Glycodendrimer coated gold nanoparticles for proteins detection based on surface energy transfer process†
Nicoleta
Bogdan
*a,
René
Roy
b and
Mario
Morin
b
aDepartment of Chemistry and Biochemistry, Concordia University, 7141 Sherbrooke St. W. H4B 1R6, Montreal, Canada. E-mail: nbogdan@alcor.concordia.ca
bDepartment of Chemistry, Université du Québec à Montréal, P.O. Box 8888, Succ. Centre-ville, H3C 3P8, Montreal, Canada
First published on 2nd December 2011
Abstract
We report here a novel and simple approach on which nanometer-size, water soluble glycodendrimer (mannose-functionalized poly(amidoamine) PAMAM generation G0 cystamine core dendrimer) coated gold nanoparticles (Au–man) allows the detection of protein–carbohydrate interactions based on surface energy transfer process (SET). This sensitive SET sensing approach enables quantitative analysis of the binding constant of a mannose-binding protein, lectin Concanavalin A (Con A) labelled with a fluorophore to a sensing Au–man in aqueous samples. Competitive binding and inhibition assays were done to investigate the SET efficiency. The binding constant of Con A to Au–man interactions of (5.6 ± 0.1) × 106 M−1 is 100-times higher than the binding constant values obtained in the interactions with the glycodendrimer G2 alone. The simple modification of the gold nanoparticles with glycodendrimers provides a convenient method to obtain biocompatible and selective carbohydrate-based SET probes for protein biorecognition.
Introduction
Multivalent carbohydrates are involved in numerous biochemical processes such as the adhesion of bacteria to cells, metastasis spreading, and communication between cells.1–8 The interactions of carbohydrates with proteins, bacteria, viruses and cancerous cells have been used to develop vaccines and drugs.9–11 Biosensors based on various detection techniques such as electrochemistry, spectroscopies, Quartz Crystal Microbalance (QCM) and microarray were also reported.12–18 However, monovalent carbohydrates ligands have shown low affinity in order of milimolar for proteins receptors.10 To increase their affinity a new type of synthetic multivalent carbohydrates, glycodendrimers (GDs), were used in numerous applications for mimicking the glycoproteins present at cellular membrane.9–11 The GDs have been found to be more efficient than simple carbohydrates for the recognition and inhibition of lectins, bacteria type-1 Escherichia Coli as well as of cancer cells.10,19–25 They were also tested as synthetic vaccines.26 The GDs were also used to study the protein–carbohydrate interactions using calorimetry20 and Förster resonance energy transfer (FRET).27,28 In FRET process the energy of an excited donor molecule is transferred nonradiatively to an acceptor molecule. However, FRET occurs efficiently only when the donor and acceptor molecules are in proximity (1–10 nm). Numerous studies by isothermal titration microcalorimetry (ITC)29–32 reported the binding constants and thermodynamic data of GDs interacting with lectins. GDs were found to have binding constants (Ka) that are 1 to 2 order of magnitude higher than monosaccharides for lectins.31,32 The higher affinity of GDs for lectins is assigned mostly to their multivalency. More detailed studies by Brewer and co-workers have shown that the increase of the Ka is related to greater positive entropy contributions to binding of multivalent carbohydrates relative to monovalent carbohydrates.29,31 However, limits were found for this multivalent approach; as the glycodendrimers become larger (dendrimer generation G > 1) their efficiency, on a per saccharide basis, decreases because of the steric hindrance between branches of the dendrimer.20In the past decade, gold nanoparticles presenting similar size to many biomolecules and multivalent configuration after grafting of various ligands at their surface offer potential application in biological recognitions.33–41 For example, alkylthiols terminated by carbohydrates adsorbed on gold nanoparticles were used to detect bacteria by TEM and a lectin by calorimetry and surface plasmon resonance (SPR).42–46 It was also reported the applications of these so-called gold glyconanoparticles as new tools in antiadhesive therapy and anticancer vaccines.47 These modified nanoparticles were found to be efficient in these applications. They provide, via their metallic core, ways to detect interactions with proteins, bacteria and cancer cells. However, no quantitative sensitive assays were done for the gold glyconanoparticles to monitor the binding interaction with the proteins and it is thus difficult to asses their potential as biosensors. As optical sensing methods, FRET assay based on the luminescence quenching of quantum dots (QDs) by gold nanoparticles and employing a streptavidin–biotin interactions was done with a good sensitivity.48 However, in FRET-based methods the dipole–dipole mechanism is limited on the length scale in order of <10 nm. Recently, the applications of the gold nanoparticles in a new sensitive optical method, the surface energy transfer (SET) from a donor fluorophore to a nanogold surface, have been reported.49 This method based on dipole–surface energy transfers enable the investigation of long range distances (>10 nm) in nucleo-protein studies.
In this study, we developed a glycodendrimer (mannose-functionalized poly(amidoamine) PAMAM generation G0 cystamine core dendrimer) coated gold nanoparticles (Au–man) as biosensor in a surface energy transfer (SET) optical signaling strategy to monitor the protein–carbohydrate interactions. This biosensor combines the multivalency and biocompatibility of glycodendrimers with the simple and robust chemistry of thiols adsorbed on gold nanoparticles. The chemical and physical properties of the glycodendrimer coated gold nanoparticles are discussed. The potential of Au–man to recognize selectively a mannose-binding protein Concanavalin A (Con A), a lectin from Conavalia enziformis, was evidenced by TEM, electrophoresis and fluorescence. The signal resulted from SET between the fluorescein-labeled Con A and the nanometal surface of Au–man was used to quantify the binding constant of lectin–carbohydrate interactions. Competitive binding and inhibition assays were done to investigate the SET efficiency.
