Surface charge effect in intracellular localization of mesoporous silica nanoparticles as probed by fluorescent ratiometric pH imaging†
Yi-Ping
Chen‡
a,
Hsueh-An
Chen‡
a,
Yann
Hung
a,
Fan-Ching
Chien
b,
Peilin
Chen
b and
Chung-Yuan
Mou
*a
aDepartment of Chemistry, National Taiwan University, Taipei, 106, Taiwan. E-mail: cymou@ntu.edu.tw; Fax: (+886) 2-23660954
bResearch Center of Applied Sciences, Academica Sinica, Taipei, 115, Taiwan
First published on 2nd December 2011
Abstract
Mesoporous silica nanoparticles (MSNs) have received intensive recent interest as multi-functional nanocarriers for biological cells and tissues. Their cell uptake and intracellular distribution depends on the surface charge they carry. We develop a new approach for monitoring their uptake and intracellular localization by measuring the local pH value of the MSNs. We synthesized a ratiometric pH-sensor loaded with mesoporous silica nanoparticles that can map the location of the MSN through the site pH in HeLa cells. Positively charged MSN-TA was found to locate in higher pH regions which are identified as cytosol mostly. On the other hand, negatively charged MSN-PPs were trapped in acidic endosomes. Evidence is given for direct penetration of membranes through a charge-mediated membrane–nanoparticle interaction mechanism.
1. Introduction
Nanoparticles can traverse biological cells by exploiting several common cellular uptake mechanisms.1Nanoparticles typically end up entrapped in intracellular vesicles, such as endosomes and lysosomes, where they eventually undergo lysosomal degradation, or are released into the cytoplasm, or are exocytosed. For a particular nanoparticle system, this is usually difficult to find out. However, rational design of an efficient gene2 or drug delivery vehicle3 requires an understanding of the steps involved in the delivery process, including the cell-uptake, endosomal escape and cytoplasmic trafficking. To investigate such processes, one needs a good probe nanoparticle system where (a) size and surface properties can be well-controlled, (b) local fluorescence sensing of pH or other indicator can be built such that one can track the nanoparticle spatially, and preferably (c) multifunctionality can be easily built on.Mesoporous silica nanoparticles (MSNs) have received rapidly increasing interest as nanocarriers for biological cells.4 Its attributes include uniform mesopores, easy functionalization, multifunctionality and significant biocompatibility. Among the factors deciding the translocation of MSN nanoparticles, the surface charge of the particles is believed to be critical.5,6 We would like to study the effect of surface charge on their cell-uptake and trafficking with the use of a local fluorescent pH-nanosensor build into the MSNs to report their intracellular location.
The pH value in cell organelles has important roles in cellular activities such as membrane perforations,7apoptosis,8 multidrug resistance9 and endocytosis.10 Many diseases are associated with an abnormal pH value in cells, such as the low pH value involved in hyperuricemia, gout11 and tumour tissues.12 A measurement of the pH value at a spatially specific site in cells may become useful to provide the immediate cellular information.13 At present, only fluorescence microscope imaging can provide the necessary spatial resolution. In the last two decades, many pH-sensitive fluorescent molecules have been developed for monitoring the pH value inside cells.14 Recent developments in pH-sensitive green fluorescent proteins further enriched the applications of intracellular pH mapping.15
Several nanoparticle-based pH sensing systems have been reported in recent years, based on SERS10,16 or fluorescence nanotags.17,18 However, quantitative mapping of pH values has rarely been demonstrated in cellulo due to a lack of sensitivity or specificity. This gap can be filled by applying a high-loading pH-sensitive ratiometric fluorescence-tagged nanoparticle system,19–21 which allows the monitoring of local changes in pH in living cells with high sensitivity by confocal microscopy. The nanoparticles used were silica19 or polyacrylamide.20,21 Recently, mesoporous silica nanoparticles (MSNs)22,23 have received considerable attention and have become popular and useful nanoparticles for carrying drug and sensor agents as a multifunctional carrier.24Conjugation of highly sensitive molecules with MSNs can easily be achieved. Thus it is very desirable to add pH-sensing capability to the multi-functional MSNs. In this work, we report a ratiometric fluorescence method based on MSNs for sensing intracellular pH values and show its application in localizing MSNs with different charges inside biological cells.
