Synthesis of band-gap tunable Cu–In–S ternary nanocrystals in aqueous solution†
Meina
Wang
a,
Xiangyou
Liu
b,
Chuanbao
Cao
*a and
Cui
Shi
a
aResearch Center of Materials Science, Beijing Institute of Technology, Beijing 100081, China. E-mail: cbcao@bit.edu.cn; Fax: +86-10-6891 3792; Tel: +86-10-6891 2001
bDalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 10th February 2012
Abstract
Cu–In–S ternary nanocrystals (NCs), with an average size of less than 10 nm, were synthesized in an aqueous solution containing bovine serum albumin (BSA). X-Ray powder diffraction (XRD) and selected-area electron diffraction (SAED) analyses showed that these NCs featured a roquesite structure. The composition of the NCs could be adjusted by controlling the molar ratio of the starting Cu/In precursors in the reaction solution, which led to a tunable band gap ranging from 1.48 eV to 2.30 eV. Cytotoxicity testing showed that the BSA-stabilized Cu–In–S NCs had little effect on the cell viability, which suggested that they are user-friendly and environmentally benign. With low cost, minimal energy input and environmental impact, this simple approach shows great potential for industrial applications.
Introduction
The increasingly severe global energy and environmental crises call for new eco-friendly and low-cost materials with high performance in energy harvesting, storage and conversion.1–4 In this context, ternary and quaternary semiconductor NCs, especially CuInS2 (CIS) and related materials, have attracted much attention recently due to their numerous advantages.5–11 First of all, bulk CIS is a direct band-gap semiconductor with a 1.5 eV band gap energy, which is close to the best band gap for solar cells.6 Secondly, CIS NCs are free from poisonous heavy metal ions (e.g. Cd, Pd, Hg), which suggests that they are environmentally benign and suitable for bio-applications (e.g. near-infrared bio-imaging).12,13 Last but not least, the band gap of CIS NCs can be readily tuned by tailoring their composition or size.14 Such a tunable band gap is of particular importance in the fabrication of optoelectronic devices.15–17 For these reasons, CIS NCs have been considered as one of the most promising materials for photovoltaic applications.Many synthesis approaches (e.g. solvothermal and hydrothermal routes)18–21 have been developed recently to prepare CIS NCs which are generally achieved in organic solvents with the addition of noxious reagents (e.g. dodecanethiol).10,11 In this case, a negative environmental impact is inevitable. Meanwhile, the synthesis processes are often under harsh conditions (e.g. high vacuum and high temperature).6–11,20,22 This undoubtedly increases the cost and energy input, and the production is hardly scaled up to manufacture large amounts of CIS particles on an industrial scale. Herein we reported a facile and low-cost approach to synthesize band-gap tunable CuxInyS0.5x+1.5y NCs in aqueous solution under mild conditions (e.g. non-vacuum and room temperature). The synthesized CuxInyS0.5x+1.5y NCs, stabilized by BSA, possessed roquesite structure and exhibited little toxicity. The band gaps of the CuxInyS0.5x+1.5y NCs could be readily tuned by varying the molar ratio of Cu/In precursors. All the advantages ensure that this synthesis approach has great potential for industrial applications.
In the aqueous synthesis of CuxInyS0.5x+1.5y NCs, appropriate capping agents or stabilizing agents are needed to prevent the aggregation of the particles and to produce small sized particles with a narrow size distribution and uniform shape. Significantly, there has been a keen interest recently in exploring natural biological macromolecules (e.g. proteins and polysaccharides) as the stabilizing agents, among which BSA attracts a great deal of attention.23–28 BSA has a strong affinity toward nanoparticles due to there being plenty of groups (e.g. –SH and –NH2) on its side chains. It can avoid the aggregation of nanoparticles and improve their colloidal stability in aqueous solution.27,28 Moreover, it confers excellent biocompatibility on the nanoparticles, which lays substantial foundation for their bio-applications.24 For these reasons, BSA was selected as the stabilizing agent in this study. In addition, an appropriate sulfur source was also critical for the successful synthesis of Cu–In–S NCs. In a pre-experiment, both thioacetamide (TAA) and Na2S were tested as the sulfur source, and TAA was finally selected as it could slowly release S2− into the reaction solution which avoided the quick growth and aggregation of the CuxInyS0.5x+1.5y particles;26 thereby small sized (even less than 10 nm) and nearly dispersed nanoparticles were obtained.
