Synthesis of cubic and hexagonal ordered mesoporous YVO4:Eu3+ and their photoluminescence properties
Qiuling
Luo
a,
Shaodian
Shen
b,
Guanzhong
Lu
*ab,
Xiuzhen
Xiao
a,
Dongsen
Mao
b and
Yanqin
Wang
a
aKey Laboratory for Advanced Materials and Research Institute of Industrial catalysis, East China University of Science and Technology, Shanghai, 200237, P. R. China. E-mail: gzhlu@ecust.edu.cn; Fax: +86-21-64253824
bResearch Institute of Applied Catalysis, School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai, 200235, P. R. China
First published on 15th November 2011
Abstract
Cubic and hexagonal ordered mesoporous Eu3+-doped yttrium vanadate (YVO4:Eu3+) have been synthesized successfully through the nanocasting route with a Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol system as a guest unit and KIT-6 or SBA-15 silica as the hard template host, and were characterized by X-ray diffraction (XRD), transmission electronic microscopy (TEM) and low-temperature nitrogen adsorption. The prepared YVO4:Eu3+ samples have the characteristic cubic (Ia3d) (or hexagonal (p6mm)) ordered mesostructure based on different hard templates, and possess high surface area, large pore volume and uniform pore size distribution. Photoluminescence (PL) measurement shows that the main red emission peaks of two mesoporous YVO4:Eu3+ samples appear at 618 nm, and different mesostructures lead to different optimum concentrations of Eu3+ dopant and different PL intensities. For the hexagonal mesoporous YVO4:Eu3+, when Eu3+ dopant is 5(mol)% the highest PL intensity can be reached; for the cubic one, optimized Eu3+ amount is 8 mol%. The interaction between Eu3+ ions in hexagonal (p6mm) mesoporous YVO4:Eu3+ is more active than that in cubic (Ia3d) mesoporous YVO4:Eu3+, thus the absorbed energy is dissipated by nonradiation; while the cubic mesoporous YVO4:Eu3+ has more Eu3+ luminescent sites, compared with the hexagonal mesostructure one.
Introduction
Rare earth doped vanadate is an important solid-state luminescent material, and it has been used widely as the phosphors and fluorescent probes,1–4catalysts,5–8 polarizers and laser host materials.9,10 In the previous work, most studies of vanadate focused on the size and morphology of the particle,11–13 and little was reported the preparation of mesoporous vanadate as porous-structural solid-state luminescent materials. With the development of the preparation techniques for mesoporous materials, multifunctional materials with mesoporous structures, photoelectric and magnetic properties, have been paid more attention. Various kinds of highly ordered mesoporous metals,14 metal oxides,15,16 carbides,17nitrides,18,19 and sulfides20 have been synthesized, which have wide potential applications. However, the studies on the synthesis and property of mesoporous solid-state luminescent materials are limited, because of the difficulty from their multi-component complexity and easy precipitation of precursors. Therefore, highly ordered mesoporous vanadate compounds with different mesoporous network topologies have not been reported yet.For mesoporous materials, numerous previous works are related to preparation of replicas (metal, metal oxides, etc.) with various mesoporous network topologies, while their characteristics (optical, electric, and magnetic properties) and applications are usually touched upon only for a single compound with only certain topological mesostructures. Few papers are related to the comparative study of multi-topological macrostructure compounds, we think, which is an important research project for the studies and applications of multi-functional mesoporous materials. Among various topological mesostructure materials, the most commonly porous network topologies are two-dimensional (2D) hexagonal (p6mm) and three-dimensional (3D) cubic (Ia3d). The mesoporous network topology of 2D hexagonal (p6mm) shows highly symmetrical tubular channels, and ones with three-dimensional (3D) cubic (Ia3d) present an interpenetrating bi-continuous network with highly branched channels. If inorganic materials with various pore network topologies can be obtained, more potentially commercial requirements may emerge. For instance, a perfect material combining the features of a “cavity” structure for the storage/delivery of specific species and photoluminescence (PL) for simultaneous tracking or monitoring these species should be designed and developed, in which the absorption amount, storage ability, and release rate could be controlled or adjusted through the different mesoporous network topologies, and the luminescent properties would vary with different internal structures. This material also could avoid steric hindrance for guests, compared with the post-loaded PL nanoparticles in the mesoporous silica or carbon.13,21–27 Therefore, a kind of multifunctional mesoporous vanadate luminescent material is vividly portrayed.
