Recognition of carbon nanotube chirality by phage display†
Ting
Yu
a,
Yingxue
Gong
b,
Tingting
Lu
a,
Li
Wei
a,
Yuanqing
Li
a,
Yuguang
Mu
c,
Yuan
Chen
a and
Kin
Liao
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore. E-mail: askliao@ntu.edu.sg; Fax: +65 6791 6905; Tel: +65 6790 5835
bResearch Centre for Molecular Biology, Institute of Life and Health Engineering, College of Life Sciences and Technology, Jinan University, Guangzhou, P. R. China
cSchool of Biological Sciences, Nanyang Technological University, Singapore
First published on 19th December 2011
Abstract
We report a virus-based biological template, M13 bacteriophage, for selecting single-walled carbon nanotubes’ (SWCNTs’) chirality, based on the premise that binding affinity between carbon nanotubes (CNTs) and peptides are sensitive to amino acid sequences. Bacteriophage pIII capsid fusion peptides, which have high binding affinity for CNTs, were identified through an evolutionary screening process by a phage display technique. Most of the binding sequences are rich in aromatic amino acids, begin with histidine, and possess hydrophobic properties. Direct evidence of binding was provided by the attachment of SWCNTs to selected bacteriophage-coated microspheres via an anti-M13 monoclonal antibody and by the attachment of CdTe quantum dots coupled with the selected peptides to SWCNTs. Efficient dispersion of debundled SWCNTs was achieved by the selected peptides whose conformation is characterized by circular dichroism. Quantitative analysis of the binding affinity was carried out using molecular dynamics simulations, demonstrating that the best binder, HESFWYLPHQSY, has the highest binding energy with (7, 6) SWCNT in the peptide pool. Computational calculations also show that the selected peptides preferentially bind to large-diameter tubes and the peptide HSNWRVPSPWQL has the best discernment on chirality. Chiral selectivity was examined by UV-vis-NIR and photoluminescence spectroscopy, showing that HSNWRVPSPWQL could shift the SWCNT size distribution to larger diameter and enrich (10, 3), (9, 5), (12, 2), (11, 4), and (10, 6). This work presents chirality-preferential dispersion of SWCNTs by phage-displayed peptides for the first time, and offers new prospects of understanding the mechanism of peptide–CNT binding and producing “designer” peptide for CNT surface recognition.
Introduction
Ever since the discovery of single-walled carbon nanotubes,1,2 these quasi-one-dimensional nanosized tubes have attracted extensive attention due to their fascinating electronic, mechanical, structural, optical, and thermal properties,3 which make them extremely promising candidates in a wide range of potential applications, such as molecular electronics,4 composite materials,5 and biotechnology.6 A major obstacle for implementing carbon nanotubes in some applications is that it is difficult to harvest CNTs of uniform chirality, which specifies the roll-up mode of a single graphite layer by the chiral indices, (n, m), and subsequently determines whether one SWCNT is metallic or semiconducting. To date, various techniques, including selective growth methods and post-synthetic sorting schemes, have been employed for SWCNT separation.7–9 The availability of single-chirality SWCNT components is still a formidable challenge, although remarkable progress has been made in obtaining narrow chirality distributions of SWCNTs in the past few years.8,10Biological molecules, such as DNA and proteins/peptides, possess inherent molecular recognition capabilities for various substrates via molecular specificity. It is possible to use biomolecules to solve the SWCNT separation problem because they may selectively bind to certain (n, m) SWCNTs. Recently, twelve highly enriched chiral SWCNT species were separated by fractionating DNA-encapsulated SWCNTs from ion exchange chromatography by Zheng et al.11 Naik and co-workers reported that 86% of (6, 5) SWCNTs were enriched by salmon genomic DNA.12 Additionally, flavin mononucleotide, a redox cofactor to various oxidoreductases, can helically wrap around SWCNTs and show specific affinity to (8, 6) SWCNTs, leading to high chiral abundance up to 85%.13Peptides have been shown to selectively bind to SWCNTs of different diameters, depending on the length of the reversible cyclic peptides,14 however, their chiral selectivity toward SWCNTs has not been demonstrated until now.
Considering the fact that currently the knowledge of protein-folding prediction and surface-binding chemistry are unable to support rational design of proteins/peptides which specifically bind SWCNTs of certain chiral structures,15 random peptide libraries, such as phage display libraries, can be screened for SWCNT chiral structure recognition. Phage display is a selection technique in which a combinatorial library of polypeptides expressed on a coat protein of bacteriophage (also known as phage) allows identification of peptides with desired binding specificities to various target molecules or substrates. This a posteriori method, which has been conventionally used to identify protein epitopes, antibodies, enzymes, and to study protein interaction, and to discover drugs, has been extensively utilized to evolutionarily select specific peptide sequences which bind to a wide range of inorganic materials and nanostructures.16
Carbon materials, including multi-walled carbon nanotubes (MWCNTs),17 SWCNTs,18–21carbon nanohorns (SWNHs),22fullerene (C60),23 and graphene,24 are also target substrates for specific peptide binding. After using surfactant-suspended MWCNTs as targets in phage display screening, some common features, such as having a motif rich in histidine (His) and tryptophan (Trp) and showing a quasi-symmetric-surfactant form (hydrophilic on the ends and hydrophobic in the middle), were found in CNT-binding peptide sequences.17,18 For instance, Suet al. identified SWCNT-binding peptides having different consensus motifs.19 In order to avoid surfactant–SWCNT interaction, other researchers immobilized SWCNTs on certain substrates before performing the phage display screening,20,21 forging a better interaction between phage-displayed peptides and the SWCNT surface. Zheng et al. presented an SWCNT-binding motif SXWWXXW (where X is any amino acid) without surfactant form, again emphasizing the importance of tryptophan.20 In a recent work of Belcher and co-workers, one peptide, DMPRTTMSPPPR, without any aromatic moieties, still has a tri-block surfactant structure. But the binding affinity is approximately four times as high as its counterpart, HGHPYQHLLRVL, which is rich in histidine and tyrosine.21 Additionally, in a cell-surface display experiment, a polypeptide having 5 repeats of the sequence of PQAQDVELPQELQDQHREVEV was the selected SWCNT binder, which was noticeably different from other SWCNT-binding peptides.25
Despite the efforts to find SWCNT-binding peptides and the fact that some sequences have been identified, the differences among these binding sequences lead us to realize that the nature of the peptide–SWCNT interaction is largely unveiled. One possible reason for the diversity of the SWCNT-binding peptides is because the SWCNTs used in all the phage-displayed screening experiments had a wide distribution of (n, m), hence different structures. It is believed that phage-displayed peptides not only have affinity to various inorganic materials, but also can recognize the difference in the crystalline face, i.e., the spatial arrangement of the atoms.15,26 Therefore, phage display technology could be one possible route to recognize SWCNTs' chiral structures. Using the technique, on one hand, the underlying mechanism of peptide–SWCNT interaction may be unveiled; on the other hand, it shows great promise in separation of SWCNTs with specific chirality, which is the Holy Grail in CNT related fields and the prerequisite for their applications in nanoelectronics.
We report a combinatorial phage display screening method to select peptides which can recognize different CNT (n, m) species. CoMoCAT (7, 5) and (7, 6) SWCNT samples with more than 30% single chirality abundance were utilized as the targets of an M13 phage display library expressing linear dodecapeptides on the pIII minor coat protein. The unique feature of our SWCNT samples compared with those used in previous studies is the narrow (n, m) distribution of SWCNTs (predominantly in the high chiral-angle region). In addition, most of the SWCNTs are small-diameter tubes (0.8—1.0 nm), showing a narrow diameter distribution as well. The SWCNT-binding affinity was demonstrated through the enrichment of binding sequences and statistical analysis for binding sequences. Directed binding was evidenced by the attachment of SWCNT to selected phage-coated microspheres via an anti-M13 monoclonal antibody and by the attachment of CdTe quantum dots coupled with the selected peptides to SWCNTs. The binding energies of all the selected peptides to SWCNT (7, 0), (8, 0), (7, 5), (7, 6), (7, 7), and (8, 8) were obtained by molecular dynamics (MD) simulations. Subsequently, the best binders selected by screening experiments and MD simulations were synthesized and used for the dispersion and separation of SWCNTs. SWCNTs can be well dispersed by selected peptides whose conformation is characterized by circular dichroism (CD) spectroscopy. It was found that the best binder selected by MD simulations preferentially disperses HiPco SWCNTs of larger diameter such as (10, 3), (9, 5), (12, 2), (11, 4), and (10, 6). To our best knowledge, it is the first time that peptides selected by phage display technique can preferentially disperse a set of (n, m) SWCNTs. We believe that if the mass production of phage-displayed peptides is realized by genetic engineering, the conception and methodology of this work will offer a new path to SWCNT chirality separation.