We first synthesized CuxInyS0.5x+1.5y NCs with a starting molar ratio of Cu/In precursors of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The synthesized NCs were then characterized with transmission electron microscopy (TEM) and high-resolution TEM (HRTEM), etc. As shown in Fig. 1(a) and (b), nearly dispersed NCs of irregular shape were obtained. The average size of the particles was statistically measured to be 7.1 ± 2.5 nm. The lattice fringes had an interplanar spacing of 0.271 nm (Fig. 1(c)), matching well with the (200) interplanar spacing of the roquesite-type nanocrystal structure (see the following analyses of the XRD results). To analyze the chemical composition of the NCs, energy dispersive X-ray spectroscopy (EDS) experiments were performed which definitely confirmed the existence of Cu, In and S elements in the synthesized NCs (Fig. 2). It was estimated from the peak intensity in Fig. 2 that the approximate atomic ratio of the three elements approached the stoichiometric ratio of CuInS2. Additionally, inductively coupled plasma-atomic emission spectrometer (ICP-AES) analyses also confirmed that the relative molar ratio of Cu/In in the as-synthesized NCs was close to 1
1. The synthesized NCs were then characterized with transmission electron microscopy (TEM) and high-resolution TEM (HRTEM), etc. As shown in Fig. 1(a) and (b), nearly dispersed NCs of irregular shape were obtained. The average size of the particles was statistically measured to be 7.1 ± 2.5 nm. The lattice fringes had an interplanar spacing of 0.271 nm (Fig. 1(c)), matching well with the (200) interplanar spacing of the roquesite-type nanocrystal structure (see the following analyses of the XRD results). To analyze the chemical composition of the NCs, energy dispersive X-ray spectroscopy (EDS) experiments were performed which definitely confirmed the existence of Cu, In and S elements in the synthesized NCs (Fig. 2). It was estimated from the peak intensity in Fig. 2 that the approximate atomic ratio of the three elements approached the stoichiometric ratio of CuInS2. Additionally, inductively coupled plasma-atomic emission spectrometer (ICP-AES) analyses also confirmed that the relative molar ratio of Cu/In in the as-synthesized NCs was close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S1 in the ESI†). Subsequently, XRD was employed to further characterize the crystalline structure of the nanoparticles. As shown in Fig. 3(a), a typical diffraction pattern corresponding to the tetragonal roquesite structure (JCPDS card No. 15-0681) was observed. The phase purity was also confirmed from Fig. 3(a). Taken together, these results gave us direct evidence that CIS NCs were successfully synthesized. In addition, SAED also verified the roquesite-type crystal structure of the CIS NCs. As shown in the inset of Fig. 1(a), three distinct diffraction rings, highly consistent with the diffractions of the (112), (220) and (312) planes of the roquesite-type nanocrystal structure could be distinguished unambiguously, and two more relatively weak rings indexed to the diffractions of the (316) and (228) planes could also be discerned. Significantly, it was reported that different capping agents could result in different crystalline structures. For example, zinc blende CIS NCs were obtained when oleic acid was chosen as the capping agent, whereas wurtzite CIS NCs were yielded under the same conditions when the oleic acid was replaced by dodecanethiol.20 In the current work, for the first time BSA was chosen as the capping agent in CIS synthesis, which gave rise to a roquesite-type structure.