In this paper, we have firstly synthesized 2D hexagonal (p6mm) and 3D cubic (Ia3d) ordered mesoporous YVO4:Eu3+, and compared the difference of PL properties between YVO4:Eu3+ samples with different pore network topologies. YVO4:Eu3+ shows strong red emission excited by UV light by an efficient energy transfer from excited VO43− complex anions to Eu3+ activator ions.1 It is an excellent red emitting material. Nevertheless, it is hard to find appropriate soluble precursors to prepare the highly ordered mesoporous YVO4:Eu3+ materials.
Herein, a strong acid was added to the precursor to make a homogeneous liquid solution (Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol). Mesoporous 2D hexagonal (p6mm) and 3D cubic (Ia3d) YVO4:Eu3+ were synthesized by using mesoporous SBA-15 and KIT-6 hard templates, and the effect of pore network topology on their PL properties was investigated. It is interesting, when the highest PL intensity of YVO4:Eu3+ was reached, the concentration of Eu3+ dopant was 5 mol% for the hexagonal mesoporous one and 8% for the cubic one. It suggests that there are large numbers of Eu3+ luminescent sites in the 3D cubic mesoporous host, due to the cubic (Ia3d) mesoporous YVO4:Eu3+ with more open mesostructure channels having a larger effective surface compared with the hexagonal (p6mm) mesostructure.
Experimental section
Preparation of samples
The mesoporous silica (KIT-6 and SBA-15) hard template was prepared according to the established procedures.28,29 Ordered mesoporous YVO4:Eu3+ (Y:Eu = 0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08) was synthesized as follows: 0.352 g (0.92 mmol) Y(NO3)3·6H2O (Aldrich), 0.036 g (0.08 mmol) Eu(NO3)3·6H2O (Aldrich), 3 ml concentrated nitrate acid, and 0.117 g (1 mmol) NH4VO3 (Aldrich) were added in sequence in 10 ml ethanol under magnetic stirring till a yellow clear solution was obtained. Then, 0.15 g KIT-6 or 0.2 g SBA-15 hard template was added to the above solution. This mixture solution was further stirred until a completely dry powder was obtained. Subsequently, the samples were heated slowly to 873 K and calcined at 873 K for 4 h to form crystalline YVO4:Eu3+ in the channel of the silica template. The obtained YVO4:Eu3+/silica composite sample was treated with hot 2 M NaOH solution 3 times to remove the silica template, followed by washing it with de-ionized water several times, and it was dried at 373 K.
0.08) was synthesized as follows: 0.352 g (0.92 mmol) Y(NO3)3·6H2O (Aldrich), 0.036 g (0.08 mmol) Eu(NO3)3·6H2O (Aldrich), 3 ml concentrated nitrate acid, and 0.117 g (1 mmol) NH4VO3 (Aldrich) were added in sequence in 10 ml ethanol under magnetic stirring till a yellow clear solution was obtained. Then, 0.15 g KIT-6 or 0.2 g SBA-15 hard template was added to the above solution. This mixture solution was further stirred until a completely dry powder was obtained. Subsequently, the samples were heated slowly to 873 K and calcined at 873 K for 4 h to form crystalline YVO4:Eu3+ in the channel of the silica template. The obtained YVO4:Eu3+/silica composite sample was treated with hot 2 M NaOH solution 3 times to remove the silica template, followed by washing it with de-ionized water several times, and it was dried at 373 K.
