Lipase-catalyzed regioselective acylation of sugar in microreactors†
Li-Hua
Du
*a and
Xi-Ping
Luo
b
aCollege of Pharmaceutical Science, ZheJiang University of Technology, Hangzhou, 310014, China. E-mail: orgdlh@yahoo.com.cn; orgdlh@zjut.edu.cn; Fax: +86 571 88320903
bDepartment of Environmental Science and Technology, ZheJiang A&F University, Hangzhou, 311300, China
First published on 10th February 2012
Abstract
The highly regioselective synthesis of sugar 6-monoesters with sugar and vinyl carboxylate in a flow microreactor using Lipozyme TL IM from Thermomyces lanuginosus as a catalyst has been developed. The important features of this method include mild reaction conditions, short reaction times (30 min), high yields and high regioselectivities.
The variety of chemical reactions performed in continuous flow microreactors is steadily increasing. Important advantages over conventional batch reactions include a generally enhanced control over selectivity and significant suppression of side product formation as a result of improved mass and heat transfer processes. An additional valuable feature of microreactor technology is that reaction screening can be efficiently carried out without using large amounts of substrate or catalyst because of the small dimensions of the reactor. The advantages of microreactors have been substantiated by a growing number of examples over the past decade.1–11 In recent years, there has been increasing interest in enzymatic synthesis in microreactors.12–19 This work prompted us to investigate the possibility of regioselective acylation of sugars such as sucrose, glucose, etc. in microreactors catalyzed by lipase.
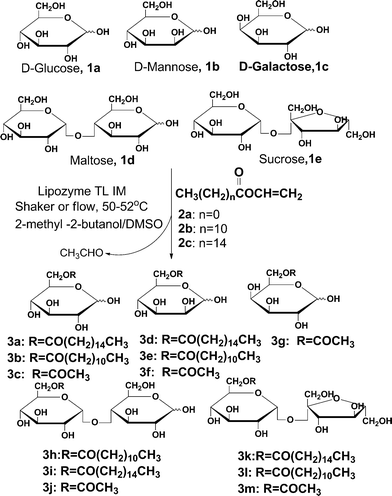
Sugars contain multiple hydroxyl groups, all of which are capable of being acylated by electrophiles. The regioselective acylation of sugars may have considerable impact in the synthesis of surfactants, sweeteners, food ingredients (e.g., emulsifiers), and chemical and pharmaceutical intermediates.20 The chemical acylation of sucrose takes place with the preference 6-OH ≥ 6′-OH > 1′-OH > secondary-OHs.21 Regioselective chemical acylation of sucrose always needs the use of exceptionally mild reaction conditions, sterically hindered acid chlorides, activated sucrose following the Mitsunobu reaction, or voluminous acylating agents in the presence of basic catalysts. The enzymatic synthesis of sugar esters is typically preferred to chemical synthesis since it is more specific and conducted under milder conditions. The most common synthetic approach to prepare sugar esters involves the transesterification of sugars with vinyl carboxylate catalyzed by lipase (Scheme 1). However, this method always requires a long reaction time (24 h) and the use of toxic solvents, which are incompatible with food applications.22–23 To avoid or reduce the amount of these solvents, and at the same time to exploit the use of lipases in these reactions, several processes have been recently reported using less harmful solvents such as tert-butanol.24 However, extreme reaction conditions are required to obtain moderate yields. Ferrer et al.25 reported the lipase-catalyzed regioselective acylation of sucrose in 2-methyl-2-butanol–DMSO mixtures; the conversion of sucrose 6-monoesters reaches a maximum in 24 h at 20% DMSO and diesters reaches a maximum at 10% DMSO.
 | ||
| Scheme 1 General sugar-vinyl carboxylate transesterification strategy to give sugar 6-monoesters. | ||
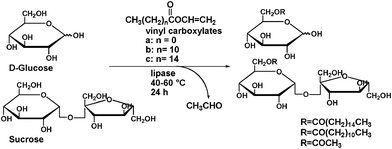
The regioselective synthesis of sugar esters in microreactors has been unexplored previously. Herein, we describe a general and scalable method for the continuous flow synthesis of sugar esters catalyzed by lipase. Key to this process is the regioselective synthesis of sugar esters, performed by the transesterification of sugar with vinyl carboxylate, and catalyzed by lipase in a continuous flow/microreactor (Fig. 1). Under optimized conditions, sugar esters are formed with quantitative conversion in residence times of 30 min, providing excellent purities (95%–99%) and yields (82%–98%) of isolated product.