Materials and methods
Preparation of SWCNTs
SWCNTs of (7, 5) and (7, 6) configuration, denoted as CNT-75 and CNT-76 respectively, were produced from Co-MCM-41 catalysis by chemical vapor deposition (CVD) and the single chirality abundance was more than 30%.27 The SWCNTs were purified by sonication and ultracentrifugation. As-received SWCNTs were homogeneously dispersed in 0.1% (v/v) Tween-20 at a concentration of 0.0015 w/v% (w/v% = 10 mg ml−1) for CNT-75 and a concentration of 0.005 w/v% for CNT-76. After 900 μl 1M NaCl was added into 100 μl SWCNT dispersion, they were quickly vortexed and allowed to stand overnight to reach equilibrium. The overnight suspension was centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 2 h. The SWCNT pellet was rinsed by TBS (tris buffered saline) several times to remove most of the attached surfactants. The SWCNTs were finally suspended in TBS with different concentration of Tween-20 (TBST, Tween-20 concentration of 0.1–0.75 v/v%) as very fine particles without precipitation in 3 h. Now the SWCNTs were in a “fine suspension” state in which they neither aggregate into bigger bundles nor precipitate in a short time. However, the SWCNTs could be pelleted by centrifugation so that the unbound phages could be separated from the bound simply by removing the supernatant.
000 g for 2 h. The SWCNT pellet was rinsed by TBS (tris buffered saline) several times to remove most of the attached surfactants. The SWCNTs were finally suspended in TBS with different concentration of Tween-20 (TBST, Tween-20 concentration of 0.1–0.75 v/v%) as very fine particles without precipitation in 3 h. Now the SWCNTs were in a “fine suspension” state in which they neither aggregate into bigger bundles nor precipitate in a short time. However, the SWCNTs could be pelleted by centrifugation so that the unbound phages could be separated from the bound simply by removing the supernatant.
Phage display
Phage display is initialized by “biopanning”, a directed evolutionary process to retain the desired phage and eliminate the undesired phages in the phage display library. The biopanning process is illustrated schematically in Fig. 1. In brief, a commercially available 12-mer phage display library (1.0 × 1013 pfu/ml, Ph.D.-12™ Phage Display Peptide Library Kit, New England BioLabs, USA) was added to the pretreated CNT-75 and CNT-76 enriched samples, respectively. The mixture was subsequently incubated with gentle agitation at room temperature for 1 h, followed by rinsing the SWCNT pellet 5 times with 0.1% TBST to remove any unbound phages and non-specific bound phages. Bound phages were eluted by 0.2 M glycine–HCl (pH 2.2) and immediately neutralized by Tris–HCl (pH 9.1). The concentration of phage eluates was determined by titering (ESI†). The phage eluate was then amplified and purified through bacterial infection. The purified phages were titered and subject to the next round of biopanning as a new “library”. The biopanning process was iterated 5 more times with stepwise increased concentration of Tween-20 (0.2, 0.3, 0.4, 0.5, and 0.75%). In each round, the phage eluates and amplified phages were titered to quantify the output phages and input phages. The input phage, which was 1.0 × 1011 pfu (plaque forming unit), was consistent in each round. The ratio of output/input phage was calculated to determine whether the binding population was enriched during the biopanning process. A control experiment was also carried out by using wild-type M13 phage (M13KE) in the first three rounds. Individual phage colonies (blue plaques) were randomly picked up from agar plates containing IPTG (isopropyl-β-D-thiogalactoside) and X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside) after 6 rounds of biopanning. Phage DNA was extracted and sequenced with −96 gIII sequencing primer after phages from individual plaques were amplified and purified.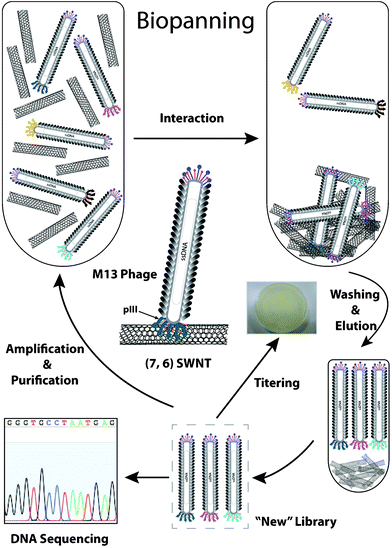 | ||
| Fig. 1 Schematic illustration of biopanning experiment against CNT-76-enriched SWCNTs. | ||
Phage-displayed peptide sequence analysis
All sequences were first analyzed by the overall frequency of amino acid occurrence (i.e., the number of times each amino acid occurs in overall selected sequences), which was compared to the overall library distribution provided by NEB to determine whether the binding sequences were significant or whether they were merely a reflection of the library. The hydrophobicity of each amino acid in each selected sequence was calculated based on the Hopp–Woods scale28 with a window size of five amino acids as a function of amino acid position. A tri-glycine (GGG) linker sequence was added to the end of each sequence to involve the influence from the phage body. The mean hydrophobicity was also calculated for all the sequences.Binding affinity assay
The binding affinity is defined as the ratio of output/input phage. The binding affinity of all the CNT-76 phage monoclones and M13KE to CNT-76 was tested. In the binding affinity assay, phages with the same input pfu, 5 × 108, were added into 1 ml SWCNTs suspended in 0.5% TBST and gently rocked at room temperature for 1 h. The SWCNT fine suspension was then pelleted by centrifugation and rinsed 5 times with 0.5% TBST to remove unbound phages. Bound phages were eluted by 0.2 M glycine–HCl (pH 2.2) and immediately neutralized by Tris-HCl (pH 9.1). The pfu of eluted phages (output phages) was obtained by titering.Peptide synthesis and purification
Selected phage-displayed peptides, including 76-4 (HSNWRVPSPWQL), 76-6 (HESFWYLPHQSY), and 76-4 dimer (HSNWRVPSPWQLHSNWRVPSPWQL), were synthesized by commercial vendor (1st BASE Pte Ltd., Singapore) with purity (HPLC) > 95%.Microsphere experiments
Polystyrene latex microspheres with a diameter of 10 μm were dispersed in an aqueous electrolyte, with concentration of 2.0 × 106 particles/ml (Coulter Corporation, USA). 400 μl microsphere dispersion was centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min to remove the electrolyte. The pelleted microspheres were resuspended by 700 μl phosphate buffered saline (PBS, pH 7.4) and 100 μl anti-M13 monoclonal antibody (100 μg ml−1, GE Healthcare, USA), which specifically reacts with the bacteriophage M13 major coat protein pVIII. The antibody was allowed to coat the microspheres by an overnight incubation with gentle agitation at 4 °C. The coated microspheres were then washed three times with PBS to remove the free antibody and resuspended by a mixture of 730 μl PBS, 80 μl bovine serum albumin (BSA, 2 mg ml−1) and 8 μl 10 v/v% Tween-20.
000 g for 10 min to remove the electrolyte. The pelleted microspheres were resuspended by 700 μl phosphate buffered saline (PBS, pH 7.4) and 100 μl anti-M13 monoclonal antibody (100 μg ml−1, GE Healthcare, USA), which specifically reacts with the bacteriophage M13 major coat protein pVIII. The antibody was allowed to coat the microspheres by an overnight incubation with gentle agitation at 4 °C. The coated microspheres were then washed three times with PBS to remove the free antibody and resuspended by a mixture of 730 μl PBS, 80 μl bovine serum albumin (BSA, 2 mg ml−1) and 8 μl 10 v/v% Tween-20.