Characterization of samples
Powder X-ray diffraction (XRD) patterns were recorded on a Panalytical X'Pert with Cu Kα radiation (λ = 0.15346 nm). N2 adsorption–desorption isotherms were recorded at 77 K on a Micromeritics ASAP 2020 instrument, and the samples were degassed at 523 K and 10−6 Torr for 10 h prior to measurement. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area of sample, and the pore size distribution was calculated by Barrett–Joyner–Halanda (BJH) method. The total pore volume (Vp) of sample was estimated based on the amount adsorbed at the relative pressure of p/p0 = 0.99. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-2100 microscope operated at 200 kV, and the sample to be measured was first dispersed in ethanol and then collected using copper grids covered with carbon film. Energy dispersive X-ray spectroscopy (EDS) patterns were performed on a Philips instrument. Photoluminescence spectra were recorded on a Varian Cary Eclipse Fluorescence Spectrophotometer at room temperature equipped with a 150 W Xe lamp in the range 200–800 nm with a resolution of 1 nm. The quantum yield data were recorded on a fluoromax-4 spectrofluorometer of Horiba Scientific.Results and discussion
Powder XRD
Cubic and hexagonal ordered mesoporous YVO4:Eu3+ were fabricated by a nanocasting route and employed a homogenous Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol solution system as a guest and mesoporous silica KIT-6 or SBA-15 as the hard template host. Fig. 1 shows the small angle X-ray diffraction (SAXRD) patterns of mesoporous silica KIT-6 and cubic ordered mesoporous YVO4:Eu3+ materials. It can be seen that the mesoporous silica KIT-6 template exhibits several obvious diffraction peaks, characteristic of a highly ordered cubic Ia3d structure. The mesoporous YVO4:Eu3+ cast from the mesoporous KIT-6 silica shows a SAXRD pattern with the same diffraction peak (211) position which was less resolved in comparison with its parents.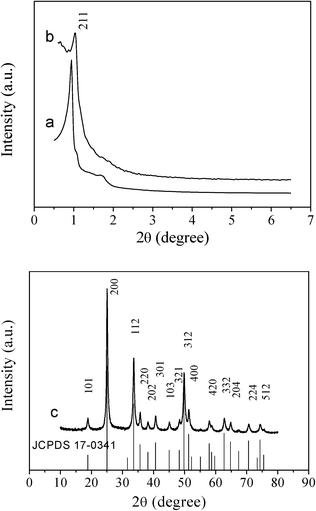 | ||
| Fig. 1 Small-angle XRD patterns of (a) cubic ordered mesoporous KIT-6 silica, (b) cubic ordered mesoporous YVO4:Eu3+, and wide-angle XRD pattern of (c) cubic ordered mesoporous YVO4:Eu3+. The standard data for phase YVO4:Eu3+ (JCPDS 17-0341) is also presented in the figure. | ||
Likewise, the SAXRD pattern of the hexagonal ordered mesoporous YVO4:Eu3+ material in Fig. 2 shows the same diffraction peak (100) positions and is less resolved compared with the hard template of hexagonal mesoporous SBA-15. These results suggest that the homogenous Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol solution can be efficiently infiltrated into the silica cavity, and the mesostructure of the sample can be maintained during the thermal treatment process. The unit cell parameter of mesoporous silica KIT-6 calculated from the (211) diffraction is 22.5 nm and SBA-15 (100) is 10.7 nm, and the unit cell parameter of corresponding mesoporous YVO4:Eu3+ is 20.3 nm and 10.0 nm, respectively. A slight shrinkage occurred and is attributed to a high degree of condensation of silicate during calcination to form the YVO4:Eu3+ mesostructure. Consequently, it can be confirmed that the mesoporous YVO4:Eu3+ products are negative replicas of mesoporous silica templates.
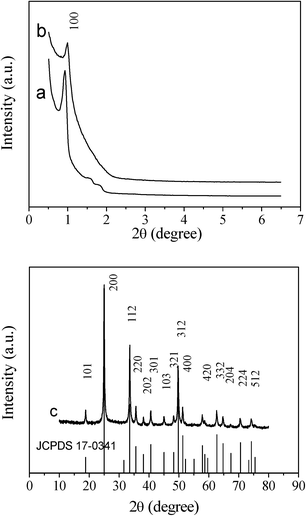 | ||
| Fig. 2 Small-angle XRD patterns of (a) hexagonal ordered mesoporous SBA-15 silica, (b) hexagonal ordered mesoporous YVO4:Eu3+, and wide-angle XRD pattern of (c) hexagonal ordered mesoporous YVO4:Eu3+. The standard data for phase YVO4:Eu3+ (JCPDS 17-0341) is also presented in figure. | ||