Materials and methods
All the chemicals were purchased from Sigma-Aldrich. Solvents were HPLC grade. All aqueous solutions were prepared with deionized water. Phosphate buffered saline (PBS) pH 7.4 contain 0.15 M NaCl.Synthesis
The synthesis steps include firstly the synthesis of the amine-terminated PAMAM cystamine core dendrimer (G0) obtained by the divergent method of Tomalia followed by their absorption on to the gold nanoparticles surface by a modified Brust method.50–52Cystamine is used as the dendrimer's core because of its disulfide bond which dissociates and then binds to gold as thiolates. Briefly, the PAMAM dendrimer G0 is added to a hydrogen tetrachloroaurate (HAuCl4) methanol solution in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After 30 min a reducing agent, sodium borohydride (NaBH4), in a molar ratio of 1
2. After 30 min a reducing agent, sodium borohydride (NaBH4), in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 for Au3+
15 for Au3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaBH4, is added. It causes the color of the solution to change from orange to brown. Stirring is continued for 2 h at room temperature. After 2 h, acetone is added to precipitate the amine-terminated PAMAM G0 coated gold nanoparticles (Au–G0) which are then filtered (0.45 μm nylon filter) and purified by rinsing extensively with a 1
NaBH4, is added. It causes the color of the solution to change from orange to brown. Stirring is continued for 2 h at room temperature. After 2 h, acetone is added to precipitate the amine-terminated PAMAM G0 coated gold nanoparticles (Au–G0) which are then filtered (0.45 μm nylon filter) and purified by rinsing extensively with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 methanol
5 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone mixture. They are then dried under vacuum. The last synthetic step consists in grafting a mannose derivative, the p-isothiocyanatophenyl α-D-mannopyranoside synthesised as described previously,53 onto the amine-terminated PAMAM G0 coated gold nanoparticles (Au–G0) by a thiourea linkage to obtain the glycodendrimer coated gold nanoparticles (Au–man). An aqueous solution of Au–G0 is slowly added to an aqueous solution of carbohydrate (1.2 equiv. per amine group on the gold nanoparticles as obtained by a titration with trinitrobenzenesulfonic acid TNBS (see below)) while stirring. The reaction is allowed to proceed for 6 h while maintaining the pH at 9 by adding a 0.1 M NaOH solution. The reaction mixture is then kept at 5 °C for 12 h. The pH of the solution is adjusted to 7 with 0.1 M HCl. Acetone is then added to the mixture to precipitate the particles. The solution is filtered using a 0.45 μm nylon filter. Unreacted carbohydrates are removed by rinsing with acetone. After drying for 7 h under vacuum at room temperature, the nanoparticles are stored at 5 °C in the dark.
acetone mixture. They are then dried under vacuum. The last synthetic step consists in grafting a mannose derivative, the p-isothiocyanatophenyl α-D-mannopyranoside synthesised as described previously,53 onto the amine-terminated PAMAM G0 coated gold nanoparticles (Au–G0) by a thiourea linkage to obtain the glycodendrimer coated gold nanoparticles (Au–man). An aqueous solution of Au–G0 is slowly added to an aqueous solution of carbohydrate (1.2 equiv. per amine group on the gold nanoparticles as obtained by a titration with trinitrobenzenesulfonic acid TNBS (see below)) while stirring. The reaction is allowed to proceed for 6 h while maintaining the pH at 9 by adding a 0.1 M NaOH solution. The reaction mixture is then kept at 5 °C for 12 h. The pH of the solution is adjusted to 7 with 0.1 M HCl. Acetone is then added to the mixture to precipitate the particles. The solution is filtered using a 0.45 μm nylon filter. Unreacted carbohydrates are removed by rinsing with acetone. After drying for 7 h under vacuum at room temperature, the nanoparticles are stored at 5 °C in the dark.
