Chem. Commun., 2011, 47, 4421 RSC.
- X. Zhang and S. K. Manohar, Chem. Commun., 2006, 2477 RSC.
- Y. E. Whang, J. H. Han, T. Motobe, T. Watanabe and S. Miyata, Synth. Met., 1991, 45, 151 CrossRef CAS.
- E. Antolini and E. R. Gonzalez, Appl. Catal., A, 2009, 365, 1 CrossRef CAS.
- M. A. A. Rahim, H. B. Hassan and R. M. A. Hameed, Fuel Cells, 2007, 7, 298 CrossRef.
- J. Zhao and A. Manthiram, J. Electrochem. Soc., 2011, 158, B208 CrossRef CAS.
- J. Wolfenstine, J. Read and J. L. Allen, J. Power Sources, 2007, 163, 1070 CrossRef CAS.
- X. Yu and S. Ye, J. Power Sources, 2007, 172, 133 CrossRef CAS.
- X. Yu and S. Ye, J. Power Sources, 2007, 172, 145 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Enlarged images and the experimental details. See DOI: 10.1039/c1nr10994d |
| This journal is © The Royal Society of Chemistry 2012 |