In the wide angle XRD patterns of mesoporous YVO4:Eu3+ samples in Fig. 1c and 2c, there are the same diffraction peaks of the tetrahedral phase YVO4 (JCPDS 17-0341), that is to say, the mesoporous YVO4:Eu3+ material with 3D cubic mesostructure exhibits the same crystalline structure as the sample with 2D hexagonal mesostructure. Also, because of the small amount of Eu3+, the wide angle XRD patterns of YVO4:Eu3+ are the almost same as that of the tetrahedral phase YVO4 and the diffraction peaks of Eu3+ compounds cannot be observed, suggesting that Eu3+ ions have been homogeneously incorporated or dispersed into the YVO4 host lattice.30
TEM
TEM images of cubic and hexagonal mesoporous YVO4:Eu3+, prepared with KIT-6 or SBA-15 hard templates, are shown in Fig. 3. The representative TEM images along [531] and [111] directions31 (Fig. 3A, B) reveal that this YVO4:Eu3+ sample consists of large ordered domains of cubic Ia3d structures which is a good replication of mesoporous KIT-6. The high-resolution TEM (HRTEM) image (Fig. 3C) shows the structures and crystal walls of the pores, which can also be confirmed by the wide angle XRD results (Fig. 1c). The spacing of the lattice fringe is ∼0.35 nm, which can be attributed to [200] direction (d = 0.3559 nm).![TEM images (A) along [531] and (B) [111] direction) and (C) HRTEM image of the cubic (Ia3d) ordered mesoporous YVO4:Eu3+ prepared by the nanocasting route with KIT-6 silica template; TEM images ((D) along [110] direction), (E) HRTEM image and (F) EDS spectrum of the hexagonal (P6mm) ordered mesoporous YVO4:Eu3+ prepared with the SBA-15 silica template.](/image/article/2012/RA/c1ra00653c/c1ra00653c-f3.gif) | ||
| Fig. 3 TEM images (A) along [531] and (B) [111] direction) and (C) HRTEM image of the cubic (Ia3d) ordered mesoporous YVO4:Eu3+ prepared by the nanocasting route with KIT-6 silica template; TEM images ((D) along [110] direction), (E) HRTEM image and (F) EDS spectrum of the hexagonal (P6mm) ordered mesoporous YVO4:Eu3+ prepared with the SBA-15 silica template. | ||
The TEM images (Fig. 3D) along the [110] direction show the hexagonal P6mm ordered mesoporous structure of YVO4:Eu3+ prepared with the SBA-15 hard template, and in the HRTEM image (Fig. 3E), we can clearly see the lattice fringes of the walls and strip channels, which is different from the cubic Ia3d structure (Fig. 3C). The spacing of the lattice fringe is ∼0.34 nm, considering the measurement error, this datum is also close to d = 0.3559 nm ([200] direction in Fig. 2c). The spectrum of energy dispersive X-ray spectroscopy (EDS) (Fig. 3F) shows that, after leaching SiO2 with hot 2 M NaOH three times, only a small amount of silica can be detected in the surface (this situation of using KIT-6 template is similar to that of using SBA-15), due to the strong interaction between the host silica template and heavy rare earth element guest.32–34 The Eu content is 1.15% (atom), Y is 17.48%, V is 16.43%, and other elements include O and a small number of Si (< 4%) and Au (this sample was treated by gold sputtering).
N2 adsorption–desorption isotherms
Fig. 4 shows the N2 adsorption–desorption isotherms and pore diameter distribution curves of hard templates and mesoporous YVO4:Eu3+ replicas, and the BET surface areas, total pore volumes, pore diameters and cell parameters of these samples are listed in Table 1. The isotherms of KIT-6 and SBA-15 templates are the typical IV-type curves with an H1-type hysteresis loop of mesoporous materials. The mesoporous silica KIT-6 has a little larger BET surface area (737 m2 g−1), higher pore volume (1.44 cm3 g−1) and lager pore size (9.5 nm), in comparison with the SBA-15 template (the BET surface area of 663 m2 g−1, pore