1 (Table S1 in the ESI†). Subsequently, XRD was employed to further characterize the crystalline structure of the nanoparticles. As shown in Fig. 3(a), a typical diffraction pattern corresponding to the tetragonal roquesite structure (JCPDS card No. 15-0681) was observed. The phase purity was also confirmed from Fig. 3(a). Taken together, these results gave us direct evidence that CIS NCs were successfully synthesized. In addition, SAED also verified the roquesite-type crystal structure of the CIS NCs. As shown in the inset of Fig. 1(a), three distinct diffraction rings, highly consistent with the diffractions of the (112), (220) and (312) planes of the roquesite-type nanocrystal structure could be distinguished unambiguously, and two more relatively weak rings indexed to the diffractions of the (316) and (228) planes could also be discerned. Significantly, it was reported that different capping agents could result in different crystalline structures. For example, zinc blende CIS NCs were obtained when oleic acid was chosen as the capping agent, whereas wurtzite CIS NCs were yielded under the same conditions when the oleic acid was replaced by dodecanethiol.20 In the current work, for the first time BSA was chosen as the capping agent in CIS synthesis, which gave rise to a roquesite-type structure.
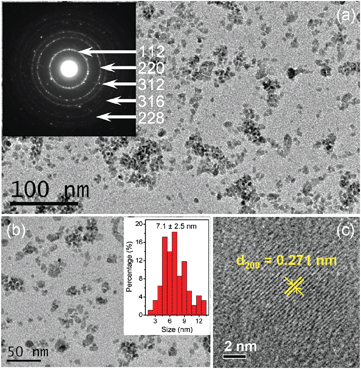 | ||
| Fig. 1 Representative TEM (a, b) and HRTEM (c) images of the synthesized CIS NCs. The insets in (a) and (b) are SAED and the size distribution histogram of the CIS NCs, respectively. | ||
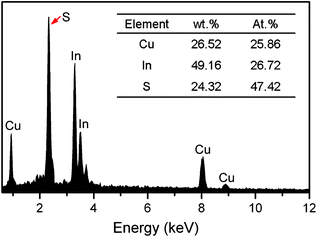 | ||
| Fig. 2 EDS spectrum of the CIS NCs. Inset shows the results of quantitative elemental analysis. | ||
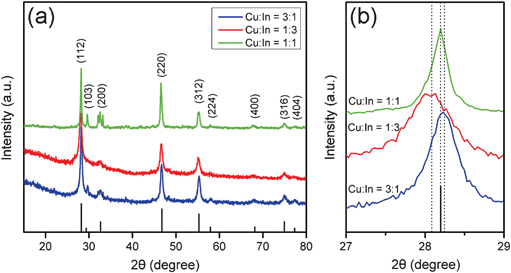 | ||
Fig. 3 (a) XRD patterns of the CuxInyS0.5x+1.5y NCs synthesized with varying molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Cu/In precursors. Reference pattern of roquesite is shown at the bottom. (b) Expanded view of the (112) peaks in (a) showing the peak shift. The dotted lines indicate the peak positions of the corresponding samples. 1) of Cu/In precursors. Reference pattern of roquesite is shown at the bottom. (b) Expanded view of the (112) peaks in (a) showing the peak shift. The dotted lines indicate the peak positions of the corresponding samples. | ||
A tunable band gap is required for photovoltaic materials to maximize their solar absorption, make full use of the energy of photons and improve their energy conversion efficiencies.15–17 Tuning the band gaps of ternary Cu–In–S NCs by changing their size or compositions in organic solution has been reported recently.14,17 However, it has never been achieved in the aqueous phase due to the difficulty of precisely controlling the composition or size of the crystals in aqueous synthesis. To address this issue, we synthesized various CuxInyS0.5x+1.5y NCs with different molar ratios of Cu/In by adjusting the starting amounts of Cu and In precursors in the reaction solution. TEM images revealed that the shape of the CuxInyS0.5x+1.5y NCs was still irregular and their size was comparable with CIS (see Fig. S1 in the ESI†), which indicated that the changes in composition of the Cu–In–S NCs had little influence on the particle shape and size. EDS and ICP-AES analyses showed that the composition of these NCs varied with the starting molar ratio of Cu/In precursors (Table 1 and Table S1 in the ESI†). Generally, a higher ratio of Cu/In precursors led to a higher Cu content in the final NCs. However, it was worth noting that a high ratio of Cu/In precursors of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 only gave rise to the NCs with a Cu/In ratio of ∼1.5
