High performance three-dimensionally ordered macroporous composite cathodes for intermediate temperature solid oxide fuel cells†
Naiqing
Zhang
ab,
Juan
Li
c,
Wei
Li
c,
Dan
Ni
c and
Kening
Sun
*ab
aAcademy of Fundamental and Interdisciplinary Sciences, Harbin Institute of Technology, Harbin, 150001, China. E-mail: keningsun@yahoo.com.cn; Fax: +86 451 86412153; Tel: +86 451 86412153
bState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin, 150090, PR China
cDepartment of Chemistry, Harbin Institute of Technology, Harbin, 150001, PR China
First published on 2nd December 2011
Abstract
The colloidal crystal templating method combined with the fast firing technique is used to prepare the three-dimensionally ordered macroporous (3-DOM) composite cathodes for solid oxide fuel cells. The 3-DOM cathodes present very low polarization resistances, showing high electrocatalytic activity at intermediate temperatures.
It is widely recognized that high operating temperature leads to complex material problems for solid oxide fuel cells (SOFCs), therefore there is a growing interest in developing intermediate temperature SOFCs (IT-SOFCs).1 Since the oxygen reduction reaction (ORR) occurs at the triple-phase boundary (TPB, the interface where air, electronic conductor and ionic conductor are in contact),2 the cathode requires high specific surface area and adequate porosity to obtain large TPBs.3 Optimum microstructure design is an effective way to improve the electrochemical performance of the cathodes.4 At present, the studies are centered on optimizing the cathode microstructure, i.e. porosity and pore size distribution, by adjusting the type and content of the pore formers.5 As a result, the microstructure can not be precisely controlled at current level.
Colloidal crystal templating method is a popular way to prepare three-dimensionally ordered macroporous (3-DOM) materials for many applications, such as optical device, chemical sensing and lithium-ion batteries, etc.6 The main advantage of this method is that the dimension of the pores in the final material is set by the size of the template, thus the microstructure can be readily tailored. Furthermore, due to the large surface to volume ratio, such 3-DOM architecture has the potential to increase the TPB length, which can dramatically improve the performance of the SOFC cathode. In addition, the ordered macropores facilitate the gas transportation processes.
Nevertheless, as far as we know, there has been no report on successful application of the 3-DOM architecture in the SOFC field. The reason is that very high sintering temperature is needed, i.e. 1000∼1200 °C, to guarantee good contact between the solid state cathode and electrolyte, whereas the 3-DOM skeleton collapses earlier, i.e. at around 700∼900 °C.7 It is the rapid grain growth and shrinkage of the skeleton which destroys the 3-DOM architecture. Thus, it is still a challenge to apply the colloidal templates to the synthesis of macroporous electrodes for SOFCs. Fast firing is a relatively non-conventional approach which is carried out by heating the samples with very fast heating and cooling rates and very short dwell time, minimizing the opportunity for grain growth during sintering.8
In this study, we prepared 3-DOM La0.8Sr0.2MnO3/YSZ (8 mol% Y2O3 doped ZrO2, LSM/YSZ) composite cathodes for SOFCs via the colloidal crystal templating method and the fast firing technique. A schematic for the preparation of 3-DOM LSM/YSZ composite cathodes is shown in Scheme 1. The experimental details are presented in the ESI.† The SEM images of the monodispersed PS spheres and the PS colloidal crystal are shown in Fig. S1†. After LSM/YSZ mixed sol impregnation, a 700 °C firing (with the heating rate of 1 °C min−1) was carried out to remove the PS templates and crystallize the solids, producing the 3-DOM LSM/YSZ skeleton, as shown in Fig. S1†. There are rather uniform and close-packing hexagonal arrangement pores with the pore size of approximately 350 nm.
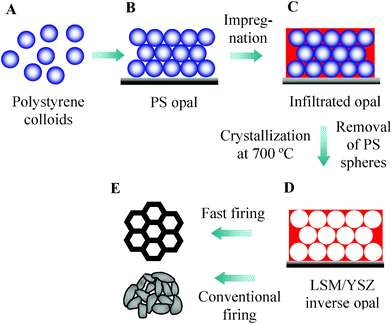 | ||
| Scheme 1 Outline of the experimental route to prepare 3-DOM LSM/YSZ composite cathodes for SOFCs. | ||
Obviously, the contact between cathode and electrolyte is weak after the 700 °C thermal treatment. Thus, in order to enhance the contact between the two layers, and thereby improve the electrochemical performance of the cathode, a subsequent fast firing was conducted. Information on the phase composition was obtained from the XRD patterns of the 3-DOM LSM/YSZ materials after fast firing at 1000 °C for 5 min and 15 min, as shown in Fig. S2†. The position of the diffraction peaks coincides well with that of the standard value and LSM/YSZ phases are successfully co-synthesized via the impregnation process.