volume of 0.93 cm3 g−1 and pore size of 7.7 nm). As a replica of SBA-15, hexagonal ordered mesoporous YVO4:Eu3+ exhibits a slightly higher BET surface area (127 m2 g−1), pore volume (0.22 cm3 g−1) and pore size (6.4 nm) than the cubic mesoporous YVO4:Eu3+ (the BET surface area of 104 m2 g−1, pore volume of 0.20 cm3 g−1 and pore size of 4.2 nm). The results above mean that the replica of KIT-6 cubic mesoporous YVO4:Eu3+ has a thicker wall, a slightly smaller pore size and pore volume than the hexagonal one; a homogenous solution can more easily infiltrate and fill the 3D cubic mesostructure silica template with larger pore size and pore volume than the 2D hexagonal template.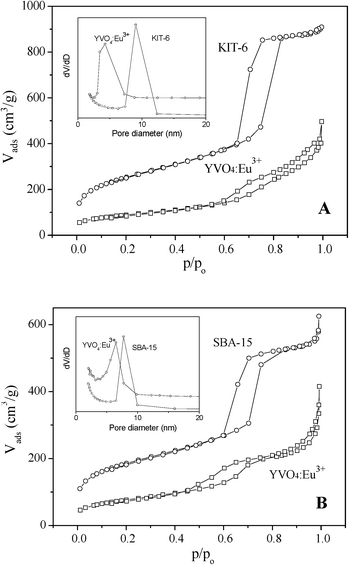 | ||
| Fig. 4 Nitrogen adsorption–desorption isotherms of (A) KIT-6 hard template and cubic ordered mesoporous YVO4:Eu3+ prepared with KIT-6 hard template, (B) SBA-15 hard template and hexagonal ordered mesoporous YVO4:Eu3+ prepared with SBA-15 hard template, and (inset) the pore-size distribution curves of samples. | ||
| Sample | SA (m2 g−1) | Pore vol. (cm3 g−1) | Pore size (nm) | a o a (nm) | d hkl (nm) |
|---|---|---|---|---|---|
| a The cell parameter, cubic a0 = d211(6)0.5; hexagonal a0 = d100(4/3)0.5. | |||||
| KIT-6 | 737 | 1.44 | 9.5 | 22.5 | 9.2 (d211) |
| Meso YVO4:Eu3+ (cubic) | 104 | 0.20 | 4.2 | 20.3 | 8.3 (d211) |
| SBA-15 | 663 | 0.93 | 7.7 | 10.7 | 9.3 (d100) |
| Meso YVO4:Eu3+ (hexagonal) | 127 | 0.22 | 6.4 | 10.0 | 8.7 (d100) |
Cubic mesoporous YVO4:Eu3+ also displays the IV-type isotherm of mesoporous material. However, the isotherm of hexagonal mesoporous YVO4:Eu3+ displays two steps at p/p0 = 0.5∼0.7 and 0.8∼1.0. The former step corresponds to the uniform mesopores replicated from SBA-15 and the latter the voids, possibly due to the incomplete filling of precursor inside the long-narrow channels of SBA-15.31,35,36
These results above suggest that well-ordered mesoporous YVO4:Eu3+ can be generated inside the mesoporous silica hard template by employing a new guest unit, in which the Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol homogenous solution can be infiltrated firstly into the mesopore silica hard template through capillary and host–guest interactions,37,38 subsequently confined growth inside the channels of the KIT-6 or SBA-15 host by the evaporation of the solvent, resulting in the formation of a YVO4:Eu3+/silica composite. After the silica template is removed from the composite, the ordered mesoporousYVO4:Eu3+ can be obtained. In the process of preparing the sample, we have found that the channels of cubic mesoporous KIT-6 are more favorable to an infiltration of the homogenous solution than that of hexagonal SBA-15.
Photoluminescence properties
Mesoporous YVO4:Eu3+ materials exhibit tunable and intense optical properties by adding an appropriate amount of Eu3+. PL spectra of cubic and hexagonal ordered mesoporous YVO4:Eu3+ have been tested at room temperature and under the same conditions, and the results are presented in Fig. 5–7.The excitation spectra of cubic and hexagonal ordered mesoporous YVO4:Eu3+ (8%), taken from the 5D0–7F2 emission at λem = 618 nm, are shown in Fig. 5 (dashed line). A broad band at 200∼350 nm can be observed in the excitation spectra, ascribed to the charge transfer from the oxygen ligands to the central vanadium ions inside the VO43−groups.39,40 The general f–f transitions within Eu3+ (4f6 electron configuration) in the longer wavelength region can hardly be observed due to their weak intensity relative to that of VO43−.40,41 Hence, an excitation of the Eu3+ ions is mainly caused by the energy transfer from the VO43−groups to Eu3+ ions.