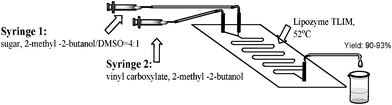 | ||
| Fig. 1 Microreactor setup for the continuous-flow synthesis of sugar esters catalyzed by Lipozyme TL IM. | ||
An evaluation of existing protocols for batch sugar ester syntheses following the general sugar-vinyl carboxylate transesterification strategy to yield sugar 6-monoesters (Scheme 1) made the challenges of converting batch into flow conditions immediately apparent (Fig. 1). Apart from the fact that most of the published procedures report reaction times of several hours (or even days) to obtain high conversions, in many instances the regioselectivities of sugar 6-monoesters were low. Solvents that can dissolve both sugars and lipids include dimethyl sulfoxide (DMSO), pyridine, and dimethylformamide, but these solvents often inactivate the enzyme and are incompatible with food applications.26 For this reason, fully homogeneous reaction media are highly desirable in continuous flow/microreactor processing. In addition, reaction times (= residence times) in the flow should ideally be on the order of a few minutes to permit high throughput. With this background, an optimization procedure was implemented selecting sucrose and vinyl palmitate as model compounds for sugar ester formation (Table 1). To run lipase-catalyzed regioselective acylation of sucrose under continuous-flow conditions, a solution of sucrose (0.4 mmol in 10 mL 2-methyl -2-butanol–DMSO=4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was mixed with a stream of vinyl palmitate (2.0 mmol in 10 mL 2-methyl-2-butanol) at a “Y” junction using silicone tubing (inner diameter = 2.0 mm). The tubing was packed with silica spheres (0.3–0.9 mm) with surface-adsorbed Lipozyme® TL IM (Novozyme). The reactant solution was flushed through the microreactor for 30 min, at a flow rate of 20.8 μL min−1 and then the product was collected. When the reactor was heated to 40 °C with a 30 min residence time, only a 35% conversion was obtained (Table 1, entry 1). Increasing the reaction temperature produced better results, with 52 °C giving the highest conversion and regioselectivity (Table 1, entry 4).
1) was mixed with a stream of vinyl palmitate (2.0 mmol in 10 mL 2-methyl-2-butanol) at a “Y” junction using silicone tubing (inner diameter = 2.0 mm). The tubing was packed with silica spheres (0.3–0.9 mm) with surface-adsorbed Lipozyme® TL IM (Novozyme). The reactant solution was flushed through the microreactor for 30 min, at a flow rate of 20.8 μL min−1 and then the product was collected. When the reactor was heated to 40 °C with a 30 min residence time, only a 35% conversion was obtained (Table 1, entry 1). Increasing the reaction temperature produced better results, with 52 °C giving the highest conversion and regioselectivity (Table 1, entry 4).
| Entry | T/°C | Conv. [%]b | Regioselectivity [%]c |
|---|---|---|---|
a Standard reactions conditions: 0.4 mmol sucrose in 10 mL 2-methyl-2-butanol–DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.0 mmol vinyl palmitate in 10 mL 2-methyl-2-butanol, 0.87 g Lipozyme TL IM in silicone tubing, 20.8 μL min−1 flow rate, residence time 30 min.
b Conversion determined using HPLC.
c Corresponding to the amount of 6-O-palmitoylsucrose to the total amount of sucrose ester. 1, 2.0 mmol vinyl palmitate in 10 mL 2-methyl-2-butanol, 0.87 g Lipozyme TL IM in silicone tubing, 20.8 μL min−1 flow rate, residence time 30 min.
b Conversion determined using HPLC.
c Corresponding to the amount of 6-O-palmitoylsucrose to the total amount of sucrose ester.