The ratiometric method is based on fluorescence intensity measurements at two different wavelengths of two fluorescent dyes, one sensitive to pH and the other insensitive to pH.15 The advantage of the ratiometric sensor is that factors such as excitation source fluctuations and sensor concentration normally do not affect the ratio between the fluorescence intensities of the indicator and reference dye.25
In this study, we prepared MSN pH-nanosensors of two different surface charges, a phosphonate functionalized negatively charged MSN (functionalized with 3-trihydroxysilylpropylmethylphosphonate, MSN-PP)26 and a quaternary ammonium functionalized positively charged MSN (functionalized with N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride, MSN-TA).25 We then monitor the pH of the sites where they reside, using a fluorescence ratiometric method. For pH-sensing, we chose two well-known fluorophores, fluorescein isothiocyanate (FITC) and rhodamine B isothiocyanate (RITC) while FITC acts as the pH sensitive dye and RITC as the reference dye. Stucky and coworkers27 first used a FITC-loaded mesoporous silica film for pH sensing. Recently, it has been shown in a test tube study that the MSN-based ratiometric sensor is sensitive to pH changes between 5 and 9.28 However, its use in cellular studies has not yet been developed. The probe conjugated pH-sensitive MSN exhibits the following properties: (a) high fluorescence intensity resulting from the high loading of dyes on the large internal surface of the MSN; (b) dual fluorophores grafted on the MSN; (c) high stability and easy chemical modification. In this work, we exploit the multi-functional property of the MSN-based pH-sensor, loaded with two fluorophors and one surface charge agent, to study the influence of surface charge of the MSN on its cellular fate. Conjugation of MSN with the three different alkoxysilanes was performed through a two-step selective surface modification as shown in Scheme 1.29 The FITC-APTMS, and RITC-APTMS were co-condensed with a silica precursor during the synthesis of the MSNs. The as-synthesized FITC&RITC@MSN, with surfactant still inside the pores, was then modified with charged silane (PP or TA in Scheme 1). After the templates were removed by acidic ethanol extraction, the large amount of functionalized fluorophores attached on the interior surface then became accessible to solution.
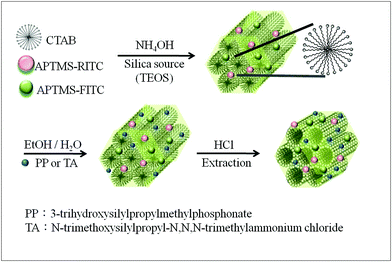 | ||
| Scheme 1 The synthesis and surface functionalization of MSNs. | ||
The synthesis of MSNs is based on a Stöber-like process developed by Mou and co-workers,22,30 which gives uniform and precise control of the size of MSN in the range 30 to 300 nm.30 Further conjugation of MSNs for the charge and fluorophor attachments follows an extraction of surfactant and silane coupling approach.