Fig. 1 shows the SEM images of the LSM/YSZ composite cathodes obtained by the fast firing and the conventional calcination process. It is indicated from Fig. 1A that after fast firing at 1000 °C for 15 min, the 3-DOM architecture is not collapsed and the LSM/YSZ skeleton is successfully retained. The reason is that the fast firing process is carried out with the heating and cooling rates of 200 °C min−1 and dwell time of 15 min. In this way, the rise and holding period is significantly shortened than the general value of 6∼8 h for the conventional calcination process. Fig. 1B shows that the 3-DOM skeleton transformed into randomly packed nanoparticles after the conventional thermal treatment. As expected, the very long rise and holding time at high temperature causes rapid grain growth and the collapse of the 3-DOM skeleton.
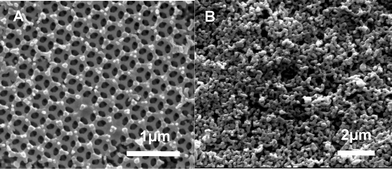 | ||
| Fig. 1 SEM micrographs of (A) the 3-DOM LSM/YSZ composite cathode fast firing at 1000 °C for 15 min and (B) the LSM/YSZ composite cathode obtained by conventional firing at 1000 °C. | ||
Fig. 2 presents the Nyquist plots of the 3-DOM LSM/YSZ composite cathode fast-firing at 1000 °C for 15 min, operating at 650 °C and 700 °C. The intercept of the semicircle on the real axis in the high-frequency represents the ohmic resistance (Rel). The overall polarization resistance was obtained by fitting the impedance spectrum with the equivalent circuit model shown inset using the ZSimpWin software. It is indicated that the polarization resistance is 0.71 Ω cm2 and 0.57 Ω cm2 at 650 °C and 700 °C, respectively. These resistance values are fairly good performance compared with the non-optimized LSM/YSZ composite electrodes.9 The inset in Fig. 2 shows the cross-section SEM image of the 3-DOM LSM/YSZ composite cathode calcined at 1000 °C for 15 min. It reveals that good adhesion between the 3-DOM LSM/YSZ composite cathode and the YSZ electrolyte is obtained.
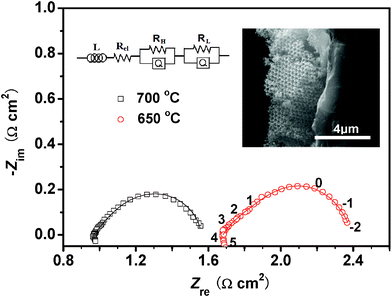 | ||
| Fig. 2 Nyquist plots of the 3-DOM LSM/YSZ composite cathode fast firing at 1000 °C for 15 min, operating at 650 °C and 700 °C. Symbols correspond to the experimental data and solid lines correspond to fitted results using the equivalent circuit shown inset. The numbers indicate the frequency (logarithm). The inset shows the cross-section SEM image of the 3-DOM LSM/YSZ composite cathode. | ||
Since it has been proved that the fast firing method is feasible for the preparation of the 3-DOM LSM/YSZ composite cathodes, the fast firing procedure is further optimized. Shown in Fig. 3 is the relationship between the ohmic and polarization resistances of the LSM/YSZ composite cathodes and the dwell time of the fast firing process, operating at 650 °C. The resistance values are obtained by fitting the Nyquist plots of the LSM/YSZ composite cathodes fast firing for 2∼12 min and 18∼30 min, as shown in Fig. S3∼Fig. S5†. It is indicated that the ohmic resistance gradually decreases from 4.62 Ω cm2 to 1.16 Ω cm2 as the dwell time increases from 2 min to 22 min, but the value increases to 1.87 Ω cm2 when the dwell time reaches 30 min. While for the polarization resistance, gradual reduction is observed with the dwell time increases from 2 min to 18 min. The polarization resistance is 0.71 Ω cm2 and 0.66 Ω cm2 for the 3-DOM LSM/YSZ composite cathode fast firing for 15 min and 18 min, respectively. However, the polarization resistance deteriorates when the dwell time further increases to 22 min and 30 min, reaching 1.3 Ω cm2 and 2.12 Ω cm2, respectively.