1 only gave rise to the NCs with a Cu/In ratio of ∼1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It seemed that Cu atoms were difficult to incorporate into the NCs, which was contrary to the cases in organic synthesis of the Cu–In–S ternary NCs.20 It was speculated that, at a higher Cu concentration, the reduction of Cu2+ to Cu+ was insufficient in aqueous solution at room temperature. The exact reason for this result is currently under research. As shown in Fig. 3(a), all the CuxInyS0.5x+1.5y NCs exhibited three representative diffraction peaks at around 2θ = 28°, 47° and 55°, which corresponded to the (112), (220) and (312) planes of the roquesite-type nanocrystal structure, respectively. Thus it was concluded that, although the ratio of Cu/In varied, all the CuxInyS0.5x+1.5y NCs possessed a similar structure, which was possibly attributed to the similar ionic radius of Cu+ (0.74 Å) and In3+ (0.76 Å).22 In addition, a slight shift for the (112) peak at around 2θ = 28° could be clearly observed from the expanded view in Fig. 3(b). Specifically, the (112) diffraction peak of the samples shifted toward higher angles with the increase of the Cu/In ratio. This trend is similar to a previous report.22 Such a slight shift in the diffraction peak was possibly attributed to the decrease in the unit-cell dimensions with the incorporation of more Cu atoms which had a smaller atomic size than In.
1. It seemed that Cu atoms were difficult to incorporate into the NCs, which was contrary to the cases in organic synthesis of the Cu–In–S ternary NCs.20 It was speculated that, at a higher Cu concentration, the reduction of Cu2+ to Cu+ was insufficient in aqueous solution at room temperature. The exact reason for this result is currently under research. As shown in Fig. 3(a), all the CuxInyS0.5x+1.5y NCs exhibited three representative diffraction peaks at around 2θ = 28°, 47° and 55°, which corresponded to the (112), (220) and (312) planes of the roquesite-type nanocrystal structure, respectively. Thus it was concluded that, although the ratio of Cu/In varied, all the CuxInyS0.5x+1.5y NCs possessed a similar structure, which was possibly attributed to the similar ionic radius of Cu+ (0.74 Å) and In3+ (0.76 Å).22 In addition, a slight shift for the (112) peak at around 2θ = 28° could be clearly observed from the expanded view in Fig. 3(b). Specifically, the (112) diffraction peak of the samples shifted toward higher angles with the increase of the Cu/In ratio. This trend is similar to a previous report.22 Such a slight shift in the diffraction peak was possibly attributed to the decrease in the unit-cell dimensions with the incorporation of more Cu atoms which had a smaller atomic size than In.
Subsequently, the optical properties of the CuxInyS0.5x+1.5y NCs were investigated. As shown in Fig. 4(a), a characteristic absorption peak at 280 nm (the absorption feature of BSA) was observed for all the samples, which confirmed the existence of BSA. In addition, it was clearly shown that, with the increase of Cu content, the absorption onsets of the CuxInyS0.5x+1.5y NCs were red-shifted from ∼560 nm to ∼870 nm (Fig. 4(b)). These distinct absorption onsets were used to estimate the optical band gaps of the samples according to an approach described previously (Fig. 4(a) inset).16 The results showed that the band gaps calculated for CuxInyS0.5x+1.5y NCs, with a starting Cu/In ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, are 1.48 eV, 1.93 eV and 2.30 eV, respectively. Obviously, the band gap increased with the decrease of the molar ratio of Cu/In. Overall, these results suggested that a tunable band gap could be achieved through adjusting the initial molar ratio of Cu/In in the reaction solution.
3, are 1.48 eV, 1.93 eV and 2.30 eV, respectively. Obviously, the band gap increased with the decrease of the molar ratio of Cu/In. Overall, these results suggested that a tunable band gap could be achieved through adjusting the initial molar ratio of Cu/In in the reaction solution.