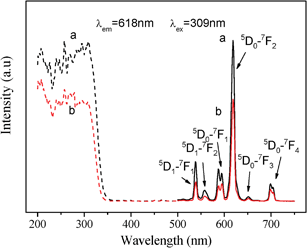 | ||
| Fig. 5 Excitation spectra (dashed line) and emission spectra (solid line) of (a) cubic and (b) hexagonal ordered mesoporous YVO4:Eu3+ (Y/Eu = 92/8, mol) materials. | ||
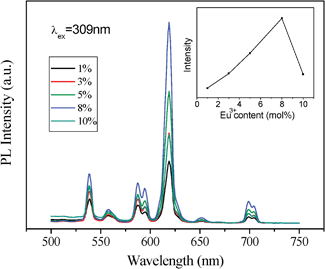 | ||
| Fig. 6 Effect of the Eu3+ dopant concentration on PL of cubic ordered mesoporous YVO4: Eu3+ material and (inset) on the intensity of peak at 618 nm. | ||
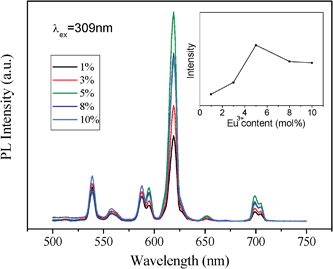 | ||
| Fig. 7 Effect of the Eu3+ dopant concentration on PL of hexagonal ordered mesoporous YVO4: Eu3+ material and (inset) on the intensity of peak at 618 nm. | ||
The emission spectra (solid line), taken at an excitation wavelength of 309 nm, shows that the mesoporous YVO4:Eu3+ materials behave the characteristic lines from low energy level 5D0 to 7FJ (J = 1, 2, 3, 4) of Eu3+42 at ∼590 nm, 618 nm, 652 nm and 698 nm, respectively. And the peaks at 537 nm and 558 nm are from 5D1 to 7FJ (J = 1, 2). The presence of emission lines from higher excited states of Eu3+ (5D1, 5D2 and 5D3) is attributed to the low vibration energy of VO43−groups (823 cm−1). The multiphonon relaxation of VO43− is not completely able to bridge the gaps between the higher energy levels (5D1, 5D2 and 5D3) and 5D0 level of Eu3+, resulting in the weak emission from these levels.43 The highest intensity at 618 nm in the PL spectra of cubic and hexagonal mesoporous YVO4:Eu3+ should attribute to the transition of 5D0–7F2. This suggests that the electric-dipole transition emission (5D0–7F2) is dominant, which results from an absence of an inversion symmetry at the Eu3+ lattice sites (D2d symmetry) for both mesoporous YVO4 hosts.44 The results in Fig. 5 also show that the positions of peaks in both spectra are of indifference, which means that the essential environment of the Eu3+ ions in the cubic mesoporous YVO4 host is almost the same as one in the hexagonal YVO4.
However, the intensity of cubic mesoporous YVO4:Eu3+ (8%) at 618 nm is higher than that of the hexagonal one (Fig. 5), and the quantum yield of cubic ordered mesoporous YVO4:Eu3+ (8%) is 47.29% and that of hexagonal one is 36.04%. This is because when the concentration of Eu3+ ions is 8%, the cubic ordered mesoporous YVO4:Eu3+ get the optimum concentration of Eu3+, and for the hexagonal ordered mesoporous YVO4:Eu3+, it is a quenching concentration of Eu3+ ions, which affect its quantum yield.
Interestingly, an obviously difference of the relative intensity with the concentration of Eu3+ ions between two YVO4:Eu3+ samples can be observed. The results in Fig. 6 and 7 show that the PL intensity of YVO4:Eu3+ varies considerably with a change of Eu3+concentration, and the highest PL intensity of the cubic mesoporous YVO4:Eu3+ at 8 mol% Eu3+ and the highest PL intensity of the hexagonal one at 5% can be observed. It can be explained by the interaction between rare earth ions because of the typical concentration quenching of lanthanide-doped system due to a mutual interaction between Eu3+ ions.45–48 The interaction between Eu3+ ions in hexagonal mesoporous YVO4:Eu3+ is stronger than that in cubic mesoporous YVO4: Eu3+, which dissipates the absorbed energy by nonradiation. Their difference is attributed to different mesoporous topology structures. The formed different surface defaults and channel arrangements make the surface available for absorbing UV light and the distance of mutual interaction Eu3+ ions can be different, which affects the number of luminescent centres in the respective emitting levels, resulting in different optimum concentrations of Eu3+ dopant.49
Conclusions
In summary, Eu3+-doped yttrium vanadate (YVO4:Eu3+) with cubic (Ia3d) and hexagonal (p6m) ordered mesopores have been synthesized successfully through the nanocasting route by employing a Y(NO3)3/Eu(NO3)3/NH4VO3/HNO3/ethanol solution system as a guest unit and KIT-6 or SBA-15 silica as the hard template host. The cubic or hexagonal ordered YVO4:Eu3+ materials possesses the typical mesoporous features, high surface area, large pore volume and uniform pore size distribution, which are the fine replication of KIT-6 or SBA-15 hard templates.Both cubic and hexagonal mesoporous YVO4:Eu3+ materials display a good luminescence property and the same characteristic lines from the low energy level 5D0 to 7FJ (J = 1, 2, 3, 4) of Eu3+. However, there are different quenching concentrations for two cubic and hexagonal mesoporous YVO4:Eu3+ samples, the highest PL intensity of the cubic mesoporous YVO4:Eu3+ at 8 mol% Eu3+ and the highest PL intensity of the hexagonal one at 5 mol%, which suggest the different mesoporous channels of the cubic and hexagonal mesoporous YVO4:Eu3+ lead to different defaults and available surfaces for absorption of the UV light. The studies on the synthesis and comparison of various mesostructure fluorescent materials might lead to better ideas on the further development of solid-state luminescent materials and other related materials (such as catalysis and energy materials), and the multifunctional YVO4:Eu3+ materials will promise a good candidate for the applications in combination of carrier and tracer.