2. Materials and methods
Experimental details are given in ESI.† Only a brief description of the experiments is given in main text. Fluorescein isothiocyanate (FITC) and tetramethylrhodamine isothiocyanate (RITC) were attached to MSN by coupling with 3-aminopropyltrimethoxysilane (APTMS) in a co-condensation synthesis. We obtained negatively charged MSNs by surface attachment of phosphonate groups (MSN-PP) and positively charged MSNs by attaching quaternary ammonium groups (MSN-TA) to FITC&RITC@MSN in a post-synthesis surface modification. The Coulombic repulsion between the charged MSNs made the aggregation of particles less likely and thus produced a well-suspended solution of functionalized MSNs.A PANalytical X'Pert PRO instrument was used for collecting XRD patterns. N2 adsorption–desorption isotherms were obtained at −196 °C on a Micrometerics ASAP 2010 apparatus. TEM images were taken using a Hitachi H-7100 instrument with an operating voltage of 100 KV. Particle size in solution and Zeta potential were obtained at room temperature on a MALVEN Nano-ZS. A Hitachi F-4500 spectrofluorometer was used for obtaining fluorescence spectra. The fluorescence images were measured by confocal laser scanning microscopy (TCS SP5, Leica) using an oil immersion objective with a magnification of 63 All fluorescence microscopy was conducted using separate excitation lines for FITC (488 nm), RITC and FM 4–64 (543 nm) of an Argon ion laser. Emission was collected sequentially for FITC (514–535 nm), RITC (550–600 nm) and FM 4–64 (710–800 nm) channels as well as a bright field image. The fluorescence intensities of the ROIs in the FITC channel and the RITC channel were integrated individually to calculate ratios between the FITC and RITC intensity. FITC and RITC fluorescence of nanosensors were analyzed by flow cytometry with a FACS Calibur flow cytometry (BD Biosciences).
3. Results and discussion
Products were characterized with TEM, XRD, N2 adsorption–desorption measurements, and zeta potential. A TEM micrograph (Fig. 1a) shows the well-ordered mesoporous structure of MSN-PP (left) and MSN-TA (right) after surface modification and the average particle size is around 50 nm. We choose this size because previously we have shown it is the optimal size for cell-uptake.30 The porous properties of the MSNs were analyzed by N2 adsorption–desorption isotherms (Fig. 1b) to give surface area (MSN-PP:1050 and MSN-TA:820 m2 g−1), pore volume (MSN-PP:0.99 and MSN-TA:0.72 cm3 g−1), and BJH pore size (MSN-PP:2.6 nm and MSN-TA:2.2 nm). They exhibited characteristic type IV BET isotherms. One notes that the pore size of MSN-TA is significantly smaller than MSN-PP, indicating the positively charged TA can exchange with the surfactant CTAB during grafting and thus distributed in the nanochannels.31,32 On the other hand, the negative charge group of PP in MSN-PP probably mostly resides on the exterior surface. Typical MSN XRD patterns with three peaks were observed for the well-ordered 2D hexagonal mesostructure of the samples (Fig. S1, ESI†). In Fig. 1c, the zeta potentials of the nanosensors (MSN-PP and MSN-TA) at various pH are shown. As expected, the zeta potential for MSN-PP is quite negative and the zeta potential of MSN-TA becomes much more positive. For the positively charged MSN-TA, the zeta potential is fairly constant in the pH range 4 to 7.5 while the zeta potential of the negatively charged MSN-PP, remains almost constant in the pH range 5.5 to 9. The highly charged MSN suspensions are thus solution stable because of the strong repulsion between the MSNs.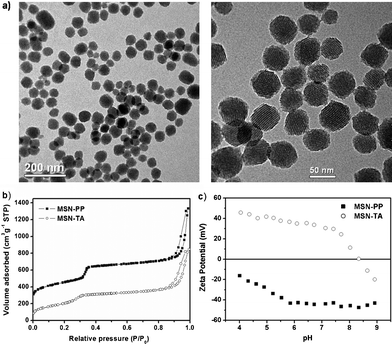 | ||
| Fig. 1 Structural characterization of MSN-TA and MSN-PP. (a) TEM images, left for MSN-PP and right for MSN-TA, (b) nitrogen adsorption isotherms, (c) zeta potential, solid square: MSN-PP, open circle: MSN-TA. | ||
We use spectrofluorometry to study the ratiometric response of MSNs to pH in sodium phosphate buffers. The fluorescence spectra excited at 490 nm (for FITC) and 540 nm (for RITC) are given in Fig. S2 and S3, ESI.† Both MSN-PP and MSN-TA show decreased fluorescence intensity of FITC with the decrease of pH. The RITC fluorescence profiles of MSN-PP and MSN-TA show little change in the pH range 5–8. These results suggested that they are highly specific for pH in sodium phosphate buffer. Fig. 2(a) (MSN-PP) and 2(b) (MSN-TA) show the plots of the fluorescence intensity ratio (FITC/RITC) versus pH value. There is a smooth sigmoid-like correlation between the fluorescence intensity ratio and the value of pH in the range 5.0–8.0. The data in Fig. 2 serve as the calibration curves for the detection of pH in a ratiometric manner, and more importantly, the detection range covers the physiological pH level inside biological cells. The calibration curve of the pH sensing roughly follows that predicted by the Henderson–Hasselbalch equation. Near both ends the readings of pH are less sensitive.