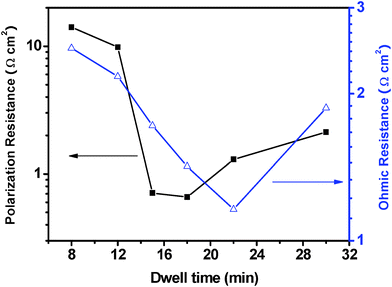 | ||
| Fig. 3 The relationship between the polarization and ohmic resistances of the 3-DOM LSM/YSZ composite cathodes and the dwell time of the fast firing process, operating at 650 °C. | ||
As shown in Fig. S6A∼C, the LSM/YSZ composite cathodes consist of continuous 3-DOM skeleton and ordered macropores. Such architecture makes the cathode yield large TPB length and adequate porosity, which significantly enhance the ORRs. As the dwell time increases, it is obvious that coarsening of the particles can be observed and the contact between cathode and electrolyte is gradually improved. Thus, the ohmic and polarization resistances decrease accordingly. However, as shown in Fig. S6D, the 3-DOM architecture collapses and transforms into randomly packed nanoparticles when the dwell time further increases to 30 min. The destruction of the 3-DOM architecture may be the main reason for the deterioration of both the ohmic and polarization resistances. Therefore, the results demonstrate that the 3-DOM architecture is beneficial for improving the electrochemical performance of the cathodes for IT-SOFCs. Furthermore, it is worth noting that the values of the polarization resistance at 650 °C found in this work are substantially lower than those reported in the literature for many manganite-based composite cathodes based on YSZ electrolyte.9
To summarize, 3-DOM LSM/YSZ composite cathodes are prepared by the colloidal crystal templating method for IT-SOFCs. Through the fast firing technique, good adhesion between the 3-DOM composite cathode and the YSZ electrolyte is achieved while the nanostructure is retained. The polarization resistance of the 3-DOM LSM/YSZ composite cathode fast firing at 1000 °C for 15 min reaches as low as 0.71 Ω cm2 and 0.57 Ω cm2 at 650 °C and 700 °C, respectively. The present work opens a path for a new generation of nanostructured electrodes. 3-DOM materials with better intrinsic properties, such as the Ba0.5Sr0.5Co0.8Fe0.2O3 or Ba(Zr0.1Ce0.7Y0.2)O3-δ,10 may exhibit an additional improvement of the cathode performance. Finally, it is worth mentioning that the 3-DOM architecture, which increases the electrochemical active sites, deserves to be investigated for anode materials since a similar enhanced performance could be obtained.
This work is funded by National Natural Science Foundation of China (No. 20906015), Funds for Creative Research Groups of China (No. 50821002) and Natural Scientific Research Innovation Foundation of Harbin Institute of Technology (No. HIT.NSRIF.2008.23).
References
- (a) D. Brett, A. Atkinson, N. P. Brandon and S. J. Skinner, Chem. Soc. Rev., 2008, 37, 1568 RSC; (b) D. K. Niakolas, J. P. Ouweltjes, G. Rietveld, V. Dracopoulos and S. G. Neophytides, Int. J. Hydrogen Energy, 2010, 35, 7898 CrossRef CAS.
- (a) S. B. Adler, Chem. Rev., 2004, 104, 4791 CrossRef CAS; (b) R. Peng, T. Wu, W. Liu, X. Liu and G. Meng, J. Mater. Chem., 2010, 20, 6218 RSC.
- (a) D. Dong, D. Li, X. Zhang, Z. Chai, K. Wang, C. Z. Li, D. Zhao and H. Wang, J. Mater. Chem., 2010, 20, 1122 RSC; (b) E. C. Brown, S. K. Wilke, D. A. Boyd, D. G. Goodwin and S. M. Haile, J. Mater. Chem., 2010, 20, 2190 RSC; (c) M. Zhi, N. Mariani, R. Gemmen, K. Gerdes and N. Wu, Energy Environ. Sci., 2011, 4, 417 RSC.