|
|||
| 1 | 40 | 35 | 100 |
| 2 | 45 | 70 | 90 |
| 3 | 50 | 86 | 96 |
| 4 | 52 | 95 | 99 |
| 5 | 55 | 91 | 98 |
Following the standard procedure given above, the influence of vinyl palmitate/sucrose on the conversion of 6-O-palmitoylsucrose was investigated (Fig. 2). The highest conversion and regioselectivity of 6-O-palmitoylsucrose can be obtained when vinyl palmitate/sucrose = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
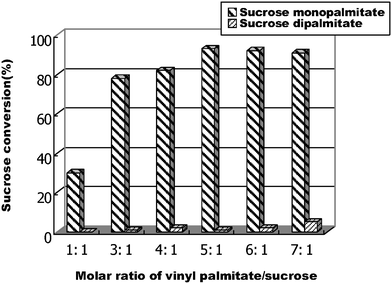 | ||
| Fig. 2 The influence of vinyl palmitate/sucrose on the conversion of 6-O-palmitoylsucrose catalysed by Lipozyme TL IM in a flow microreactor. | ||
Furthermore, the influence of reaction time/flow rate on the conversion of 6-O-palmitoylsucrose was also studied. Fig. 3 shows that the best conversion and regioselectivity of 6-O-palmitoylsucrose was observed for a residence time of 30 min and at a flow rate of 20.8 μL min−1. Longer residence times resulted in the conversion to diesters.
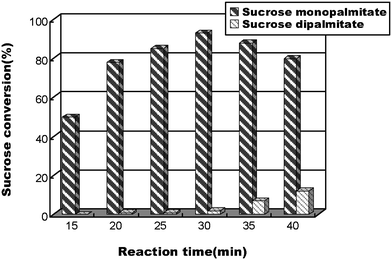 | ||
| Fig. 3 The influence of reaction time on the conversion of 6-O-palmitoylsucrose catalysed by Lipozyme TL IM in a flow microreactor. | ||
Finally, to explore the scope and limitations of this new high-speed sugar ester synthesis, a set of 5 sugars 1a–e and 3 vinyl carboxylates 2a–c was subjected to the general reaction conditions, both using a single-mode shaker reactor and continuous flow/microreactor processing. For the shaker experiments, reaction times always need to be about 24 h or even more to obtain ideal conversion (Method A). Moreover, the percentage of DMSO (v/v) greatly affected the purities and yields of 6-monoesters; the conversion of sucrose 6-monoesters reaches a maximum at 20% DMSO. On the other hand, using lipase-catalyzed regioselective acylation of sugar under continuous-flow conditions, 13 sugar monoesters were synthesized in parallel in a single experiment at the same flow rate (Methods B, C and D); the results were better with the flow/microreactor processing than with the single-mode shaker (Table 2, entries 1–13). Importantly, applying continuous flow/microreactor processing, a conversion of 95% or even more to 6-monoester can be obtained, with negligible formation of diesters. This allows us to control the production of 6-monoesters and simplify the purification of products. Furthermore, application of continuous flow/microreactor processing reduced the percentage of DMSO to 10% with greatly reduced reaction times, from about 24 h to 30 min.
| Entry | Product | Methoda | t | Yield [%]b | Regioselectivity [%]c |
|---|---|---|---|---|---|
a Method A: shaker reactor, 2-methyl-2-butanol–DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (4 mL), 0.17 g Lipozyme TL IM (43 mg mL−1), 24 h. Method B; continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4 1 (4 mL), 0.17 g Lipozyme TL IM (43 mg mL−1), 24 h. Method B; continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 10.4 μL min−1 feed II (0.20 M solution of vinyl palmitate in 2-methyl-2-butanol) at 52 °C (residence time 30 min), Lipozyme TL IM 0.87 g. Method C: continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4 1) and 10.4 μL min−1 feed II (0.20 M solution of vinyl palmitate in 2-methyl-2-butanol) at 52 °C (residence time 30 min), Lipozyme TL IM 0.87 g. Method C: continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 10.4 μL min−1 feed II (0.44 M solution of vinyl laurate in 2-methyl-2-butanol) at 55 °C (residence time 30 min), Lipozyme TL IM 0.87 g. Method D: continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4 1) and 10.4 μL min−1 feed II (0.44 M solution of vinyl laurate in 2-methyl-2-butanol) at 55 °C (residence time 30 min), Lipozyme TL IM 0.87 g. Method D: continuous flow microreactor, 10.4 μL min−1 feed I (0.04 M solution of sugar in 2-methyl-2-butanol–DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 10.4 μL min−1 feed II (0.72 M solution of vinyl acetate in 2-methyl-2-butanol) at 52 °C (residence time 30 min), Lipozyme TL IM 0.87 g.