 | ||
Fig. 4 (a) UV-Vis-NIR absorption spectra of the CuxInyS0.5x+1.5y NCs synthesized with varying molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Cu/In precursors. Inset shows the plots of (αhν)2versus photon energy (hν) of corresponding samples. (b) Expanded view of the region between 500 nm and 900 nm in (a) which shows the different absorption onsets of the samples. 1) of Cu/In precursors. Inset shows the plots of (αhν)2versus photon energy (hν) of corresponding samples. (b) Expanded view of the region between 500 nm and 900 nm in (a) which shows the different absorption onsets of the samples. | ||
Finally, we tested the biocompatibility of the BSA-stabilized CuxInyS0.5x+1.5y NCs. Even though the CuxInyS0.5x+1.5y NCs are not imperatively applied in living systems, it is still necessary to test their potential hazards to the organisms since direct contact or inhalation of these particles by the researchers/users during the fabrication/applications cannot be completely avoided. To this end, the cytotoxicity test was performed. As shown in Fig. 5, at low Cu concentrations (<10 μM), the particles in the experimental and control groups both showed good biocompatibility. When the Cu concentration was over 10 μM, a significant dose-dependent decrease of the cell viability in the control was observed. However, in the experimental groups, more than 60% of the cells still survived after they were treated with 125 μM Cu–In–S NCs. We have mentioned above that BSA could stabilize and disperse the particles. Herein the cytotoxicity results further emphasized the importance of BSA which could obviously improve the biocompatibility of the nanomaterials. It should be mentioned that Cd-containing quantum dots (e.g. CdS, CdSe and CdTe) under similar conditions were much more toxic.29 For example, 20 μM CdTe quantum dots (with an average size of 6 nm) could result in a ∼50% decrease of the viability of the human hepatoma cell line HepG2 cells after incubation for 48 h.30 However, 20 μM CIS NCs only caused 28% decrease under the same conditions (Fig. 5). Moreover, it was also concluded from Fig. 5 that adjusting the composition of the NCs did not influence the cell viability greatly and all the BSA-stabilized CuxInyS0.5x+1.5y NCs showed good biocompatibility.
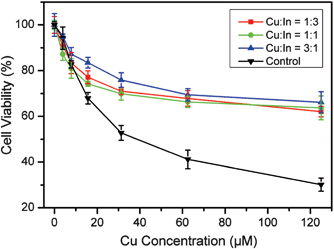 | ||
Fig. 5 Cytotoxicity analysis of the CuxInyS0.5x+1.5y NCs synthesized with varying molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Cu/In precursors. Bulk particles synthesized with no BSA added were used as the control. Experimental results are given as mean ± SD of three independent experiments. 1) of Cu/In precursors. Bulk particles synthesized with no BSA added were used as the control. Experimental results are given as mean ± SD of three independent experiments. | ||
Conclusions
Sub-10 nm and irregularly-shaped Cu–In–S ternary NCs with roquesite-type structure were successfully synthesized in aqueous solution. The composition of the NCs could be adjusted by controlling the molar ratio of the starting Cu/In precursors in the reaction solution. XRD and SAED confirmed the tetragonal roquesite structure and phase purity of the CuxInyS0.5x+1.5y NCs. Optical absorption of the NCs showed distinct onsets from ∼560 nm to ∼870 nm with the increase of Cu content, which corresponded to the tunable band gaps ranging from 2.30 eV to 1.48 eV. To the best of our knowledge, this is the first report to synthesize Cu–In–S ternary NCs with tunable band gaps in aqueous solution. In addition, these BSA-stabilized NCs showed little cytotoxicity, suggesting that they are user-friendly and environmentally benign. It is believed that such a simple synthesis approach is also adapted to synthesize other ternary and even quaternary semiconductor NCs (e.g. Cu2ZnSnS4). Further study to gain insights of the formation mechanisms of the BSA-stabilized ternary NCs and to expand the applications of the presented approach is currently underway.Experimental section
Materials