UV-VIS spectroscopy
The UV-visible spectrum of aqueous solution of the Au–man (0.1 mg mL−1) is recorded between 300 and 600 nm with a Cary 100 Bio UV-VIS Spectrometer from Varian. Water is used as background.FTIR spectroscopy
Diffuse reflectance IR spectra is recorded with a Thermo Nicolet Avatar FTIR spectrometer between 3500 and 1050 cm−1 using a resolution of 2 cm−1 by using the KBr method.Thermo gravimetric analysis (TGA)
The thermogram for Au–man (3 mg) is recorded using an Exstar6000 Thermal Instrument from Seiko. The sample is kept under an argon atmosphere. The temperature is initially maintained at 120 °C for 30 min to dehydrate the sample. The heating rate is set at 10 °C min−1 between 120 °C and 800 °C.X-ray photoelectron spectroscopy (XPS)
The XPS measurements are done with a VG Escalab 200i spectrometer. The spectra are recorded at a photoemission angle of 0° using 400 W achromatic Al X-ray source (1486.7 eV). The analyzer pass energy is fixed at 20 eV in CAE mode for the high-resolution scans. The subtraction of a linear background is made with the CasaXPS® software. The C 1s level at 284.9 eV is used as an internal reference. The relative atomic% of the elements is determined from the peak areas of the XPS bands of each element after subtraction of a linear background and correction for the transmission function using the CasaXPS® software. This software is also used to deconvolute overlapping XPS peaks.Transmission electron microscopy (TEM)
Samples for TEM are prepared by drop casting one drop of Au–man (1 mg mL−1) or mixed in a 1 to 1 proportion with a Con A (1 mg mL−1) solution in PBS for 30 s onto a copper grid (300 mesh). The excess is removed and the support is dried in air for at least 1 h. The images are obtained with a JEM-2000FX electron microscope.Titration of terminal amino groups
The quantification of the primary amines on the gold nanoparticles before and after reaction with the mannose derivative is done using trinitrobenzenesulfonic acid (TNBS) which reacts specifically with primary amines.54 The yield of the mannose grafted on the surface of amines terminated Au–G0 is determined by taking the ratio of the number of moles of NH2 mg−1 of gold nanoparticle before and after the grafting of the mannose derivative. The resulting amine–TNBS complex is yellow and is quantified by measuring its absorbance at 350 nm. An aqueous solution of Au–G0 or Au–man (0.1 to 0.3 μmol mL−1) is mixed with 1 mL borate buffer solution of pH 9 (boric acid 0.1 M and KOH 0.5 M) and with 1 mL 0.1% TNBS. The solutions are kept at 70 °C for 2 h and 0.5 mL 1 M HCl is then added to all the solutions. The UV-visible spectra are recorded between 300 and 500 nm with a Cary 100 Bio UV-VIS Spectrometer from Varian. A calibration curve is obtained using cystamine solutions ranging from 0.05 to 0.2 μmol mL−1.SDS-PAGE electrophoresis
For SDS-PAGE electrophoresis stained by Coomassie Brilliant Blue, the Au–man (1 mg mL−1 in PBS) is mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 proportion with Concanavalin A (Con A), Wheat Germ Agglutinin (WGA) and a mixture of two lectins: Con A and WGA. The concentration for each lectin is 1 mg mL−1 in PBS. The precipitate obtained after 1 h is separated from the supernatant. The both fractions (precipitate and supernatant) and native lectins are analyzed by SDS-PAGE. For this 0.075 mL sample solution is mixed with 0.075 mL bromophenol blue 0.04% used for proteins separation by SDS-PAGE analysis at 160 V and 350 mA. SDS-PAGE is carried out in a 12% gel on a Mini-Protean II apparatus (Bio-Rad) and stained with a solution of 0.025% Coomassie Brilliant Blue, 40% methanol, 10% acetic acid). For the standard (BroadRange®, 6.5–200 kDa, BioRad), 10 μL of a dilution solution 1
2 proportion with Concanavalin A (Con A), Wheat Germ Agglutinin (WGA) and a mixture of two lectins: Con A and WGA. The concentration for each lectin is 1 mg mL−1 in PBS. The precipitate obtained after 1 h is separated from the supernatant. The both fractions (precipitate and supernatant) and native lectins are analyzed by SDS-PAGE. For this 0.075 mL sample solution is mixed with 0.075 mL bromophenol blue 0.04% used for proteins separation by SDS-PAGE analysis at 160 V and 350 mA. SDS-PAGE is carried out in a 12% gel on a Mini-Protean II apparatus (Bio-Rad) and stained with a solution of 0.025% Coomassie Brilliant Blue, 40% methanol, 10% acetic acid). For the standard (BroadRange®, 6.5–200 kDa, BioRad), 10 μL of a dilution solution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 is applied to the gel. To obtain the dilution, 0.05 mL of standard is mixed with 0.45 mL water and 0.5 mL sample buffer (0.5 M Tris–HCl, pH 6.8, glycerol 1.0 mL 5%, bromophenol blue 0.04%) containing 1% β-mercaptoethanol.
20 is applied to the gel. To obtain the dilution, 0.05 mL of standard is mixed with 0.45 mL water and 0.5 mL sample buffer (0.5 M Tris–HCl, pH 6.8, glycerol 1.0 mL 5%, bromophenol blue 0.04%) containing 1% β-mercaptoethanol.
Spectrofluorometry
The fluorescence is recorded using a 96-well microplate of 0.3 mL using a fluorescence microplate reader (SPECTRA Max Gemini, Molecular Devices, Sunnyvale, CA). The excitation and emission slits widths are set at 5 nm. Each measurement is carried out in triplicate in phosphate buffer saline (PBS, pH 7.4) solutions. The sample solutions are incubated for 15 min at room temperature.The specific interaction between the fluorescent lectins fluorescein or tetrarhodamine-labeled Con A (FITC–Con A or RITC–Con A) (donor) and Au–man is investigated by fluorescence in a surface energy transfer (SET) signalling strategy. A tetrarhodamine-labeled WGA (RITC–WGA) lectin that recognizes sialic acid and N-acetylglucosaminyl carbohydrate was used as negative control.
In SET assay, FITC–Con A or RITC–Con A/RITC–WGA, was mixed with Au–man and the absorbance was recorded at 520 nm or 580 nm after an excitation at 490 nm or 550 nm. The final concentration of Au–man was 2.2 μM and for lectins was 320 nM. The decrease in donor fluorescence was used to quantify the SET efficiency.
To calculate the binding constant (Ka) of interaction between FITC–Con A and Au–man and to estimate the stoichiometry of the complex, a concentration of 320 nM (after mixing) of lectin was titrated with various concentrations of Au–man in the range of 0.011–2.2 μM (after mixing). In this range of concentrations no precipitation was observed and the solutions remained transparent. The fluorescence was recorded at 520 nm after an excitation at 490 nm.