4 aliquots of the microsphere suspension were dispensed into 4 wells of a 24-well plate for 4 parallel experiments. Subsequently, different phage samples were added into each well (Well A: 1 μl selected phage monoclone [after six rounds of biopanning]; Well B: 1 μl phage eluate after only one round of biopanning; Well C: no phage added; Well D: 1 μl wild-type phage). The phages were gently shaken at room temperature for 1 h to allow the interaction with anti-M13 antibody. The unbound phages were removed by centrifugation at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min. After resuspending in a 200 μl CNT-76 dispersion, the precipitated microsphere–antibody–phage complexes were transferred to fresh wells and incubated with gentle agitation at room temperature for 1 h. The unbound SWCNTs were removed by centrifugation at 16
000 g for 10 min. After resuspending in a 200 μl CNT-76 dispersion, the precipitated microsphere–antibody–phage complexes were transferred to fresh wells and incubated with gentle agitation at room temperature for 1 h. The unbound SWCNTs were removed by centrifugation at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min. The non-specific bound SWCNTs were removed by washing the microspheres 4 times with 0.1% TBST. To remove the unwanted salt which might affect subsequent imaging, the microspheres were washed by water. The samples were suspended in 10 μl deionized (DI) water and examined by a field-emission scanning electron microscope (FESEM, JSM-6700, JEOL, Japan) at 5 kV. The microspheres were sputter coated with a thin layer of platinum prior to observation.
000 g for 10 min. The non-specific bound SWCNTs were removed by washing the microspheres 4 times with 0.1% TBST. To remove the unwanted salt which might affect subsequent imaging, the microspheres were washed by water. The samples were suspended in 10 μl deionized (DI) water and examined by a field-emission scanning electron microscope (FESEM, JSM-6700, JEOL, Japan) at 5 kV. The microspheres were sputter coated with a thin layer of platinum prior to observation.
Coupling quantum dots to SWCNTs
5 mg (7, 6)-enriched SWCNTs (SWeNT ® SG76, SouthWest NanoTechnologies Inc., USA) were added to H2SO4/HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 ml) and bath-sonicated for 3 h. The SWCNT dispersion was diluted 10 times, filtrated through 0.22 μm mixed cellulose ester membrane and washed with DI water five times. Dried SWCNTs were suspended in DI water. 0.01 mM highly fluorescent mercaptosuccinic acid (MSA)-capped CdTe quantum dots (QDs) reacted with 0.5 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Sigma-Aldrich) and 0.1 mM N-hydroxysuccinimide (NHS, Sigma-Aldrich) to form a CdTe-NHS ester complex. In brief, QDs, EDC, and NHS were mixed in PBS and vortexed for 1 h. 600 μl ethanol was added to the mixture, vortexed briefly and centrifuged at 8000 g for 10 min. 900 μl TBS was used to resuspend the QDs and mixed with 100 μl synthetic peptide 76-6 (HESFWYLPHQSY). After reacting at room temperature for 1 h, the QD–peptide complex was incubated at 4 °C overnight. The carboxyl-modified SWCNTs were then mixed with QD–peptide complex with gentle agitation at room temperature for 1 h. The unbound QD–peptide complex was removed by centrifugation prior to observation by confocal microscope (Zeiss LSM 510 Meta, Germany).
1, 20 ml) and bath-sonicated for 3 h. The SWCNT dispersion was diluted 10 times, filtrated through 0.22 μm mixed cellulose ester membrane and washed with DI water five times. Dried SWCNTs were suspended in DI water. 0.01 mM highly fluorescent mercaptosuccinic acid (MSA)-capped CdTe quantum dots (QDs) reacted with 0.5 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Sigma-Aldrich) and 0.1 mM N-hydroxysuccinimide (NHS, Sigma-Aldrich) to form a CdTe-NHS ester complex. In brief, QDs, EDC, and NHS were mixed in PBS and vortexed for 1 h. 600 μl ethanol was added to the mixture, vortexed briefly and centrifuged at 8000 g for 10 min. 900 μl TBS was used to resuspend the QDs and mixed with 100 μl synthetic peptide 76-6 (HESFWYLPHQSY). After reacting at room temperature for 1 h, the QD–peptide complex was incubated at 4 °C overnight. The carboxyl-modified SWCNTs were then mixed with QD–peptide complex with gentle agitation at room temperature for 1 h. The unbound QD–peptide complex was removed by centrifugation prior to observation by confocal microscope (Zeiss LSM 510 Meta, Germany).
Molecular dynamics simulations
Six SWCNT (n, m) models, namely (7, 0), (8, 0), (7, 5), (7, 6), (7, 7), and (8, 8), were constructed with the same length of 10 nm. Peptide models of extended forms were built in a DeepView – Swiss–PdbViewer. The N and C termini were capped with acetyl and N-methylamide, respectively. One peptide and one SWCNT were placed in a simulation box (12 × 5 × 5 nm) with a minimum distance of 1 nm between the peptide and the SWCNT in order to eliminate interference. Simple point charged (SPC) water molecules were added to completely fill up the simulation box and counter ions were added to neutralize the system. Motions of the SWCNT were restrained in the simulations while the peptide was allowed to move freely. All simulations, employing the all-atom AMBER force field, were conducted by GROMACS package version 4.0.329 at 300 K for 20 ns, and the data from the last 10 ns were used for analyzing binding energies. The binding energies were estimated by the Generalized Born (GB) implicit solvent model30 in sander module of the AMBER 9 package.31SWCNT dispersion by selected peptides
The selected phage-displayed synthetic peptides were first dissolved in 1 ml DI water or deuterium oxide (D2O, Sigma-Aldrich) at a concentration of 1000 μM, 500 μM, or 100 μM. The peptide solution was added to HiPco and SG76 SWCNTs (0.25 mg), respectively. The SWCNTs were dispersed by probe sonication (ultrasonicator, VCX-130, SONICS, USA) at 10 W for 1 h in an ice bath. The dispersion was centrifuged at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 1 h to remove nanotube bundles and the top 75% supernatant was collected for subsequent measurements.
000 g for 1 h to remove nanotube bundles and the top 75% supernatant was collected for subsequent measurements.
AFM characterization
Atomic force microscopy (AFM) characterization was conducted on a MFP 3D microscope (Asylum Research, Santa Barbara, USA) in AC mode. SWCNTs dispersed by peptide 76-4 were deposited onto silicon wafer and dried in a desiccator prior to observation.Secondary structure of selected peptides
Peptides 76-4 and 76-6 were dissolved in both DI water and KH2PO4 buffer (100 μM, pH 7.1) at concentrations of 500 μM and 100 μM for CD measurements. CD spectrometry was also employed to study the conformation of HiPco SWCNTs dispersed by sodium dodecyl benzene sulfonate (SDBS, 0.1 w/v%), 76-4, 76-4 dimer, and 76-6. All the CD spectra were measured between 180 nm and 280 nm with quartz cuvettes having a 0.1 mm path length by a Chirascan™ Circular Dichroism Spectrometer (Applied Photophysics, Surrey, United Kingdom). The final spectra were obtained by subtracting the solvent baseline from the average value of triplicate measurements for each sample. The CD spectra were analyzed by an algorithm called SELCON in order to quantitatively determine the contents of each secondary structure.UV-vis-NIR and fluorescence spectroscopy
Ultraviolet (UV)-visible (vis)-near-infrared (NIR) absorption spectra were obtained by a Varian Cary 5000 UV-vis-NIR spectrophotometer in transmission mode and quartz cells with a 1 cm path length. Absorption data were collected every nanometre between 400 and 1600 nm. Fluorescence measurements were conducted on a Jobin-Yvon Nanolog-3 spectrofluorometer with an InGaAs detector by scanning the excitation wavelength from 500 to 800 nm and the emission wavelength from 875 to 1450 nm.Results
To obtain peptides specifically binding to (7, 5) and (7, 6) SWCNTs, biopanning experiments were performed by a 12-mer M13 phage display library, as shown in Fig. 1. SWCNTs were in a state of “fine suspension” for simple separation of bound phage from unbound phage by centrifugation.Enrichment of binding peptides
In each round of biopanning against (7, 5) and (7, 6) enriched SWCNT samples by 12-mer M13 phage display library, the phages were titered right after they were eluted from SWCNTs. The ratio of output phage to input phage (output/input ratio) increased gradually because selection stringency was increased by raising the Tween-20 concentration stepwise from 0.1% to 0.75%, indicating that the desired sequences were enriched during the biopanning process (Fig. 2A). The recovery percentage was improved by 18.5-fold and 15.7-fold for CNT-75 and CNT-76 specific phage, respectively (Table S1, ESI†).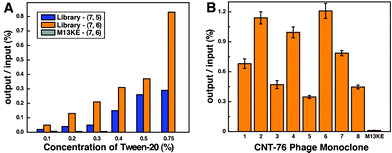 | ||
| Fig. 2 (A) The ratio of output phage/input phage as a function of the concentration of Tween-20 for 6-round biopanning of 12-mer phage library against CNT-75 and CNT-76. No significant increase was observed in the first three rounds when M13KE phage was used as a library in the biopanning against CNT-76. (B) Binding affinity for CNT-76 of selected CNT-76 specific phages. The ratio of output/input is proportional to the binding affinity. | ||
Wild-type phages can also bind targets through their pVIII major coat protein, thus exhibit non-specific affinity to the targets. Therefore, it cannot always be certain whether bound phage have specific affinity through the displayed peptides or the major coat protein. In the control experiment, M13KE phage was exposed to CNT-76 samples and three rounds of biopanning experiments were conducted. The titer of eluted M13KE was less than 1/12 of CNT-76-specific eluate in the first round and less than 1/45 of CNT-76-specific eluate in the third round. After amplified and exposed to SWCNTs again, M13KE eluate produced the same titer in the second and third rounds because they are genetically identical. This indicates that although background binding (i.e., through pVIII major coat protein) did contribute to the affinity, the engineered pIII 12-mer peptides played the major role in SWCNT binding.