Copper(II) sulfate pentahydrate (CuSO4·5H2O, 99.99%), indium(III) chloride (InCl3, 99.99%) and TAA (C2H5NS, ≥99.0%) were all purchased from Aladdin reagent company. BSA (purity ≥99%) was purchased from Amresco. All the other chemicals were commercial available and used without further purification.Synthesis of CIS NCs
In a typical process, BSA (60 mg) was dissolved in 45 mL phosphate buffered saline (PBS buffer, pH 7.4) to form a clear solution. Then CuSO4·5H2O (125 μmol) and InCl3 (125 μmol) were added and the solution was kept stirring for 12 h at room temperature. Subsequently 5 mL TAA aqueous solution (50 mM) was added dropwise. After stirring for another 24 h at room temperature, a brown solution was obtained. This solution was kept static for 72 h at 4 °C, then centrifuged at 4 °C for 20 min. The sediment was discarded, and the supernatant was dialyzed in order to remove the free ions. Finally the CIS solution was concentrated by lyophilization. Other CuxInyS0.5x+1.5y NCs were also synthesized according to the procedures, only except that the starting amounts of Cu and In precursors varied. In order to study the influence of BSA on the formation of CIS NCs as well as on the biocompatibility of the particles, a control experiment was performed according to the same procedures as described above except that no BSA was added.Characterization of CIS NCs
For TEM and HRTEM observation, the sample solution was dropped onto a nickel grid covered with a thin carbon film, then dried in air at room temperature. TEM images and SAED were obtained on an FEI Tecnai G2 F20 microscope operating at an accelerating voltage of 200 kV. HRTEM images were taken on an FEI Tecnai G2 F30 S-TWIN microscope operating at an accelerating voltage of 300 kV. EDS spectra were acquired using a Hitachi S-4800 scanning electron microscope equipped with a Bruker AXS XFlash detector 4010. Besides EDS, ICP-AES (Teledyne Leeman Labs) was also employed to analyze the nanocrystal composition. The UV-Vis-NIR spectra of the sample solutions were recorded with a Hitachi U-4100 spectrophotometer. XRD measurements were performed on a PANalytical X'Pert PRO X-ray diffractometer with Cu-Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA.Cell culture and cytotoxicity analysis
HepG2 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO, Invitrogen) supplemented with 10% (v/v) newborn calf serum (Invitrogen) and 1% penicillin–streptomycin (Beyotime, China) in a humidified incubator at 37 °C with 5% CO2.A Cell Counting Kit-8 (CCK-8) assay kit (Beyotime, China) was employed to evaluate the toxicity of the samples according to the manufacturer's instructions. Briefly, the cells were seeded into a 96-well plate (Corning, USA) and grown to a density of 104 cells/well. Then serial dilutions of the samples were added and co-incubated with the cells for 48 h. Subsequently, CCK-8 solution (20 μL/well) was added and the plate was further incubated for 30 min. The absorbance of each well at 450 nm was finally measured using a microplate reader (Infinite M200, Tecan).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 50972017 and No. 20471007).References
- C. Steinhagen, M. G. Panthani, V. Akhavan, B. Goodfellow, B. Koo and B. A. Korgel, J. Am. Chem. Soc., 2009, 131, 12554–12555 CrossRef CAS.
- X. Ge, L. Zhang, Y. Fang, J. Zeng and S. H. Chan, RSC Adv., 2011, 1, 715–724 RSC.
- H. W. Hillhouse and M. C. Beard, Curr. Opin. Colloid Interface Sci., 2009, 14, 245–259 CrossRef CAS.
- M. Sahal, B. Marí and M. Mollar, Thin Solid Films, 2009, 517, 2202–2204 CrossRef CAS.
- F. Yakuphanoglu, Sol. Energy, 2011, 85, 2518–2523 CrossRef CAS.
- M. Kruszynska, H. Borchert, J. Parisi and J. Kolny-Olesiak, J. Am. Chem. Soc., 2010, 132, 15976–15986 CrossRef CAS.
- S. T. Connor, C. M. Hsu, B. D. Weil, S. Aloni and Y. Cui, J. Am. Chem. Soc., 2009, 131, 4962–4966 CrossRef CAS.
- M. G. Panthani, V. Akhavan, B. Goodfellow, J. P. Schmidtke, L. Dunn, A. Dodabalapur, P. F. Barbara and B. A. Korgel, J. Am. Chem. Soc., 2008, 130, 16770–16777 CrossRef CAS.