In the SET competitive assays, a solution of FITC–Con A, (320 nM) was incubated with mannan (0.0033 to 3.33 μM), a mannose–polysaccharide, and prior to adding Au–man (2.2 μM) to the sample. The fluorescence is recorded at 520 nm after an excitation at 490 nm. The decrease in donor fluorescence was used to quantify the SET efficiency.
A binding inhibition assays based on decreasing the binding efficiency between FITC–Con A and Au–man in the presence of a monomeric carbohydrate, D-mannose, was developed. In this assay, Au–man (2.2 μM) was preincubated with FITC–Con A (320 nM). After 15 min different concentrations of D-mannose (0.01 to 0.55 M) were added to the complex. The optical emission signal is recorded at 520 nm after an excitation at 490 nm.
Results and discussion
The synthesis of Au–man shown in Scheme 1 was done in two steps. The first step consisted of using amine-terminated PAMAM generation G0 dendrimers with a cystamine disulfide core in the synthesis of monolayer protected gold nanoparticles (Au–G0). In the second step, p-isothiocyanatophenyl α-D-mannopyranoside was grafted on the terminal amino group of the PAMAM G0 coated-gold nanoparticles by a thiourea linkage to obtain the glycodendrimer gold nanoparticles (Au–man).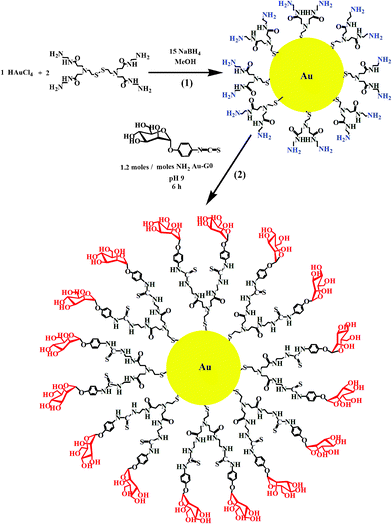 | ||
| Scheme 1 Synthesis of (1) Au–G0 and (2) Au–man. | ||
The chemical and physical properties of the Au–man were first determined. The grafting of p-isothiocyanatophenyl α-D-mannopyranoside on amine-terminated PAMAM G0 adsorbed on gold nanoparticles was followed by FTIR spectroscopy (supporting information, Figure S1†). The FTIR spectrum of the reactant, Au–G0 display amide bands at 1635 cm−1 and at 1566 cm−1. The spectrum of the other reactant, p-isothiocyanatophenyl α-D-mannopyranoside, shows a large isothiocyanate stretching band at 2123 cm−1. The presence of the amide bands, related of the PAMAM, the C–S band at 1502 cm−1 typical of a thiourea group and the absence of the isothiocyanate group in the spectrum of the product show that mannose-coated glycodendrimer gold nanoparticles (Au–man) was formed.
The XPS spectra of Au–man (supporting information, Figure S2†) display two bands related to the gold, 4f7/2 and 4f5/2 levels, at approximately 83.2 and 86.9 eV indicating that the gold is in a zero valence.55,56 The doublet 2p3/2 and 2p1/2 of sulphur is at 161.7 and 162.9 eV and is assigned to thiolate and thiourea species. The absence of a peak at 168 eV shows that the sulphurs are not oxidized. A single 1s peak for nitrogen at 398.9 eV is assigned to amines, amides, and thiourea species and is observed in spectra. A N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio of (2.0 ± 0.1)
S ratio of (2.0 ± 0.1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is obtained from the XPS data. This ratio is very close to the predictestoichiometryry (2.3
1 is obtained from the XPS data. This ratio is very close to the predictestoichiometryry (2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for a defect-free glycodendrimer gold nanoparticles.
1) for a defect-free glycodendrimer gold nanoparticles.
The gold fraction of the Au–man evaluated from TGA (supporting information, Figure S3†) is 53 ± 3%. From this value and a method described previously,57 the average size of gold particles, the number of mannose terminal groups adsorbed on the gold surface and the average number of gold atoms in the nanoparticles were estimated. A 53% of gold fraction for Au–man is compatible with a particle of 1 nm particle made of 28 gold atoms.57 The 47% organic fraction of Au–man would correspond to 5 PAMAM branches with 10 mannose terminal groups adsorbed at the gold surface.
The yield of the grafting of the mannose derivative at the surface of the amine-terminated PAMAM G0 adsorbed on gold nanoparticles obtained from the TNBS titration methods was (87 ± 1)%. This result agrees with the XPS measurements.
The visible spectrum of Au–man (supporting information, Figure S4†) shows no gold surface plasmon band (SP) at approximately 520 nm. As it was reported by Hostetler et al., the surface plasmon band decreases in intensity and energy with decreasing the gold cluster size and the numbers of gold atoms.57 Very small clusters, with diameter <2 nm and containing less than 50 gold atoms, exhibit a strong UV absorption feature which decays exponentially into the visible with no observed SP at around 520 nm. The undetectable SP band in the spectrum of Au–man is an indication of the quantum size effects of nanoparticles and suggests that the gold particles have diameters of 2 nm or less.57 This result is consistent with the small number of gold atoms of Au–man estimated from TGA measurements and with the size of the nanoparticles determined by TEM.