CNT-75 and CNT-76 binding sequences and statistical analysis
Phage ssDNA was extracted from amplified monoclonal phage after six rounds of biopanning. Fifteen monoclonal ssDNA samples of both CNT-75-specific and CNT-76-specific phage were identified by automated cycle sequencing (Table 1 and Table 2). A few sequences appeared as both CNT-75-specific and CNT-76-specific sequences, such as HSSWYIQHFPPL, HESFWYLPHQSY, and HWRQTMPMTSAP. This is not surprising because CNT-75 and CNT-76 are structurally similar and some sequences might not be specific enough to distinguish close SWCNT chiral structures. It also reflects that these sequences have high affinity to SWCNTs because they were obtained from two totally independent selection experiments. The existence of recurrent sequences probably indicates evidence of supreme binding, such as peptides 75-10, 75-11, and 76-6, whose binding energies are among the highest ones in the MD simulation results (Table 1 and Table 2). The sequence HESFWYLPHQSY appeared five times in the CNT-76-specific pool, while for CNT-75, it was isolated only once, implying that this peptide possessed high affinity to both CNT-75 and CNT-76, but with higher affinity to CNT-76 than to CNT-75. It is confirmed by MD simulation results that HESFWYLPHQSY has higher binding energy with CNT-76 than CNT-75. The highest binding affinity of peptide 76-6 (HESFWYLPHQSY) with CNT-76 is also proved by a binding affinity assay (Fig. 2B).| Phage | Peptide Sequence | Occurrence | Binding energy (kcal mol−1) |
|---|---|---|---|
| 75-1 | HKLATSPWWPPI | 2 | −41.2 ± 2.6 |
| 75-2 | HSTSYQSLRWGA | 1 | −36.6 ± 3.0 |
| 75-3 | FPPWWHNSSGAP | 1 | −50.4 ± 1.0 |
| 75-4 | HWGNHSKSHPQR | 2 | −43.1 ± 1.3 |
| 75-5 | IYAHSLANSHNS | 1 | −49.3 ± 4.7 |
| 75-6 | HSAFWQISPPST | 1 | −51.9 ± 3.1 |
| 75-7 | LPPWKHKTSGVA | 1 | −56.7 ± 2.6 |
| 75-8 | HWRQTMPMTSAP | 1 | −62.2 ± 4.4 |
| 75-9 | SVSVGMKPSPRP | 1 | −48.1 ± 2.8 |
| 75-10 | HSSQWHPMAVHR | 2 | −55.2 ± 4.4 |
| 75-11 | HESFWYLPHQSY | 2 | −59.6 ± 5.3 |
| 75-12 | HSSWYIQHFPPL | 1 | −57.4 ± 3.2 |
| Phage | Peptide Sequence | Occurrence | Binding energy (kcal mol−1) |
|---|---|---|---|
| 76-1 | HSSWYIQHFPPL | 3 | −34.6 ± 5.0 |
| 76-2 | HLWTYSAPTVPT | 1 | −61.4 ± 3.9 |
| 76-3 | HTQNMRMYEPWF | 1 | −50.9 ± 4.4 |
| 76-4 | HSNWRVPSPWQL | 1 | −54.9 ± 3.9 |
| 76-5 | HTMSSWWAGHAT | 1 | −46.0 ± 4.7 |
| 76-6 | HESFWYLPHQSY | 5 | −62.5 ± 4.2 |
| 76-7 | HNRWSSWDEHTP | 1 | −42.9 ± 4.1 |
| 76-8 | HWRQTMPMTSAP | 2 | −52.2 ± 4.1 |
The frequency of each amino acid in CNT-75-specific pool and CNT-76-specific pool was calculated respectively and compared to the overall amino acid distribution of the Ph.D.-12 library provided by NEB (Table S2 and Table S3, ESI†). The most dramatic differences from the library averages are the increase in histidine, tryptophan, and serine, and the decrease in leucine and threonine. The result confirms that the selected peptides are SWCNT-specific sequences. If there is no distinct difference from the overall library distribution, the binders were probably randomly picked from the library, resulting in merely a reflection of the library. On the contrary, these amino acids, whose frequency is significantly different from that of the library, probably serve as the binding parts in the sequences. Similar analyses were also performed based on the amino acid grouping after the twenty amino acids were grouped into several equivalency sets according to their chemical and structural properties (Table S4, ESI†). Ring group amino acids, including histidine, tryptophan, phenylalanine, and tyrosine, underwent the most significant change (Fig. S1, ESI†). A plausible reason why amino acids with ring groups manifested themselves in SWCNT binding is that they led to strong interactions with the hydrophobic surface of SWCNTs via π-stacking.
Many of the selected peptides in our work possess similar features in terms of amino acids as those from Wang and co-workers,17 and Naik and coworkers.18 For example, the binding sequences are rich in aromatic amino acids and begin with histidine. This confirms that these peptides have binding affinity to CNTs. However, the positions at which aromatic amino acids occur are not consistent with Wang's and Naik's results. In addition, the CNT-75-specific and CNT-76-specific sequences are also rich in hydroxyl amino acids and proline. These differences may result from different CNT samples used for our phage display screening experiments. The CNTs used in our work were SWCNTs having a narrow diameter distribution and high single chirality abundance, while Wang et al. used MWNTs, and Naik's group used SWCNTs with a wide diameter distribution. However, chirality recognition depends not only on the functional groups of amino acids, but also the positions where they occur, implying the importance of the conformation of binding peptides.