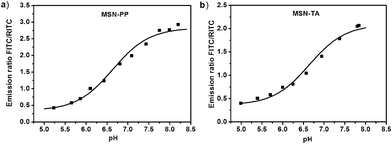 | ||
| Fig. 2 Ratios of normalized fluorescence intensities between the indicator (FITC) and reference (RITC) dyes in sodium phosphate buffers at varying pH (pH range 5.0 to 8.0). The fluorescence was measured at peaks of 515 nm (FITC) and 575 nm (RITC), respectively. (a) MSN-PP, (b) MSN-TA. | ||
We then demonstrate the application of the MSN pH-nanosensors in the cell-uptake in living cells. We delivered them into HeLa cells to find out the uptake efficiency and changes in pH value. HeLa cells were incubated with various concentrations of nanosensors (0, 70 and 100 μg mL−1) in a serum-free medium for 4 h and then the cells were washed and harvested by trypsinization. We examined the cell-uptake efficiency of nanosensors by flow cytometry for both MSN-PP (Fig. 3(a)) and MSN-TA (Fig. 3(b)). Since the red emission of RITC (second row) is pH-independent, its intensity indicates the uptake efficiency. We found that, from the flow cytometry results, the uptake efficiency for MSN-PP and MSN-TA are roughly at the same level. The charge of MSN nanosensors did not interfere much with the efficiency of cell delivery apparently. Most likely, they entered the cell through the same mechanism of endocytosis. From the top rows, we found the FITC fluorescence intensity of MSN-PP was much weaker at various concentrations as compared to MSN-TA (Fig. 3) implying MSN-PP is located in a more acidic environment in the cell. Thus, cellular localization of the sensor is important for determining the difference in FITC intensity.
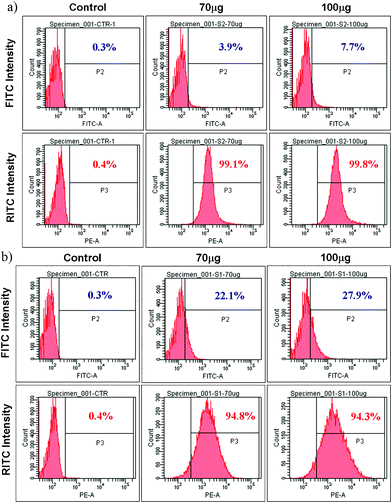 | ||
| Fig. 3 The effect of surface charge on the uptake and fluorescence intensity of MSNs by flow cytometry analysis of cell-uptake. HeLa cells were incubated separately at 37 °C with MSN-PP or MSN-TA for 4 h. Top rows are for green emission (FITC) and bottom rows are for red emission (RITC). (a) Negatively charged MSN-PP. (b) Positively charged MSN-TA. | ||
We then map the intracellular pH levels of MSN-PP and MSN-TA in HeLa cellsvia ratiometric imaging of MSNs. As shown in Fig. 4, the ratios of fluorescence at various intracellular locations are measured using confocal microscopy. The reference dye (RITC) acts not only as an internal standard for pH measurements but also as an indicator of particle location throughout the cells.