The TEM image in Fig. 1a, show that Au–man sample is monodisperse and exhibit a particle size distribution in the range of 1.5 ± 0.5 nm. In Fig. 1b, the addition of Con A to Au–man causes the formation of large aggregates related to the formation of cross-linked clusters.29
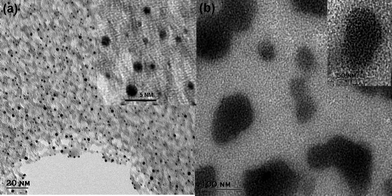 | ||
| Fig. 1 Transmission electron microscopy (TEM) images of (a) Au–man (1 mg mL−1); Inset: higher magnification and (b) complex formation between Au–man (1 mg mL−1) and Con A (1 mg mL−1) mixed in a 1 to 1 proportion; Inset: higher magnification. | ||
Further immunoprecipitation assays were undertaken to evidence the formation of clusters and to confirm the selectivity of interactions between Con A and Au–man. After mixing Con A with Au–man, a complex is formed instantaneously. A complete precipitation is obtained in 30 min (supporting information, Figure S5†). The complex obtained between Con A and Au–man was dissolved by adding an excess of D-mannose but not in the presence of an excess of D-galactose. In the presence of the WGA lectin that is a carbohydrate-binding protein that selectively recognizes sialic acid and N-acetylglucosaminyl carbohydrate, the solution of Au–man remains transparent indicating that no aggregation or visible precipitation occurs. These experiments show the specificity and reversibility of interaction between Con A and Au–man.
The selectivity of the Au–man for Con A was also evidenced by the electrophoresis SDS-PAGE assay shown in Fig. 2. Under the experimental conditions that were used, the Con A tetramers are broken into smaller fragments of 31, 12 and 14.4 kDa20,58 as shown in Fig. 2b. For WGA (Fig. 2c) the fragments were of 21 and 36 kDa.20,59Fig. 2d corresponds to the supernatant of a mixture of Con A and WGA after the addition of Au–man. It only shows two bands corresponding to WGA. Finally, Fig. 2e reveals only the bands corresponding to Con A as the addition of Au–man produce a selective precipitation of lectin. This results evidences the potential of Au–man to isolate and detect a single from a complex mixture.
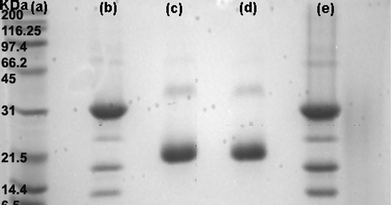 | ||
| Fig. 2 Electrophoresis SDS-PAGE: (a) protein markers (Broad Range); (b) Con A; (c) WGA; (d) supernatant of a mixture of Con A and WGA after the addition of Au–man; (e) precipitate of the mixture of Con A and WGA after the addition of Au–man. | ||
The potential of Au–man to detect a fluorescent protein in SET-based sensing approach was further examined. The decrease in the donor fluorescence was used to quantify the SET efficiency. Fig. 3 shows that in the presence of Au–man the fluorescence of FITC–Con A or RITC–Con A was reduced by more than 60%. However, the fluorescence of FITC–WGA in presence of Au–man was reduced by 3%. Similar attenuations of the fluorescence of the two different fluorophores labeled-Con A caused by Au–man confirms the selectivity of the mannose-glycodendrimer coated nanoparticles biosensor for the mannose binding receptors.
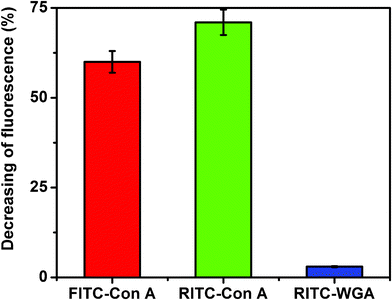 | ||
| Fig. 3 Decreasing of fluorescence (%) of FITC–Con A, RITC–Con A and RITC–WGA (320 nM) after addition of Au–man (2.22 μM); for FITC: λex = 490 nm, λem = 520 nm, for RITC: λex = 550 nm, λem = 580 nm. | ||
The binding constant of FITC–Con A to Au–man was calculated considering the decrease of the donor fluorescence in SET sensing mechanism. The intensity of the fluorescence at 520 nm of FITC–Con A after addition of Au–man decreases exponentially as is shown in Fig. 4. This behaviour is typical of a first order reaction. Hence, these data were analyzed using a model where one Au–man reacts with one Con A receptor.
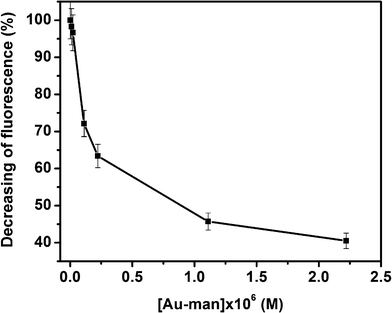 | ||
| Fig. 4 Decreasing of fluorescence (%) of FITC–Con A (320 nM) with the concentration of Au–man (0.011–2.2 μM). | ||
Analyzing the data shown in Fig. 4 by the method of Scatchard,60 a stoechiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Con A
1 for Con A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au–man is obtained (supporting information, Figure S6†) which confirms the use of this model to calculate the binding constant (Ka) between FITC–Con A and Au–man in Eqn (1).61,62
Au–man is obtained (supporting information, Figure S6†) which confirms the use of this model to calculate the binding constant (Ka) between FITC–Con A and Au–man in Eqn (1).61,62
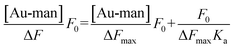 | (1) |
By plotting 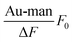 against [Au–man] and fitting it with Eqn (1), as is shown in Fig. 5, the binding constant Ka is obtained.
against [Au–man] and fitting it with Eqn (1), as is shown in Fig. 5, the binding constant Ka is obtained.