Microsphere experiments
The association between phages and SWCNTs was demonstrated in the following figures. In Fig. 3A, the microspheres were coated with selected phage clone after six rounds of biopanning. This clone is believed to have affinity to CNT-76 so that it captured many SWCNTs. In Fig. 3B, the phage eluate after the first round of biopanning was used to coat the microspheres. Much fewer SWCNTs were observed because the first round eluate possessed lower affinity to SWCNTs compared with the selected clone. If no phage were added to the microspheres (Fig. 3C), a few tiny SWCNT bundles still remained on microspheres due to the van der Waals attraction between SWCNTs and antibody encapsulated microspheres. In the last control experiment (Fig. 3D), the selected phage clone was substituted by phage clone without pIII fusion peptides. Similar to Fig. 3C, a few SWCNT bundles appear because of either the van der Waals force or background binding, or both of the two types of interaction.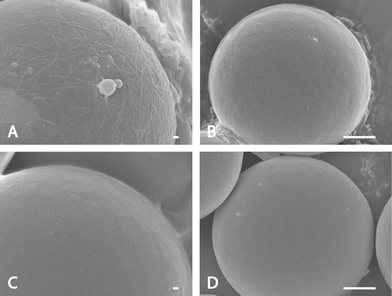 | ||
| Fig. 3 FESEM images of microsphere experiments showing that SWCNTs bind to phage coated microspheres through different phages. (A) Microspheres coated with selected phage clone after 6-round biopanning. The white threads are SWCNT bundles while the while particles are believed to be salt. (B) Microspheres coated the phage eluate after the first round of biopanning. Tiny SWCNT bundles can be observed. (C) No phage coating on microspheres. Very tiny SWCNT bundles can be seen due to the van der Waals force. (D) Wild-type phage coating on microspheres. Very tiny SWCNT bundles can be seen due to van der Waals force or background binding. The scale bars are 100 nm in A and C, and 1 μm in B and D. | ||
QD/SWCNT conjugation
QDs were attached to SWCNTs viapeptide 76-6 (HESFWYLPHQSY) to demonstrate their affinity to SWCNTs. Fig. 4A illustrates the conjugation of QDs to SWCNTs by a noncovalent chemical approach. SWCNTs are dissolvable in DI water after they are carboxyl-functionalized. The selected phage-displayed peptide was chemically conjugated to CdTe QDs through EDC/NHS coupling. CdTe QDs were attached onto the SWCNTs via the peptide as a linker. As shown in Fig. 4, the black clump is composed of SWCNTs and they are luminous due to the conjugated QDs—direct evidence that the selected peptide has binding affinity to SWCNTs.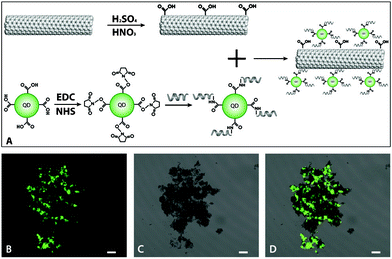 | ||
| Fig. 4 Optical tracking SWCNTs via a QD-functionalized synthetic phage-displayed peptide. (A) Schematic illustration of the process of QD/SWCNT coupling through a peptide. (B) Confocal fluorescence image of QDs coupled to SWCNTs due to noncovalent interaction between QD-modified peptides and SWCNTs. (C) Transmission image of B. The black clumps are SWCNTs. (D) The merged image of B and C. In B, C, and D, all the scale bars are 10 μm. | ||
The CNT-binding peptides may find practical applications in optically tracking CNTs and linking specific materials onto CNTs via a bi-functional peptide, in which one end is a selected CNT-binding peptide and the other end is an additional functional domain or epitope. For instance, a bi-functional peptide, containing a SWCNT-binding domain and a trinitrotoluene (TNT)-binding domain, was integrated into a chemical sensor for detecting TNT.32 We believe that such CNT-binding peptides can be also utilized for biosensors, enzyme immobilization, etc., particularly in a bi-functional manner, and eventually make CNTs “application tunable” materials.
Hydrophobicity analysis
The hydrophobicity of each amino acid and the mean hydrophobicity of all of the selected phage-displayed sequences were calculated based on the Hopp–Woods scale (Table S5 and S6, ESI†). The hydrophobicity of some representative sequences, whose occurrence is more than 1, or that is selected by MD simulations (with the best discernment of binding energy), are plotted in Fig. 5 for CNT-75-specific and CNT-76-specific sequences, respectively. Most of the amino acids in this analysis are hydrophobic (with value less than 0). No typical symmetric-surfactant form, which was reported by Wang et al., was found in our results, although some curves (76-6 in Fig. 5, bottom) have two hydrophobic flanks and one base with even higher hydrophobicity. It may result from the surfactant-free surface of SWCNTs in our experiment, in contrast to the surfactant-coated MWNTs in Wang's screening experiments.17 We speculate that the hydrophobic property may render the peptides more suitable to interact with the hydrophobic surface of SWCNTs. The symmetric-surfactant form may be necessary for oligopeptides themselves to attach SWCNTs, however, for phage-displayed peptides, because they have already been linked to a hydrophilic phage body, they can be even more hydrophobic to maximize affinity to SWCNTs. Zheng et al. reported the similar phenomenon,20 however, their hydrophobic phage-displayed peptides could not disperse SWCNTs. Contrarily, although our peptides are overall hydrophobic, they are dissolvable in water and capable of dispersing SWCNTs, thus enabling themselves to be utilized as a tool for SWCNT separation.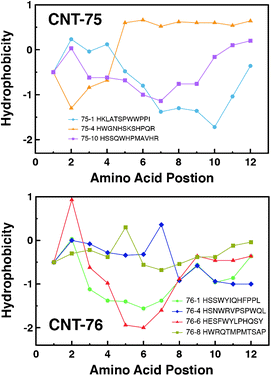 | ||
| Fig. 5 Amino acid hydrophobicity by position for representative CNT-75-specific (top) and CNT-76-specific (bottom) sequences using the Hopp–Woods scale. Positive values represent hydrophobic regions. Connecting lines indicate data trend. | ||
MD simulations
To understand the nature of recognition of SWCNTs and the chiral selectivity of SWCNTs by peptides, MD simulations were performed on all the selected phage-displayed peptides and SWCNTs, whose chiralities are (7, 0), (8, 0), (7, 5), (7, 6), (7, 7), and (8, 8). The initial state of the peptides was set to be the extended form according to the results from CD spectroscopy and the minimum distance between peptides and SWCNTs was larger than 10 Å. During the 20 ns simulations, peptides absorbed onto SWCNTs. All of the peptides exhibit the same binding conformation, and most amino acids are in close contact with SWCNTs with a distance of 3 ± 0.5 Å. A representative binding conformation of peptide 76-4 on a (7, 6) SWCNT is shown in Fig. 6. Most parts of a peptide attach to and interact with the SWCNT surface in a random coil form, which is consistent with peptides' secondary structures obtained by CD spectroscopy after binding to SWCNTs. Ring groups align with the SWCNT surface in a face-to-face manner to form π-stacking, which is the favorable and dominant interaction between SWCNTs and biomolecules, such as DNA33 and peptides.34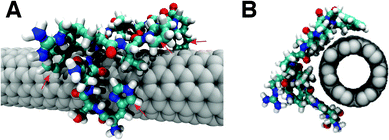 | ||
| Fig. 6 (A) Side view and (B) front view of binding conformation of peptide 76-4 (HSNWRVPSPWQL) on a (7, 6) SWCNT. Red arrows in A indicate the ring groups parallel to the SWCNT surface. | ||
The binding energy of the peptide–SWCNT complex, EB, can be estimated with the following equation:
| EB = Epeptide/SWCNT − (ESWCNT + Epeptide) |
| Peptide–(n, m) SWCNT | Diameter (nm) | Binding energy (kcal mol−1) |
|---|---|---|
| 76-4/(7, 0) | 0.566 | −35.0 ± 1.1 |
| 76-4/(8, 0) | 0.635 | −49.6 ± 5.0 |
| 76-4/(7, 5) | 0.829 | −55.3 ± 3.4 |
| 76-4/(7, 6) | 0.895 | −54.9 ± 3.9 |
| 76-4/(7, 7) | 0.963 | −64.0 ± 5.2 |
| 76-4/(8, 8) | 1.100 | −71.4 ± 3.6 |
| 76-6/(7, 0) | 0.566 | −45.3 ± 2.9 |
| 76-6/(8, 0) | 0.635 | −56.1 ± 4.0 |
| 76-6/(7, 5) | 0.829 | −59.6 ± 5.3 |
| 76-6/(7, 6) | 0.895 | −62.5 ± 4.2 |
| 76-6/(7, 7) | 0.963 | −64.0 ± 6.5 |
| 76-6/(8, 8) | 1.100 | −63.4 ± 3.7 |
Peptide 76-6, the best binder selected from the biopanning experiment, has the highest binding energy with (7, 6) in our peptide pool, yet it shows poor discernment on chirality. In contrast, as a result of its best distribution of the binding energy and the favorable energetic interaction with large-diameter tubes, peptide 76-4 is promising to preferentially select SWCNTs with large sizes, thereby narrowing the diameter distribution and distinguishing certain SWCNT chiralities. Therefore, peptides 76-4 and 76-6 were chosen for the following characterizations to study their capability of dispersing SWCNTs and enriching certain (n, m) species. Dimeric 76-4 was also synthesized to investigate whether longer peptide sequences have better SWCNT dispersibility and chiral selectivity.