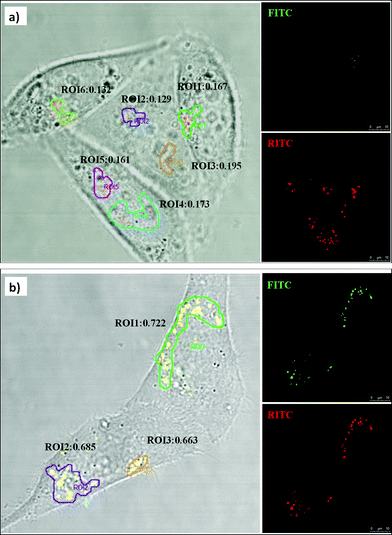 | ||
| Fig. 4 Ratiometric imaging of pH in various intracellular compartments using confocal microscopy. HeLa cells were incubated at 37 °C with MSN-PP and MSN-TA for 4 h, respectively. The images (overlaid on bright field) of pH sensors in HeLa cells showing (a) MSN-PP, and (b) MSN-TA. | ||
We located a few region of interest (ROI) and calculated the ratio of emission at wavelength of 515 nm over that at 575 nm (Fig. 4). Distinct differences of fluorescence ratio responses in HeLa cells were observed. With the sensors, we found that the pH of the intracellular locations of MSN-PP is more acidic (pH < 5) than those of MSN-TA (pH∼6). According to the calibration curves in Fig. 2, the site pH of MSN-TA is in a range 6.0 to 6.3, but the pH of MSN-PP is certainly less than 5. This qualitatively agrees with the flow cytometry results. Because of the pH detection range limitation, we are not able to give exact pH values for the MSN-PP. We can only conclude that the locations of the negatively charged MSN-PP are fairly acidic resemble those in lysosomes or late endosomes.
FM4-64 dyes are widely used to study endocytosis, vesicle trafficking and organelle organization in living eukaryotic cells. The nanosensors were co-cultured with FM4-64 (blue, Molecular Probes) to track the distribution of the nanoparticles in living HeLa cells. The confocal images (Fig. 5) show that MSNs are in the perinuclear region in discrete distribution. Merging the red color of RITC@MSN and green color of FITC@MSN gives yellow spots completely (not shown here), meaning RITC and FITC did not dissociate from the MSN. Further merging with FM4-64, finds only magenta spots with no yellow spot left (see Fig. 5). This means that MSM-PP were trapped inside the endosome/lysosome vesicles. On the other hand, MSN-TA has a different distribution pattern from that of intracellular endocytic vesicles stained with FM4-64 as compared to MSN-PP. In the merged image of the positively charged MSN-TA with FM4-64 (Fig. 6), we observed lots of yellow spots with few magenta spots found. This indicates most of the MSN-TA may have escaped from the endosomes or have directly entered the cells to reside in the cytosol. In order to escape from the endosomes, some proton sponge mechanism is usually necessary. However, N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride is a quaternary ammonium which does not exhibit the proton sponge effect. Thus at least some fraction of MSN-TA may have penetrated the cell membrane directly into the cytoplasm, or more likely penetrated the endosome membrane through a charge-mediated mechanism, which explains its dramatic increase of FITC fluorescence in our study.