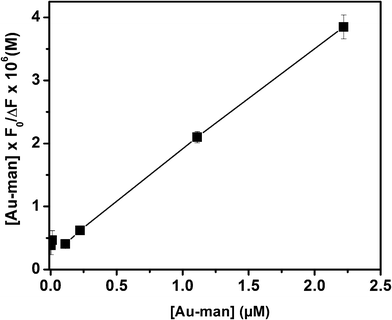 | ||
| Fig. 5 Plot of the data of Fig. 4 with Eqn 1 to calculate the binding constant (Ka) of FITC–Con A (320 nM) and Au–man interactions. | ||
The value of Ka for the binding of Con A to Au–man of (5.6 ± 0.1) × 106 M−1 was 100-times larger than for the glycodendrimer (mannose-functionalized PAMAM dendrimer generation G2).32 However, there are approximately 10 mannose per glycodendrimer coated gold nanoparticle whereas there are 32 mannose per glycodendrimer G2. This result confirms the higher affinity of Au–man than the glycodendrimer alone per mannose for Con A. The glycodendrimer coated gold nanoparticles are as efficient as the more structurally complex multivalent oligosaccharides which have Ka of the order of 106–107 M−1.29,31
The high affinity of the glycodendrimer coated gold nanoparticles (Au–man) is most probably related to its structure which differs from the glycodendrimers alone for which Ka have been reported. The glycodendrimers alone have a more flexible structure. However, the adsorption of the dendrimers to the gold surface which is followed by grafting of the mannose derivative constraints all the carbohydrates to be at the surface and confers a more rigid structure of Au–man. This is not an entropically favorable situation for the solvation of the glycodendrimer coated gold nanoparticles especially since there is a hydrophobic phenyl group in the mannose derivative. The interaction of the Au–man with Con A should be favorable since the lectin has hydrophobic regions. Thus, the entropy should play an important role in the interaction between the Au–man and Con A. These considerations make the suggested entropy-driven process likely.
A SET competitive assays was done to determine the efficiency of Au–man to bind to lectin Con A in the presence of mannan, a mannose based polysaccharide with high affinity for Con A. In accordance with our expectation, the mannan is capable of binding to FITC–Con A for the first time and Au–man compete poorly on the Con A binding sites, which results in low SET efficiency between FITC–Con A and Au–man. As it is shown in Fig. 6, in absence of mannan, the fluorescence of FITC–Con A is quenched by 60% after addition of Au–man. In the presence of a high concentration of mannan, the fluorescence of FITC–Con A increases to the point that it reaches a value corresponding to 90% of the level in absence of Au–man (a decrease of only 10% after addition of Au–man). This result demonstrated that the Au–man are not in proximity of lectin and the efficiency of the SET decrease in the presence of mannan.
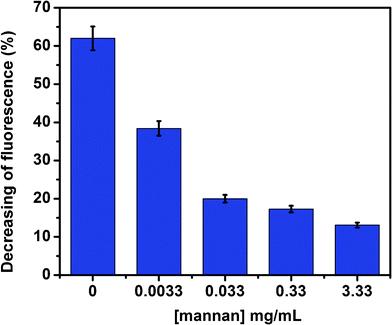 | ||
| Fig. 6 SET competitive assays in presence of mannan (0.0033 to 3.33 mg mL−1) for the complex between FITC–Con A (320 nM) and Au–man (2.2 μM). | ||
Further experiments were done to confirm the effect of Au–man in a sensing SET process. Furthermore, the complex FITC–Con A–Au–man obtained in Fig. 3 was disturbed in the presence of inhibitor, a monosaccharideD-mannose. Fig. 7 show that upon the addition of an excess of D-mannose (0.55 M), the emission recovered at 520 nm is recuperated up to 80% of that of free FITC–Con A, thus indicating that the D-mannose added disturbed the complex and the gold nanoparticles are far from the mannose binding receptors of lectin. These results demonstrate the reversibility of the molecular recognition and the effect of the gold nanoparticle in the SET sensing process.
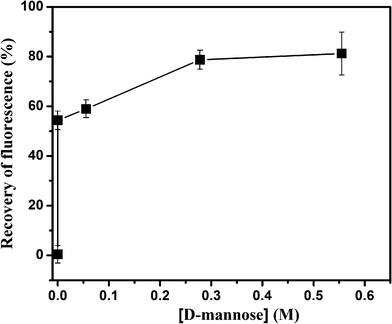 | ||
| Fig. 7 Recovery of fluorescence (%) after addition of D-mannose to the complex between FITC–Con A (320 nM) and Au–man (2.2 μM). | ||
Conclusions
In summary, nanometer-size, water soluble glycodendrimer coated gold nanoparticles were synthesised and used for the first time to monitor the protein–carbohydrate interactions by surface energy transfer (SET) process. Immunoprecipitation and electrophoresis assays shows the selectivity of Au–man for Con A. SET sensing approach was used to monitor the interaction of fluorescent lectin FITC–Con A with nanogold surface of Au–man resulting in a decrease of FITC–Con A fluorescence by 60%. The binding constant of Con A to Au–man interactions of (5.6 ± 0.1) × 106 M−1 is 100-times higher than the binding constant values obtained in the interactions with the glycodendrimer G2 alone. It was demonstrated that the efficiency of SET decreases when mannan, a mannose polysaccharide, blocks efficiently the receptors of lectin as only 10% of fluorescence of FITC–Con A decrease after addition of Au–man. In addition, the efficiency of SET decreases in the presence of an inhibitor as the fluorescence of FITC–Con A in complex with Au–man is recuperated up to 80% after addition of the monomeric D-mannose used in order of molar. For further application, this novel SET sensing glycodendrimer coated gold nanoparticles could be used in bacteria and cancer detection as well as in protein microarray assays.Acknowledgements
The financial support of FQRNT and NSERC is gratefully acknowledged. N. B. thanks FQRNT and UQAM for graduate scholarships.References
- N. Sharon, FEBS Lett., 1987, 217, 145 CrossRef CAS.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637 CrossRef CAS.