SWCNT solubilization
After being probe-sonicated for 1 h followed by centrifugation at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 1 h, HiPco SWCNTs were solubilized in DI water as a homogenous dark dispersion (Fig. 7D, left) by peptides 76-4, 76-4 dimer, and 76-6. However, Only peptides with high concentration (500 μM) could disperse SWCNTs. SWCNTs remained as black flocci after sonication when a 100 μM peptide solution was used (Fig. 7D, right). The AFM image of SWCNT–peptide dispersion further confirms that our peptides can disperse SWCNTs into small nanotube bundles and individual nanotubes (Fig. 7A, 7B, and 7C). SWCNT dispersions are stable without visible aggregates for weeks in 500 μM peptide solution and for months in 1000 μM peptide solution.
000 g for 1 h, HiPco SWCNTs were solubilized in DI water as a homogenous dark dispersion (Fig. 7D, left) by peptides 76-4, 76-4 dimer, and 76-6. However, Only peptides with high concentration (500 μM) could disperse SWCNTs. SWCNTs remained as black flocci after sonication when a 100 μM peptide solution was used (Fig. 7D, right). The AFM image of SWCNT–peptide dispersion further confirms that our peptides can disperse SWCNTs into small nanotube bundles and individual nanotubes (Fig. 7A, 7B, and 7C). SWCNT dispersions are stable without visible aggregates for weeks in 500 μM peptide solution and for months in 1000 μM peptide solution.
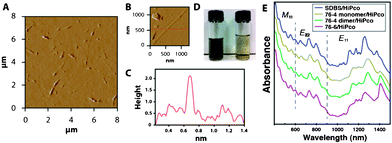 | ||
Fig. 7 (A) AFM micrograph of well dispersed HiPco SWCNTs by peptide 76-4 dimer. (B) An individually dispersed SWCNT by peptide 76-4 dimer at 500 μM. (C) AFM height profile along the red line crossing a SWCNT shown in B. (D) The photograph of HiPco SWCNTs dispersed by peptide 76-4 dimer. Left: 500 μM 76-4 dimer. Supernatant after centrifugation at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 1 h. Right: 100 μM 76-4 dimer. (E) UV-vis-NIR absorption spectra of SDBS/HiPco, 76-4/HiPco, 76-4 dimer/HiPco, and 76-6/HiPco. 000 g for 1 h. Right: 100 μM 76-4 dimer. (E) UV-vis-NIR absorption spectra of SDBS/HiPco, 76-4/HiPco, 76-4 dimer/HiPco, and 76-6/HiPco. | ||
CD spectroscopy
CD spectroscopy was used to study the original conformation of selected peptides and their secondary structure on the SWCNT surface. Peptides 76-4 and 76-6 were first dissolved in DI water and KH2PO4 buffer (500 μM, pH 7.1), respectively. The CD spectra of peptides in DI water and in buffer are almost identical (data not shown), indicating that salt is unnecessary here. The CD spectra of 100 μM peptide solutions are slightly different from those of 500 μM peptide solutions due to background noise. Remarkably weak signals were detected when no peptides were in the sample (SWCNTs dispersed by SDBS, Fig. 8B), showing that the influence of SWCNTs on the secondary structure of peptides is negligible.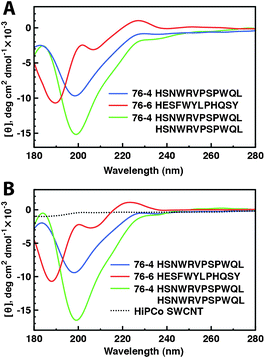 | ||
| Fig. 8 The secondary structure of SWCNT-binding peptides. (A) CD spectra of free peptides 76-4, 76-6, and a dimer of 76-4 in water at neutral pH. (B) CD spectra of peptides 76-4, 76-4 dimer, 76-6 after dispersing SWCNTs in water at neutral pH. | ||
The CD spectra of free peptides 76-4 monomer, 76-4 dimer, and 76-6 at neutral pH are shown in Fig. 8A. Peptide 76-4 monomer exhibits a typical “random coil” conformation, which has been reported as an SWCNT binding conformation.19,20Peptide 76-6 contains mainly random coil and β structure. Despite some discrepancies, we believe that both 76-4 monomer and 76-6 interact with SWCNTs in an extended form, which is verified by quantitative analysis by SELCON, showing that less than 10% peptides present in α-helical structure for both 76-4 monomer and 76-6. 76-4 dimer presents the same features of CD spectrum as the 76-4 monomer except that the signal strength is stronger. The CD spectra of all tested peptides after binding to SWCNTs are almost the same as those of the free peptide (Fig. 8B). So it is highly possible that the peptides just simply attached to the SWCNT surface without any conformational changes. It should be noted that sonication enhances solubilisation of peptides and may cause potential hydrolysis of peptides. However, in this study, probe sonication gives rise to no degradation of the peptides as the spectra are unchanged after sonication. It is reported that there was no adverse effect on the stability of 12-mer phage-displayed peptides even after tip sonication for 3 h at a power level of 20 W.35
UV-vis-NIR spectroscopy
HiPco SWCNTs were solubilized in deuterium oxide (D2O) by peptides for UV-vis-NIR examination to determine whether peptides are capable of selectively dispersing SWCNTs of certain chiralities without losing their intrinsic electronic properties. It is shown in Fig. 7E that the spectral peaks of peptide-dispersed SWCNTs shift to longer wavelength as compared to those of SDBS-dispersed SWCNTs. We attribute the red shifts to the changes of dielectric properties around the SWCNTs, reflecting a more polarizable local environment.36–38 Clear first (E11, 800–1600 nm), second (E22, 550–900 nm) van Hove transitions, and van Hove transitions of the metallic nanotubes (M11, 400–600 nm) are presented in our SWCNT samples, indicating that our peptides non-covalently bind to SWCNTs and their intrinsic electronic properties are preserved. It is also verified by Raman spectra (Fig. S2, ESI†). However, SWCNT chirality separation cannot be discerned from Fig. 7E because some peaks are broadened. Fluorescence spectroscopy was performed to identify (n, m) species in peptide-dispersed SWCNTs.Fluorescence spectroscopy
The chirality distribution of SDBS-dispersed and peptide-dispersed HiPco SWCNT samples was measured by photoluminescence excitation/emission (PLE) spectroscopy. Particular (n, m) species can be assigned on the two-dimensional emission-excitation maps as fluorescence peaks when debundled SWCNTs’ E11 matches E22.39 The spectral intensities are proportional to the corresponding (n, m) species abundance, so the relative abundance of certain (n, m) species can be deduced from the relative intensities of corresponding peaks in the emission-excitation maps. As shown in Fig. 9A,HiPco SWCNTs dispersed in SDBS have a wide distribution of chirality and the most abundant species are (8, 6) and (7, 6). Compared to SDBS dispersion, SWCNTs dispersed in 76-4 monomer and dimer show a significant red shift on the emission peaks, which is also evident in the UV-vis-NIR spectra. The most abundant chiral species in peptide-dispersed samples are (9, 5), (10, 3), and (7, 6), while (8, 6) is depleted. Some large-diameter tubes, such as (10, 6), (11, 4), and (12, 2) are enriched; on the contrary, these species are insignificant in SDBS-dispersed SWCNTs. The relative abundance for each (n, m) species was shown in the graphene sheet map (Fig. 10) and Table 4. An over 20% increase on total abundance of selected (n, m) species in peptide-dispersed samples was achieved compared to SDBS-dispersed ones, indicating that the peptides preferentially bind to large-diameter tubes (above 0.916 nm), especially those with medium or small chiral angles, as highlighted in bold in Table 4. The diameter selection was also demonstrated by MD simulations (Table 3), showing that 76-4 monomer has much higher binding energy with (8, 8) than (7, 6). However, the selection of chiral angle is not consistent with our experimental results. 76-4 monomer and dimer show no differences on dispersion preferences, implying that simply doubling or tripling the length of peptide might not be effective on chirality separation. It is noteworthy that the PLE signal of peptide-dispersed SWCNTs has more noise than that of SDBS-dispersed SWCNTs at the same concentration of SWCNT because the concentration of peptide (2 mg ml−1) is much lower than that of SDBS (10 mg ml−1). No spectrofluorometric signals could be detected on peptide 76-6-dispersed SWCNT samples for reasons unknown. The selected peptides are not effective for metallic/semiconducting SWCNT separation (Fig. S2, ESI†) and more details are available from Raman spectra in the ESI.†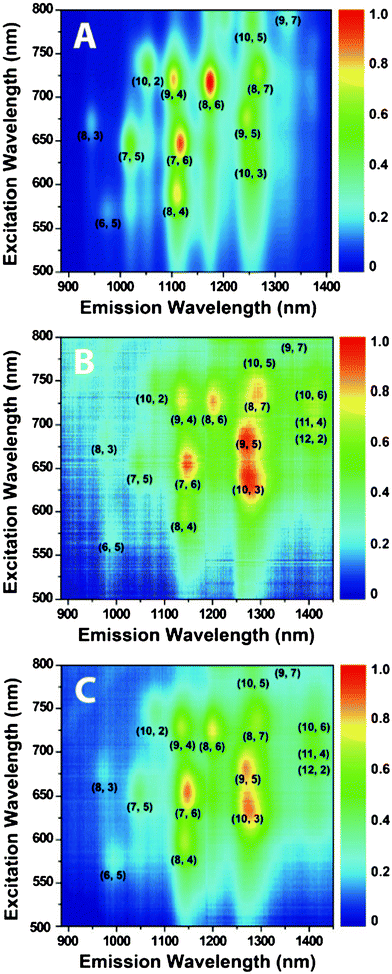 | ||