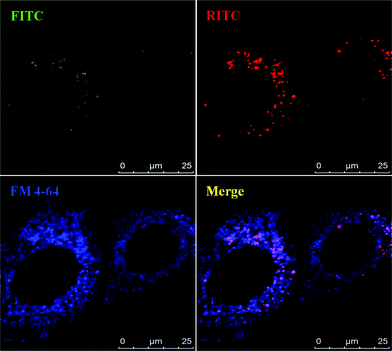 | ||
| Fig. 5 Confocal microscopy analysis of MSN-PP in HeLa cells. The living unfixed cells were co-treated with endosome-specific marker FM 4–64 (5 μg ml−1) and analyzed by confocal microscopy for an endosomal colocalization image. The fluorescent images show the MSNs (green, FITC and red, RITC) and FM 4-64-labeled endosomes (blue). | ||
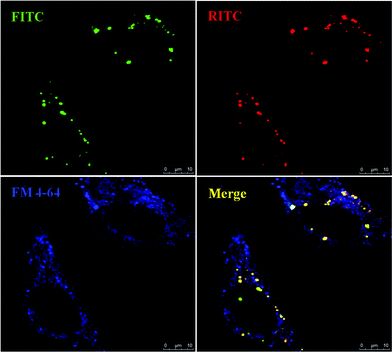 | ||
| Fig. 6 Confocal microscopy analysis of MSN-TA in HeLa Cells. The living unfixed cells were co-treated with endosome-specific marker FM 4–64 (5 μg ml−1) and analyzed by confocal microscopy for an endosomal co-localization image. The fluorescent images show the MSNs (green, FITC and red, RITC) and FM 4-64-labeled endosomes (blue). | ||
Recently, there have been several physicochemical studies, either experimental33 or theoretical,34 of membrane-particle interactions. A direct charge-mediated penetration mechanism has been proposed as an alternative to the proton-sponge mechanism for the penetration of nanoparticles through the cell membrane33 or endosome.34 Since our MSN-TA is very highly charged in the pH range of the endosome (pH = 4 to 6), the new endosome escape mechanism seems to be reasonable for MSN-TA. On the other hand, the zeta potential for the MSN-PP is not so high that they are trapped inside endosome. However, a definite answer to this problem can be reached if the MSN pH sensor can be tracked in real time which will be our future goal.
Since the positively charged MSN-TA shows a predominant cytosol distribution, it could be further functionalized to target specific intracellular organelles, such as mitochondria, for mapping their local pH values. A future potential development of our multifunctional MSN-TA (and to a lesser extent MSN-PP) pH sensor would be real-time live-cell monitoring of pH value as MSNs are uptaken in a biological cell. Cargos such as drug or DNA may be simultaneously loaded for delivery. However, for this difficult task, several improvements of the MSN materials are needed. Firstly, other pH-sensitive fluorescence molecules may also be loaded onto MSNs.14,25 Ratiometric pH-sensitive fluorescence molecules that emit in the near IR range would also be desirable.35 Moreover, in this work, the FITC fluorophor is not particularly responsive at a low pH range less than 4.5. A third dye may be added to extend the dynamic range of pH-sensing.36,37 Or, other dyes with lower pKa could be employed for the purpose of sensing the endosome–lysosome pathway.38
Finally, an issue about our MSN-based ratiometric method should be discussed. In the emission spectra excited at 490 nm, there is a 570 nm band due to FRET (ESI,† Fig. S3). Previously, Lei et al.28 exploited this FRET effect and constructed a pH-sensor. In order to have strong FRET effect, the loading of dyes in ref. 28 is about 10 times higher than ours. This, however, leads to many undesirable characteristics such as (a) self-quenching (b) sensitive to details of sample preparation because of changes in the distribution of FITCvs.RITC. The double excitation ratiometric method employed in this work is more robust where the reference emission of RITC is fairly pH-independent. In the future, separate compartmentalization, such as a core–shell arrangement,39 of the two fluorophores may be needed to avoid a pH-dependent emission of the reference dye.