Acknowledgements
This project was supported financially by the National Basic Research Program of China (2010CB732300), and the National Natural Science Foundation of China (20971087/B0101), the Science and Technology Commission of Shanghai Municipality (08ZR1418800) and the Education Commission of Shanghai Municipality (J51503).References
- K. Riwotzki and M. Haase, J. Phys. Chem. B, 2001, 105, 12709 CrossRef CAS.
- Y. J. Sun, H. J. Liu, X. Wang, X. G. Kong and H. Zhang, Chem. Mater., 2006, 18, 2726 CrossRef CAS.
- A. Huignard, T. Gacoin and J. P. Boilot, Chem. Mater., 2000, 12, 1090 CrossRef CAS.
- P. P. Yang, Z. W. Quan, Z. Y. Hou, C. X. Li, X. J. Kang, Z. Y. Cheng and J. Lin, Biomaterials, 2009, 30, 4786 CrossRef CAS.
- Y. M. He, Y. Wu, H. Guo, T. L. Sheng and X. T. Wu, J. Hazard. Mater., 2009, 169, 855 Search PubMed.
- M. Oshikiri, M. Boero, A. Matsushita and J. H. Ye, J. Electroceram., 2009, 22, 114 CrossRef CAS.
- Z. M. Fang, Q. Hong, Z. H. Zhou, S. J. Dai, W. Z. Weng and H. L. Wan, Catal. Lett., 1999, 61, 39 CrossRef CAS.
- W. D. Zhang, C. T. Au and H. L. Wan, Appl. Catal., A, 1999, 181, 63 Search PubMed.
- E. A. Maunders and L. G. Deshazer, J. Opt. Soc. Am., 1971, 61, 684 Search PubMed.
- R. A. Fields, M. Birnbaum and C. L. Fincher, Appl. Phys. Lett., 1987, 51, 1885 CrossRef CAS.
- N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197 CrossRef CAS.
- J. W. Park, S. S. Park, Y. Kim, I. Kim and C. S. Ha, ACS Nano, 2008, 2, 1137 CrossRef CAS.
- P. P. Yang, S. S. Huang, D. Y. Kong, J. Lin and H. G. Fu, Inorg. Chem., 2007, 46, 3203 CrossRef CAS.
- Y. Yamauchi and K. Kuroda, Chem.–Asian J., 2008, 3, 664 CrossRef CAS.
- Y. Q. Wang, C. M. Yang, W. Schmidt, B. Spliethoff, E. Bill and F. Schuth, Adv. Mater., 2005, 17, 53 CrossRef CAS.
- F. Jiao, J. C. Jumas, M. Womes, A. V. Chadwick, A. Harrison and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS.
- P. Krawiec, E. Kockrick, L. Borchardt, D. Geiger, A. Corma and S. Kaskel, J. Phys. Chem. C, 2009, 113, 7755 CrossRef CAS.
- Y. F. Shi, Y. Wan, R. Y. Zhang and D. Y. Zhao, Adv. Funct. Mater., 2008, 18, 2436 CrossRef CAS.
- Y. F. Shi, Y. Wan, B. Tu and D. Y. Zhao, J. Phys. Chem. C, 2008, 112, 112 CrossRef CAS.
- F. Gao, Q. Y. Lu and D. Y. Zhao, Adv. Mater., 2003, 15, 739 CrossRef CAS.
- P. P. Yang, Z. W. Quan, L. L. Lu, S. S. Huang, J. Lin and H. G. Fu, Nanotechnology, 2007, 18, 235703 CrossRef.
- E. DeOliveira, C. R. Neri, O. A. Serra and A. G. S. Prado, Chem. Mater., 2007, 19, 5437 CrossRef CAS.
- Y. J. Li and B. Yan, Inorg. Chem., 2009, 48, 8276 CrossRef CAS.
- Y. Li and B. Yan, Nanoscale Res. Lett., 2010, 5, 701 Search PubMed.