| Fig. 9 PLE intensity maps for HiPco SWCNTs dispersed in (A) SDBS/D2O, (B) 76-4/D2O, and (C) 76-4 dimer/D2O. | ||
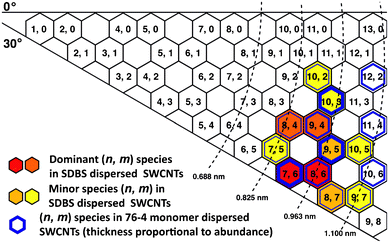 | ||
| Fig. 10 Chirality map of HiPco SWCNTs dispersed by SDBS and peptide 76-4. Red and orange hexagons are dominant (n, m) species of SDBS-dispersed SWCNTs. The thickest blue hexagons are dominant (n, m) species of 76-4-dispersed SWCNTs. The relative abundance is deduced from Fig. 9. | ||
| SWCNT species | Diameter (nm) | Chiral angle (Deg) | Relative abundance | |
|---|---|---|---|---|
| HiPco/SDBS | HiPco/76-4 | |||
| (7, 5) | 0.829 | 24.5 | 7.8% | 5.6% |
| (8, 4) | 0.840 | 19.1 | 11.5% | 7.0% |
| (10, 2) | 0.884 | 8.9 | 6.9% | 5.6% |
| (7, 6) | 0.895 | 27.5 | 13.3% | 9.6% |
| (9, 4) | 0.916 | 17.5 | 11.9% | 8.3% |
| (10, 3) | 0.936 | 12.7 | 8.3% | 10.0% |
| (8, 6) | 0.966 | 25.3 | 15.2% | 8.8% |
| (9, 5) | 0.976 | 20.6 | 9.2% | 9.7% |
| (8, 7) | 1.032 | 27.8 | 9.0% | 8.6% |
| (10, 5) | 1.050 | 19.1 | 6.7% | 5.8% |
| (12, 2) | 1.041 | 7.6 | / | 7.2% |
| (11, 4) | 1.068 | 14.9 | / | 7.2% |
| (10, 6) | 1.111 | 21.8 | / | 6.8% |
| Total abundance of selected (n, m) species | 24.2% | 46.7% | ||
Discussion
The importance of ring groups for SWCNT binding
Amino acids with ring groups were demonstrated to be critical for peptide–SWCNT affinity via the π–stacking interaction from our binding sequence analysis and MD simulations. Such interaction is known to exist between aromatic compounds and graphite. Peptides with ring groups can interact with the sidewalls of carbon nanotubes in a similar manner via π electrons because a monoelectronic π orbital orthogonal to the three σ orbitals (directed along the C–C bonds) is constructed on each carbon atom, although carbon atoms are distributed in a nonplanar system.40 It is reported that among the aromatic amino acids based on the conventional categorization (including phenylalanine, tyrosine and tryptophan), tryptophan possesses the highest affinity for CNTs, followed by tyrosine. Phenylalanine has the lowest affinity for CNTs.41 The results are consistent with ours that tryptophan is the most frequent aromatic amino acid and phenylalanine appears least often in our selected sequences. The imidazole ring of histidine contains six π electrons and thus can form π-stacking interactions as well. Therefore, it also has high affinity for carbon nanotubes and is one of the most prevalent amino acids in our peptide pool.Limitations of phage display technique on SWCNT (n, m) selection
Although phage display technology is powerful to identify peptides that have affinity to numerous materials42 including CNTs, it is noteworthy that the nature of phage-displayed peptides and SWCNTs impedes the ideal interaction between them. The ideal condition for SWCNT recognition by phage-displayed peptides is that phage can interact with individual SWCNTs. However, the high hydrophobicity of SWCNTs drives them to aggregate into bundles in aqueous environment, where the phage display selection occurs. In order to solubilize individual SWCNTs in water, amphiphiles, such as surfactants, polymers, and DNA, must be used to encapsulate the surface of SWCNTs. There is no doubt that SWCNTs can be dispersed in water by this method. Nevertheless, it leads to two other problems. First, the dispersant molecules may interfere the interaction between peptides and SWCNTs. Second, even if phage-displayed peptides can attach to the surface of surfactant encapsulated-SWCNTs, thorough separation of bound phage from unbound phage is very difficult by methods such as centrifugation, filtration, and dialysis.Some surfactants/co-surfactants,43 biomolecules44 or polymers45 can selectively bind to certain SWCNT species because they have dual effects in the selection process, i.e., dispersants and selective encapsulants. However, phages cannot play such dual roles owing to their poor ability to disperse SWCNTs. Since SWCNT dispersion and selection cannot be achieved at the same time by phage, we can realize selection first by using SWCNT bundles suspended in water or immobilized on certain supporting substrates.20,21 The limitation of this method is that in fact the phage-displayed peptides can just recognize different SWCNT surfaces rather than wrap around the SWCNTs because SWCNT bundles are too big for a 12-mer peptide. In addition, if the SWCNTs cannot totally cover the supporting substrate, i.e., phage can also interact with the substrate, a negative selection is necessary to remove the substrate-binding phage in advance. Another possibility is to use magnetic separation, which was adopted by Shiba and co-workers in phage display screening experiments on single-walled carbon nanohorns.22 The drawbacks of the method are: (1) the covalent sidewall functionalization necessary for magnetic separation may introduce perturbation to electronic and optical properties of SWCNTs, and (2) the experimental procedures are complicated.
For (n, m) SWCNT selective binding of phage-displayed peptides, the critical problem is lack of high (n, m)-enriched SWCNT samples. So far, it is uncertain whether such (n, m) selection is based on surface recognition or diameter-related selection. If it is the latter, SWCNT films having individual nanotubes without any substrate may be necessary for the screening experiments. Unfortunately, currently the SWCNT films are not robust enough because they may disassemble in the selection experiments. Thus, an alternative for (n, m) selection by phage display is to use a SWCNT-coated substrate as the target. The substrate should be completely covered by individual SWCNTs and SWCNT bundles as small as possible so that the phage-displayed peptide can wrap around SWCNTs.
Conclusions
In this work, phage display technology was used to select peptides having affinity to different chiral structures of SWCNTs. After six rounds of biopanning against (7, 5) and (7, 6)-abundant SWCNT samples, 15 specific binding sequences were identified, respectively. Many of these SWCNT-binding peptide sequences are rich in aromatic amino acids, begin with histidine, and possess hydrophobic properties. The SWCNT-binding affinity was demonstrated through the enrichment of binding sequences, statistical analysis for binding sequences, and binding affinity assay. MD simulations confirm that the best binder selected by biopanning experiments has the highest binding energy with (7, 6) SWCNTs in our peptide pool. Direct evidence of SWCNT binding was provided by the attachment of SWCNTs to selected bacteriophage-coated microspheres and the conjugation of QDs to SWCNTs. The selected peptides were in an extended form that remained unchanged after they attached to the SWCNTs' surface. MD simulations show that most peptides favorably bind to large-diameter SWCNTs. One peptide, HSNWRVPSPWQL, which has the best chiral discernment in MD simulations, preferentially dispersed large-diameter SWCNTs, such as (10, 3), (9, 5), (12, 2), (11, 4), and (10, 6). Thus SWCNTs' (n, m)-preferential dispersion has been achieved for the first time viapeptides selected by phage display experimentally.Acknowledgements
We thank Dr Gang Ma, Dr Shucong Yu, Dr Liang Wang, Tianyi Yang and Tao Peng for technical assistance.References
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603–605 CrossRef CAS.