- R. A. Laine, Glycosciences: Status and Perspectives, Eds: H.-J. Gabius, S. Gabius, Chapman & Hall, Weinheim, London, 1997, 1–14 Search PubMed.
- E. E. Simanek, G. J. McGarvey, J. A. Jablonowski and C.-H. Wong, Chem. Rev., 1998, 98, 833 CrossRef CAS.
- B. Madison, I. Ofek, S. Clegg and S. N. Abraham, Infect. Immun., 1994, 62, 843 CAS.
- A. Karlsson, M. Markfjäll, H. Lundqvist, N. Strömberg and C. Dahlgren, Anal. Biochem., 1995, 224, 390 CrossRef CAS.
- C. Sun, Y. Zhang, Y. Fan, Y. Li and J. J. Li, J. Inorg. Biochem., 2004, 98, 925 CrossRef CAS.
- Y. Makimura, G. Zhonghong and R. Roy, Int. Congr. Ser., 2001, 1223, 45 CrossRef CAS.
- M. Touaibia and R. Roy, Mini-Rev. Med. Chem., 2007, 7, 1270 CrossRef CAS.
- Y. M. Chabre and R. Roy, The Sugar Code. Fundamentals of glycosciences, Eds: H.-J. Gabius, Wiley-VCH, Weinheim, Germany, 2009 Search PubMed.
- Y. M. Chabre and R. Roy, Adv. Carbohydr. Chem. Biochem., 2010, 63, 165 CrossRef CAS.
- R. Jelinek and S. Kolusheva, Chem. Rev., 2004, 104, 5987 CrossRef CAS.
- J. Anzai, Y. Kobayashi, N. Nakamura and T. Hoshi, Sens. Actuators, B, 2000, 65, 94 CrossRef.
- B. Hong and K. A. Kang, Biosens. Bioelectron., 2006, 21, 1333 CrossRef CAS.
- Z. Pei, H. Anderson, T. Aastrup and O. Ramström, Biosens. Bioelectron., 2005, 21, 60 CrossRef CAS.
- C.-Y. Wu, P.-H. Liang and C.-H. Wong, Org. Biomol. Chem., 2009, 7, 2247 CAS.
- J. C. Manimala, T. A. Roach, Z. Li and J. C. Gildersleeve, Angew. Chem., Int. Ed., 2006, 45, 3607 CrossRef CAS.
- S. Park, M.-R. Lee and I. Shin, Chem. Commun., 2008, 4389 RSC.
- D. Pagé and R. Roy, Int. J. Biochromatogr., 1997, 3, 231 Search PubMed.
- D. Pagé and R. Roy, Bioconjugate Chem., 1997, 8, 714 CrossRef.
- M. J. Cloninger, Curr. Opin. Chem. Biol., 2002, 6, 742 CrossRef CAS.
- K. Woller, E. D. Watler, J. R. Morgan, D. J. Singel and M. J. Cloninger, J. Am. Chem. Soc., 2003, 125, 8820 CrossRef.
- R. A. Roy, Trends Glycosci. Glycotechnol., 2003, 15, 291 CrossRef CAS.
- R. Autar, A. S. Khan, M. Schad, J. Hacker, R. M. J. Liskamp and R. J. Pieters, ChemBioChem, 2003, 4, 1317 CrossRef CAS.
- T. K. Lindhorst, M. Dubber, U. Krallmann-Wenzel and S. Ehlers, J. Org. Chem., 2000, 2027 CAS.
- R. Roy, Drug Discovery Today: Technol., 2004, 1, 327 CrossRef CAS.
- B. L. Ibey, H. T. Beier, R. M. Rounds and G. L. Cote, Anal. Chem., 2005, 77, 7039 CrossRef CAS.
- N. Bogdan, F. Vetrone, R. Roy and J. A. Capobianco, J. Mater. Chem., 2010, 20, 7543 RSC.
- T. K. Dam, R. Roy, S. K. Das, S. Oscarson and C. F. Brewer ., J. Biol. Chem., 2000, 275, 14223 CrossRef CAS.
- R. Roy and M. G. Baek, J. Biotechnol., 2002, 90, 291 CAS.
- T. K. Dam and C. F. Brewer, Chem. Rev., 2002, 102, 387 CrossRef CAS.
- S. L. Mangold and M. J. Cloninger, Org. Biomol. Chem., 2006, 4, 2458 CAS.