- X. S. Qu, H. W. Song, X. Bai, G. H. Pan, B. Dong, H. F. Zhao, F. Wang and R. F. Qin, Inorg. Chem., 2008, 47, 9654 CrossRef CAS.
- D. L. Shi, J. Lian, W. Wang, G. K. Liu, P. He, Z. Y. Dong, L. M. Wang and R. C. Ewing, Adv. Mater., 2006, 18, 189 CrossRef CAS.
- S. Lee, K. Park, S. Y. Lee, J. H. Ryu, J. W. Park, H. J. Ahn, I. C. Kwon, I. C. Youn, K. Kim and K. Choi, Bioconjugate Chem., 2008, 19, 1743 Search PubMed.
- Y. G. Wang, F. Y. Zhang, Y. Q. Wang, J. W. Ren, C. L. Li, X. H. Liu, Y. Guo, Y. L. Guo and G. Z. Lu, Mater. Chem. Phys., 2009, 115, 649 CrossRef CAS.
- T. W. Kim, F. Kleitz, B. Paul and R. Ryoo, J. Am. Chem. Soc., 2005, 127, 7601 CrossRef CAS.
- F. He, P. P. Yang, N. Niu, W. X. Wang, S. L. Gai, D. Wang and J. Lin, J. Colloid Interface Sci., 2010, 343, 71 Search PubMed.
- B. Z. Tian, X. Y. Liu, L. A. Solovyov, Z. Liu, H. F. Yang, Z. D. Zhang, S. H. Xie, F. Q. Zhang, B. Tu, C. Z. Yu, O. Terasaki and D. Y. Zhao, J. Am. Chem. Soc., 2004, 126, 865 CrossRef CAS.
- B. Liu and R. T. Baker, J. Mater. Chem., 2008, 18, 5200 RSC.
- J. Sauer, F. Marlow, B. Spliethoff and F. Schuth, Chem. Mater., 2002, 14, 217 CrossRef CAS.
- W. B. Yue, X. X. Xu, J. T. S. Irvine, P. S. Attidekou, C. Liu, H. Y. He, D. Y. Zhao and W. Z. Zhou, Chem. Mater., 2009, 21, 2540 CrossRef CAS.
- T. W. Kim, R. Ryoo, K. P. Gierszal, M. Jaroniec, L. A. Solovyov, Y. Sakamoto and O. Terasaki, J. Mater. Chem., 2005, 15, 1560 RSC.
- A. H. Lu, A. Kiefer, W. Schmidt and F. Schuth, Chem. Mater., 2004, 16, 100 CrossRef CAS.
- Y. Wan, H. F. Yang and D. Y. Zhao, Acc. Chem. Res., 2006, 39, 423 CrossRef CAS.
- H. F. Yang and D. Y. Zhao, J. Mater. Chem., 2005, 15, 1217 RSC.
- G. A. Jia, Y. H. Song, M. Yang, Y. J. Huang, L. H. Zhang and H. P. You, Opt. Mater., 2009, 31, 1032 CrossRef CAS.
- Y. H. Zhou and J. Lin, Opt. Mater., 2005, 27, 1426 CrossRef CAS.
- C. Hsu and R. C. Powell, J. Lumin., 1975, 10, 273 CrossRef CAS.
- M. Yu, J. Lin and J. Fang, Chem. Mater., 2005, 17, 1783 CrossRef CAS.
- M. Yu, J. Lin, Z. Wang, J. Fu, S. Wang, H. J. Zhang and Y. C. Han, Chem. Mater., 2002, 14, 2224 CrossRef CAS.
- A. Huignard, V. Buissette, A. C. Franville, T. Gacoin and J. P. Boilot, J. Phys. Chem. B, 2003, 107, 6754 CrossRef CAS.
- M. G. Kwak, J. H. Park and S. H. Shon, Solid State Commun., 2004, 130, 199 Search PubMed.
- P. K. Sharma, M. H. Jilavi, R. Nass and H. Schmidt, J. Lumin., 1999, 82, 187 CrossRef.
- G. K. Das and T. T. Y. Tan, J. Phys. Chem. C, 2008, 112, 11211 CrossRef CAS.
- C. S. Park, M. G. Kwak, S. S. Choi, Y. S. Song, S. J. Hong, J. I. Han and D. Y. Lee, J. Lumin., 2006, 118, 199 Search PubMed.
- A. M. Pires, S. Heer, H. U. Gudel and O. A. Serra, J. Fluor., 2006, 16, 461 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