4. Conclusion
We have described a new strategy for monitoring the cell-uptake and intracellular fate of MSNs by monitoring intracellular pH with a ratiometric pH sensor, carried by two kinds of mesoporous silica nanoparticles, MSN-PP and MSN-TA. We stained the endosomes with a fluorescent endosome marker (FM 4-64) and monitored the confocal fluorescence micrographs. The results reveal yellow fluorescent spots, indicating the presence of positively charged MSN-TA in the cytoplasm; but the negatively charged MSN-PP is probably trapped in the endosome/lysosome. The more positively charged MSN-TA appears to be able to penetrate directly into the cytoplasm, mostly independent of endocytosis. Moreover, the nanosensors can be used to differentiate the pH value between endosome and cellular cytoplasm at a single cell level by using confocal microscopy. Recent rapid developments in instrumentation may also allow real time tracking of nanoparticles inside cells.40 Mesoporous silica nanoparticles are a good platform to build further capabilities in intracellular pH-sensing which will help one to understand better the trafficking of nanoparticles inside a biological cell. Also, sensors for other biologically significant ions such as superoxide or chloride may be built on the platform of MSN taking advantage of its multi-functionality.Acknowledgements
The authors thank the National Project of Nanotechnology under National Science Council of Taiwan for the support of this work. We thank Ms. Yi-Fan Su for technical help.References
- F. Zhao, Y. Zhao, Y. Liu, X. L. Chang, C. Y. Chen and Y. L. Zhao, Small, 2011, 7, 1322–1337 CrossRef CAS.
- M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259–302 CrossRef CAS.
- Y. Malam, E. J. Lim and A. M. Seifalian, Curr. Med. Chem., 2011, 18, 1067–1078 CrossRef CAS.
- (a) J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, Small, 2010, 6, 1952–1967 CrossRef CAS; (b) S. H. Wu, Y. Hung and C. Y. Mou, Chem. Commun., 2011, 47, 9972–9985 RSC.
- (a) T. H. Chung, S. H. Wu, M. Yao, C. W. Lu, Y. S. Lin, Y. Hung, C. Y. Mou, Y. C. Chen and D. M. Huang, Biomaterials, 2007, 28, 2959–2966 CrossRef CAS; (b) T. Xia, M. Kovochich, M. Liong, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 85–96 CrossRef CAS.
- I. Slowing, B. G. Trewyn and V. S.-Y. Lin, J. Am. Chem. Soc., 2006, 128, 14792–14793 CrossRef CAS.
- L. M. Shaughnessy, A. D. Hoppe, K. A. Christensen and J. A. Swanson, Cell. Microbiol., 2006, 8, 781–792 CrossRef CAS.
- A. Ishaque and M. Al-Rubeai, J. Immunol. Methods, 1998, 221, 43–57 CrossRef CAS.
- H. C. Kang, O. Samsonova and Y. H. Bae, Biomaterials, 2010, 31, 3071–3078 CrossRef CAS.
- A. Pallaoro, G. B. Braun, N. O. Reich and M. Moskovits, Small, 2010, 6, 618–622 CrossRef CAS.
- S. Takahashi, T. Inokuchi, T. Kobayashi, T. Ka, Z. Tsutsumi, Y. Moriwaki and T. Yamamoto, Horm. Metab. Res., 2007, 39, 511–514 CrossRef CAS.
- H. Izumi, T. Torigoe, H. Ishiguchi, H. Uramoto, Y. Yoshida, M. Tanabe, T. Ise, T. Murakami, T. Yoshida, M. Nomoto and K. Kohno, Cancer Treat. Rev., 2003, 29, 541–549 CrossRef CAS.
- N. M. Walker, J. E. Simpson, R. C. Levitt, K. T. Boyle and L. L. Clarke, J. Pharmacol. Exp. Ther., 2006, 317, 275–283 CrossRef CAS.
- R. Wang, C. W. Yu, F. B. A. Yu and L. X. Chen, TrAC, Trends Anal. Chem., 2010, 29, 1004–1013 CrossRef CAS.
- R. Bizzarri, M. Serresi, S. Luin and F. Beltram, Anal. Bioanal. Chem., 2009, 393, 1107–1122 CrossRef CAS.
- J. Kneipp, H. Kneipp, B. Wittig and K. Kneipp, Nanomed.: Nanotechnol., Biol. Med., 2010, 6, 214–226 CrossRef CAS.
- A. Burns, H. Ow and U. Wiesner, Chem. Soc. Rev., 2006, 35, 1028–1042 RSC.