- J. M. de la Fuente, A. G. Barrientos, T. C. Rojas, J. Rojo, J. Cañada, A. Fernández and S. Penadés, Angew. Chem., Int. Ed., 2001, 40, 2257 CrossRef.
- H. Otsuka, Y. Akiyama, Y. Nagasaki and K. J. Kataoka, J. Am. Chem. Soc., 2001, 123, 8226 CrossRef CAS.
- G. Barrientos, J. M. Fuente, T. C. Rojas, A. Fernandez and S. Penades, Chem.–Eur. J., 2003, 9, 1909 CrossRef.
- B. Nolting, J.-J. Yu, G.-Y. Liu, S.-J. Cho, S. Kauzlarich and J. Gervay-Hague, Langmuir, 2003, 19, 6465 CrossRef CAS.
- C.-C. Lin, Y.-C. Yeh, C.-Y. Yang, G.-F. Chen, Y.-C. Chen, Y.-C. Wu and C.-C. Chen, Chem. Commun., 2003, 2920 RSC.
- J. M. de la Fuente and S. Penadés, Glycoconjugate J., 2004, 21, 149 CrossRef CAS.
- S. A. Svarovsky, Z. Szekely and J. J. Barchi, Tetrahedron: Asymmetry, 2005, 16, 587 CrossRef CAS.
- J. Rojo, V. Díaz, J. M. de la Fuente, I. Segura, A. G. Barrientos, H. H. Riese, A. Bernad and S. Penadés, ChemBioChem, 2004, 5, 291 CrossRef CAS.
- J. M. de la Fuente and S. Penadés, Biochim. Biophys. Acta, Gen. Subj., 2006, 1760, 636 CrossRef CAS.
- C.-C. Lin, Y.-C. Yeh, C.-Y. Yang, C.-L. Chen, G.-F. Chen, C.-C. Chen and Y.-C. Wu, J. Am. Chem. Soc., 2002, 124, 3508 CrossRef CAS.
- K. Aslan, J. R. Lakowicz and C. D. Geddes, Anal. Biochem., 2004, 330, 145 CrossRef CAS.
- C.-S. Tsai, T.-B. Yu and C.-T. Chen, Chem. Commun., 2005, 4273 RSC.
- K. M. Halkes, A. C. de Souza, C. E. P. Maljaars, G. J. Gerwig and J. P. Kamerling, Eur. J. Org. Chem., 2005, 17, 3650 CrossRef.
- D. C. Hone, A. H. Haines and D. A. Russell, Langmuir, 2003, 19, 7141 CrossRef CAS.
- G. Barrientos, J. M. de la Fuente, M. Jiménez, D. Solís, F. Javier Cañada, M. Martín-Lomas and S. Penadés, Carbohydr. Res., 2009, 344, 1474 CrossRef.
- E. Oh, M.-Y. Hong, D. Lee, S.-H. Nam, H. C. Yoon and H.-S. Kim, J. Am. Chem. Soc., 2005, 127, 3270 CrossRef CAS.
- S. Yun, A. Javier, T. Jennings, M. Fisher, S. Hira, S. Peterson, B. Hopkins, N. O. Reich and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 3115 CrossRef.
- D. A. Tomalia, B. Huang, D. R. Swanson, H. M. Brothers and J. W. Klimash, Tetrahedron, 2003, 59, 3799 CrossRef CAS.
- F. Perreault, N. Bogdan, M. Morin, J. Claverie and R. Popovic, Nanotoxicology, 2011 Search PubMed.
- M. Brust, M. Walker, D. Betheel, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC.
- S. P. Colowick and N. O. Kaplan, Methods in Enzymology, Vol. XXVIII.; Part B, Acad. Press, New York, 1972 Search PubMed.
- T. Hermanson, Bioconjugate Technique. Academic Press, San Diego, CA, 1996 Search PubMed.
- M. M. Maye, J. Luo, Y. Lin, M. H. Engelhard, M. Hepel and C.-J. Zhong, Langmuir, 2003, 19, 125 CrossRef CAS.
- A. Manna, T. Imae, T. Yogo, K. Aoi and M. J. Okazaki, J. Colloid Interface Sci., 2002, 256, 297 CrossRef CAS.
- M. J. Hostetler, J. E. Wingate, C.-J. Zhong, J. E. Harris, R. W. Vachet, M. R. Clark, J. D. Londono, S. J. Green, J. J. Stokes, G. D. Wignall, G. L. Glish, M. D. Porter, N. D. Evans and R. W. Murray, Langmuir, 1998, 14, 17 CrossRef CAS.
- E. Lieiner, N. Sharon and I. J. Goldstein, The Lectins: Properties, Functions, and Applications in Biology and Medicine, Academic Press, New York, 1986 Search PubMed.
- K. Kamemura and S. Kato, Anal. Biochem., 1998, 258, 305 CrossRef CAS.
- G. Scatchard, Ann. N. Y. Acad. Sci., 1949, 51, 660 CrossRef CAS.
- Tinoco, K. Sauer and J. C. Wang, Principles and applications in biological sciences. Phys. Chem., Third Edition; New Jersey, 1995 Search PubMed.
- T. Hasegawa, S. Kondoh, K. Matsuura and K. Kobayashi, Macromolecules, 1999, 32, 6595 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of FTIR spectra, XPS spectra, TGA measurements, UV-visible spectra, immunoprecipitation tests and Scatchard plot. See DOI: 10.1039/c1ra00904d |
| This journal is © The Royal Society of Chemistry 2012 |