- J. Xu, C. S. Lee, Y. B. Tang, X. Chen, Z. H. Chen, W. J. Zhang, S. T. Lee, W. X. Zhang and Z. H. Yang, ACS Nano, 2010, 4, 1845–1850 CrossRef CAS.
- X. T. Lu, Z. B. Zhuang, Q. Peng and Y. D. Li, CrystEngComm, 2011, 13, 4039–4045 RSC.
- L. A. Li, A. Pandey, D. J. Werder, B. P. Khanal, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2011, 133, 1176–1179 CrossRef CAS.
- K. T. Yong, I. Roy, R. Hu, H. Ding, H. X. Cai, J. Zhu, X. H. Zhang, E. J. Bergey and P. N. Prasad, Integr. Biol., 2010, 2, 121–129 RSC.
- T. Pons, E. Pic, N. Lequeux, E. Cassette, L. Bezdetnaya, F. Guillemin, F. Marchal and B. Dubertret, ACS Nano, 2010, 4, 2531–2538 CrossRef CAS.
- X. L. Wang, D. C. Pan, D. Weng, C. Y. Low, L. Rice, J. Y. Han and Y. F. Lu, J. Phys. Chem. C, 2010, 114, 17293–17297 CAS.
- D. C. Pan, X. L. Wang, Z. H. Zhou, W. Chen, C. L. Xu and Y. F. Lu, Chem. Mater., 2009, 21, 2489–2493 CrossRef CAS.
- P. C. Dai, X. N. Shen, Z. J. Lin, Z. Y. Feng, H. Xu and J. H. Zhan, Chem. Commun., 2010, 46, 5749–5751 RSC.
- Q. H. Liu, Z. C. Zhao, Y. H. Lin, P. Guo, S. J. Li, D. C. Pan and X. L. Ji, Chem. Commun., 2011, 47, 964–966 RSC.
- J. Xiao, Y. Xie, R. Tang and Y. Qian, J. Solid State Chem., 2001, 161, 179–183 CrossRef CAS.
- J. Xiao, Y. Xie, Y. Xiong, R. Tang and Y. Qian, J. Mater. Chem., 2001, 11, 1417–1420 RSC.
- D. Pan, L. An, Z. Sun, W. Hou, Y. Yang, Z. Yang and Y. Lu, J. Am. Chem. Soc., 2008, 130, 5620–5621 CrossRef CAS.
- W. Yue, S. Han, R. Peng, W. Shen, H. Geng, F. Wu, S. Tao and M. Wang, J. Mater. Chem., 2010, 20, 7570–7578 RSC.
- Q. Guo, G. M. Ford, H. W. Hillhouse and R. Agrawal, Nano Lett., 2009, 9, 3060–3065 CrossRef CAS.
- A. V. Singh, B. M. Bandgar, M. Kasture, B. L. V. Prasad and M. Sastry, J. Mater. Chem., 2005, 15, 5115–5121 RSC.
- A. V. Singh, R. Patil, M. B. Kasture, W. N. Gade and B. L. V. Prasad, Colloids Surf., B, 2009, 69, 239–245 CrossRef CAS.
- P. Huang, Z. M. Li, H. Y. Hu and D. X. Cui, J. Nanomater., 2010 Search PubMed.
- L. Yang, H. Y. Yang, Z. X. Yang, Y. X. Cao, X. M. Ma, Z. S. Lu and Z. Zheng, J. Phys. Chem. B, 2008, 112, 9795–9801 CrossRef CAS.
- J. P. Xie, Y. G. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS.
- L. Yang, R. M. Xing, Q. M. Shen, K. Jiang, F. Ye, J. Y. Wang and Q. S. Ren, J. Phys. Chem. B, 2006, 110, 10534–10539 CrossRef CAS.
- J. L. Pelley, A. S. Daar and M. A. Saner, Toxicol. Sci., 2009, 112, 276–296 CrossRef CAS.
- Y. B. Zhang, W. Chen, J. Zhang, J. Liu, G. P. Chen and C. Pope, J. Nanosci. Nanotechnol., 2007, 7, 497–503 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and Table S1. See DOI: 10.1039/c2ra00034b |
| This journal is © The Royal Society of Chemistry 2012 |