- (a) M. G. Bellino, J. G. Sacanell, D. G. Lamas, A. G. Leyva and N. E. Walsöe de Reca, J. Am. Chem. Soc., 2007, 129, 3066 CrossRef CAS; (b) A. Princivalle, D. Perednis, R. Neagu and E. Djurado, Chem. Mater., 2005, 17, 1220 CrossRef CAS; (c) J. R. Wilson, A. T. Duong, M. Gameiro, H. Y. Chen, K. Thornton, D. R. Mumm and S. A. Barnett, Electrochem. Commun., 2009, 11, 1052 CrossRef CAS; (d) Y. Zhang, S. Zha and M. Liu, Adv. Mater., 2005, 17, 487 CrossRef CAS.
- (a) A. Sanson, P. Pinasco and E. Roncari, J. Eur. Ceram. Soc., 2008, 28, 1221 CrossRef CAS; (b) M. Liu, B. Yu, J. Xu and J. Chen, Int. J. Hydrogen Energy, 2010, 35, 2670 CrossRef CAS; (c) J. Piao, K. Sun, N. Zhang and S. Xu, J. Power Sources, 2007, 175, 288 CrossRef.
- (a) B. T. Holland, C. F. Blanford and A. Stein, Science, 1998, 281, 538 CrossRef CAS; (b) T. Endo, Y. Yanagida and T. hatsuzawa, Sens. Actuators, B, 2007, 125, 589 CrossRef; (c) W. Cui, H. Liu, C. Wang and Y. Xia, Electrochem. Commun., 2008, 10, 1587 CrossRef CAS; (d) H. Yan, C. F. Blanford, W. H. Smyrl and A. Stein, Chem. Commun., 2000, 16, 1477 RSC; (e) S. M. Yang and G. A. Ozin, Chem. Commun., 2000, 24, 2507 RSC.
- (a) Y. Zhang, S. Zha and M. Liu, Adv. Mater., 2005, 17, 487 CrossRef CAS; (b) A. Lashtabeg, J. Drennan, R. Knibbe, J. L. Bradley and G. Q. Lu, Microporous Mesoporous Mater., 2009, 117, 395 CrossRef CAS.
- (a) M. G. Bellino, D. G. Lamas and N. E. Walsöe de Reca, Adv. Mater., 2006, 18, 3005 CrossRef CAS; (b) M. G. Bellino, D. G. Lamas and N. E. Walsöe de Reca, Adv. Funct. Mater., 2006, 16, 107 CrossRef CAS.
- (a) E. P. Murray and S. A. Barnett, Solid State Ionics, 2001, 143, 265 CrossRef; (b) H. S. Song, S. Lee, S. H. Hyun, J. Kim and J. Moon, J. Power Sources, 2009, 187, 25 CrossRef CAS; (c) H. S. Song, S. H. Hyun, J. Moon and R. H. Song, J. Power Sources, 2005, 145, 272 CrossRef CAS; (d) V. A. C. Haanappel, J. Mertens, D. Rutenbeck, C. Tropartz, W. Herzhof, D. Sebold and F. Tietz, J. Power Sources, 2005, 141, 216 CrossRef CAS; (e) Q. Zhang, B. E. Martin and A. Petric, J. Mater. Chem., 2008, 18, 4341 RSC; (f) T. Suzuki, M. Awano, P. Jasinski, V. Petrovsky and H. U. Anderson, Solid State Ionics, 2006, 177, 2071 CrossRef CAS; (g) T. Z. Sholklapper, V. Radmilovic, C. P. Jacobson, S. J. Visco and L. C. De Jonghe, J. Power Sources, 2008, 175, 206 CrossRef CAS; (h) J. Piao, K. Sun, N. Zhang and S. Xu, J. Power Sources, 2008, 175, 288 CrossRef CAS.
- (a) Z. Shao and S. M. Haile, Nature, 2004, 431, 170 CrossRef CAS; (b) L. Yang, C. Zuo, S. Wang, Z. Cheng and M. Liu, Adv. Mater., 2008, 20, 3280 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00837d/ |
| This journal is © The Royal Society of Chemistry 2012 |