- L. N. Sun, H. S. Peng, M. I. J. Stich, D. Achatz and O. S. Wolfbeis, Chem. Commun., 2009, 5000–5002 RSC.
- (a) A. Burns, P. Sengupta, T. Zedayko, B. Baird and U. Wiesner, Small, 2006, 2, 723–726 CrossRef CAS; (b) T. Doussineau, A. Schulz, A. Lapresta-Fernandez, A. Moro, S. Korsten, S. Trupp and G. J. Mohr, Chem.–Eur. J., 2010, 16, 10290–10299 CrossRef CAS.
- P. G. Coupland, S. J. Briddon and J. W. Aylott, Integr. Biol., 2009, 1, 318–323 RSC.
- H. Sun, A. M. Scharff-Poulsen, H. Gu and K. Almdal, Chem. Mater., 2006, 18, 3381–3384 CrossRef CAS.
- Y. S. Lin, C. P. Tsai, H. Y. Huang, C. T. Kuo, Y. Hung, D. M. Huang, Y. C. Chen and C. Y. Mou, Chem. Mater., 2005, 17, 4570–4573 CrossRef CAS.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC.
- M. Vallet-Regi, M. Colilla and B. Gonzalez, Chem. Soc. Rev., 2011, 40, 596–607 RSC.
- X. J. Wan, D. Wang and S. Y. Liu, Langmuir, 2010, 26, 15574–15579 CrossRef CAS.
- J. Lu, M. Liong, J. I. Zink and F. Tamanoi, Small, 2007, 3, 1341–1346 CrossRef CAS.
- G. Wirnsberger, B. J. Scott and G. D. Stucky, Chem. Commun., 2001, 119–120 RSC.
- J. Lei, L. Wang and J. Zhang, Chem. Commun., 2010, 46, 8445–8447 RSC.
- D. Bruhwiler, Nanoscale, 2010, 2, 887–892 RSC.
- F. Lu, S. H. Wu, Y. Hung and C. Y. Mou, Small, 2009, 5, 1408–1413 CrossRef CAS.
- A. B. Bourlinos, T. Karakostas and D. Petridis, J. Phys. Chem. B, 2003, 107, 920 CrossRef CAS.
- N. Gartmann and D. Brühwiler, Angew. Chem., Int. Ed., 2009, 48, 635 CrossRef.
- J. Chen, J. A. Hessler, K. Putchakayala, B. K. Panama, D. P. Khan, S. Hong, D. G. Mullen, S. C. DiMaggio, A. Som, G. N. Tew, A. N. Lopatin, J. R. Baker, Jr, M. M. B. Holl and B. G. Orr, J. Phys. Chem. B, 2009, 113, 11179–11185 CrossRef CAS.
- C. L. Ting and Z. G. Wang, Biophys. J., 2011, 100, 1288–1297 CrossRef CAS.
- S. A. Hilderbrand, K. A. Kelly, M. Niedre and R. Weissleder, Bioconjugate Chem., 2008, 19, 1635–1639 CrossRef CAS.
- R. V. Benjaminsen, H. Sun, J. R. Henriksen, N. M. Christensen, K. Almdal and T. L. Andresen, ACS Nano, 2011, 5, 5864–5873 CrossRef CAS.
- H. Sun, K. Almdal and T. L. Andresen, Chem. Commun., 2011, 47, 5268–5270 RSC.
- J. Han and K. Burgess, Chem. Rev., 2010, 110, 2709–2728 CrossRef CAS.
- T. Doussineau, M. Smaihi and G. J. Mohr, Adv. Funct. Mater., 2009, 19, 117–122 CrossRef CAS.
- M. Lakadamyali, M. J. Rust, H. P. Babcock and X. Zhuang, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9280–9285 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental details of materials synthesis and characterization, XRD, fluorescence spectra of MSN-PP and MSN-TA. See DOI: 10.1039/c1ra00586c/ |
| ‡ These two authors contribute equally. |
| This journal is © The Royal Society of Chemistry 2012 |