- D. S. Bethune, C. H. Kiang, M. S. De Vries, G. Gorman, R. Savoy, J. Vazquez and R. Beyers, Nature, 1993, 363, 605–607 CrossRef CAS.
- M. S. Dresselhaus, Dresselhaus, G., Avouris, P., Eds, Carbon Nanotubes Synthesis, Structure, Properties, and Application., Springer, Berlin, Germany, 2001 Search PubMed.
- A. Javey, J. Guo, Q. Wang, M. Lundstrom and H. Dai, Nature, 2003, 424, 654–657 CrossRef CAS.
- Y. Ren, F. Li, H. M. Cheng and K. Liao, Carbon, 2003, 41, 2177–2179 Search PubMed.
- Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47–52 CrossRef CAS.
- S. M. Bachilo, L. Balzano, J. E. Herrera, F. Pompeo, D. E. Resasco and R. B. Weisman, J. Am. Chem. Soc., 2003, 125, 11186–11187 CrossRef CAS.
- M. C. Hersam, Nat. Nanotechnol., 2008, 3, 387–394 CrossRef.
- C. H. Liu, Y. Y. Liu, Y. H. Zhang, R. R. Wei and H. L. Zhang, Phys. Chem. Chem. Phys., 2009, 11, 7257–7267 RSC.
- C. H. Liu and H. L. Zhang, Nanoscale, 2010, 2, 1901–1918 RSC.
- X. Tu, S. Manohar, A. Jagota and M. Zheng, Nature, 2009, 460, 250–253 CrossRef CAS.
- S. N. Kim, Z. Kuang, J. G. Grote, B. L. Farmer and R. R. Naik, Nano Lett., 2008, 8, 4415–4420 CrossRef CAS.
- S. Y. Ju, J. Doll, I. Sharma and F. Papadimitrakopoulos, Nat. Nanotechnol., 2008, 3, 356–362 CrossRef CAS.
- A. Ortiz-Acevedo, H. Xie, V. Zorbas, W. M. Sampson, A. B. Dalton, R. H. Baughman, R. K. Draper, I. H. Musselman and G. R. Dieckmann, J. Am. Chem. Soc., 2005, 127, 9512–9517 CrossRef CAS.
- M. Sarikaya, C. Tamerler, A. K. Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577–585 CrossRef CAS.
- U. Kriplani and B. K. Kay, Curr. Opin. Biotechnol., 2005, 16, 470–475 CrossRef CAS.
- S. Wang, E. S. Humphreys, S. Y. Chung, D. F. Delduco, S. R. Lustig, H. Wang, K. N. Parker, N. W. Rizzo, S. Subramoney, Y. M. Chiang and A. Jagota, Nat. Mater., 2003, 2, 196–200 CrossRef CAS.
- M. J. Pender, L. A. Sowards, J. D. Hartgerink, M. O. Stone and R. R. Naik, Nano Lett., 2006, 6, 40–44 CrossRef CAS.
- Z. Su, T. Leung and J. F. Honek, J. Phys. Chem. B, 2006, 110, 23623–23627 CrossRef CAS.
- L. Zheng, D. Jain and P. Burke, J. Phys. Chem. C, 2009, 113, 3978–3985 CrossRef CAS.
- Y. J. Lee, H. Yi, W. J. Kim, K. Kang, D. S. Yun, M. S. Strano, G. Ceder and A. M. Belcher, Science, 2009, 324, 1051–1055 CAS.
- D. Kase, J. L. Kulp Iii, M. Yudasaka, J. S. Evans, S. Iijima and K. Shiba, Langmuir, 2004, 20, 8939–8941 CrossRef CAS.
- Y. Morita, T. Ohsugi, Y. Iwasa and E. Tamiya, J. Mol. Catal. B: Enzym., 2004, 28, 185–190 CrossRef CAS.
- Y. Cui, S. N. Kim, S. E. Jones, L. L. Wissler, R. R. Naik and M. C. McAlpine, Nano Lett., 2010, 10, 4559–4565 CrossRef CAS.
- S. Brown, T. S. Jespersen and J. Nygård, Small, 2008, 4, 416–420 Search PubMed.
- S. R. Whaley, D. S. English, E. L. Hu, P. F. Barbara and A. M. Belcher, Nature, 2000, 405, 665–668 CrossRef CAS.
- B. Wang, C. H. P. Poa, L. Wei, L. J. Li, Y. H. Yang and Y. Chen, J. Am. Chem. Soc., 2007, 129, 9014–9019 CrossRef CAS.
- T. P. Hopp and K. R. Woods, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 3824–3828 CAS.
- D. Van der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark and H. J. C. Berendsen, J. Comput. Chem., 2005, 26, 1701–1718 CrossRef CAS.
- A. Onufriev, D. Bashford and D. A. Case, Proteins: Struct., Funct., Bioinf., 2004, 55, 383–394 Search PubMed.
- D. A. Case, T. E. Cheatham, T. Darden, H. Gohlke, R. Luo, K. M. Merz, A. Onufriev, C. Simmerling, B. Wang and R. J. Woods, J. Comput. Chem., 2005, 26, 1668–1688 CrossRef CAS.
- Z. F. Kuang, S. N. Kim, W. J. Crookes-Goodson, B. L. Farmer and R. R. Naik, ACS Nano, 2010, 4, 452–458 CrossRef CAS.
- R. R. Johnson, A. T. C. Johnson and M. L. Klein, Small, 2010, 6, 31–34 CrossRef CAS.
- W. J. Fan, J. Zeng and R. Q. Zhang, J. Chem. Theory Comput., 2009, 5, 2879–2885 CrossRef CAS.
- Z. H. Gao, C. Y. Zhi, Y. Bando, D. Golberg and T. Serizawa, J. Am. Chem. Soc., 2010, 132, 4976–4977 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. P. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593–596 CrossRef CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge, R. E. Smalley, J. Schmidt and Y. Talmon, Nano Lett., 2003, 3, 1379–1382 CrossRef CAS.
- Y. Ohno, S. Iwasaki, Y. Murakami, S. Kishimoto, S. Maruyama and T. Mizutani, Phys. Status Solidi B, 2007, 244, 4002–4005 CrossRef CAS.
- S. M. Bachilo, M. S. Strano, C. Kittrell, R. H. Hauge, R. E. Smalley and R. B. Weisman, Science, 2002, 298, 2361–2366 CrossRef CAS.
- F. Tournus, S. Latil, M. I. Heggie and J. C. Charlier, Phys. Rev. B Condens. Matter., 2005, 72.
- H. Xie, E. J. Becraft, R. H. Baughman, A. B. Dalton and G. R. Dieckmann, J. Pept. Sci., 2008, 14, 139–151 CrossRef CAS.
- J. W. Kehoe and B. K. Kay, Chem. Rev., 2005, 105, 4056–4072 CrossRef CAS.
- L. Wei, B. Wang, T. H. Goh, L. J. Li, Y. H. Yang, M. B. Chan-Park and Y. Chen, J. Phys. Chem. B, 2008, 112, 2771–2774 CrossRef CAS.
- X. M. Tu and M. Zheng, Nano Res., 2008, 1, 185–194 CrossRef CAS.
- A. Nish, J. Y. Hwang, J. Doig and R. J. Nicholas, Nat. Nanotechnol., 2007, 2, 640–646 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Protocol of phage titering, sequence characterization, and Raman spectroscopy; individual amino acid frequency of CNT-75-specific and CNT-76-specific pool; amino acid grouping frequency of CNT-75-specific, CNT-76-specific, and Ph.D.-12 library; the hydrophobicity of each amino acid for CNT-75-specific and CNT-76-specific sequences; Raman spectra of SWCNTs dispersed by SDBS, 76-4 monomer, and 76-4 dimer. See DOI: 10.1039/c1ra00581b/ |
| This journal is © The Royal Society of Chemistry 2012 |