b Isolated yield.
c Corresponding to the amount of 6-O-sugar ester to the total amount of sugar esters. 1) and 10.4 μL min−1 feed II (0.72 M solution of vinyl acetate in 2-methyl-2-butanol) at 52 °C (residence time 30 min), Lipozyme TL IM 0.87 g.
b Isolated yield.
c Corresponding to the amount of 6-O-sugar ester to the total amount of sugar esters.
|
|||||
| 1 | 3a | A | 24 h | 82 | 80 |
| B | 30 min | 95 | 100 | ||
| 2 | 3b | A | 24 h | 80 | 78 |
| C | 30 min | 94 | 100 | ||
| 3 | 3c | A | 24 h | 83 | 75 |
| D | 30 min | 96 | 99 | ||
| 4 | 3d | A | 24 h | 78 | 82 |
| B | 30 min | 98 | 99 | ||
| 5 | 3e | A | 24 h | 80 | 80 |
| C | 30 min | 97 | 99 | ||
| 6 | 3f | A | 24 h | 88 | 75 |
| D | 30 min | 96 | 99 | ||
| 7 | 3g | A | 24 h | 80 | 70 |
| D | 30 min | 90 | 99 | ||
| 8 | 3h | A | 24 h | 65 | 70 |
| C | 30 min | 82 | 95 | ||
| 9 | 3i | A | 24 h | 70 | 78 |
| B | 30 min | 84 | 96 | ||
| 10 | 3j | A | 24 h | 88 | 80 |
| D | 30 min | 97 | 99 | ||
| 11 | 3k | A | 24 h | 80 | 90 |
| B | 30 min | 93 | 98 | ||
| 12 | 3l | A | 24 h | 78 | 85 |
| C | 30 min | 92 | 99 | ||
| 13 | 3m | A | 24 h | 81 | 78 |
| D | 30 min | 93 | 99 | ||
In conclusion, we have demonstrated that sugar 6-monoesters can be synthesized with unprecedented efficiency using a flow microreactor approach. The large surface-area-to-volume ratios of the catalyst, Lipozyme® TL IM adsorbed on silica particles, is key to the success of this protocol. The adsorbed catalyst permits the substrate sugar and vinyl carboxylate to make efficient contact and react within the microreactor environment. The salient features of this method include mild reaction conditions, fast reaction times (30 min), high yields as well as high regioselectivities that make our methodology a valuable contribution to the field of sugar monoester synthesis. The method of enzymatic synthesis in a microreactor environment described here may have general applications to synthetic organic chemistry by enzymatic catalysis in the future.
The authors would like to thank the Natural Science Foundation of Zhejiang Province (Y4080126), the Science and Technology Research Program of Zhejiang Province (2008C12084) as well as the Natural Science Foundation of Zhejiang University of Technology (116004029) for financial support.
References
- D. Belder, M. Ludwig, L. W. Wang and M. T. Reetz, Angew. Chem., Int. Ed., 2006, 45, 2463 CrossRef CAS.
- G. N. Doku, W. Verboom, D. N. Reinhoudt and A. Vandenberg, Tetrahedron, 2005, 61, 2733 CrossRef CAS.
- M. Ferrer, M. A. Cruces, M. Bernabé, A. Ballesteros and F. J. Plou, Biotechnol. Bioeng., 1999, 65, 10 CrossRef CAS.
- F. Ganske and U. T. Bornscheuer, J. Mol. Catal. B: Enzym., 2005, 36, 40 CrossRef CAS.
- K. Geyer, J. D. C. Codee and P. H. Seeberger, Chem.–Eur. J., 2006, 12, 8434 CrossRef CAS.
- V. Hessel and H. Lowe, Chem. Eng. Technol., 2005, 28, 267 CrossRef.
- T. Honda, M. Miyazaki, H. Nakamura and H. Maeda, Chem. Commun., 2005, 5062 RSC.
- K. Kanno, H. Kawazumi, M. Miyazaki, H. Maeda and M. Fujii, Aust. J. Chem., 2002, 55, 687 CrossRef CAS.
- K. Kanno, H. Maeda, S. Izumo, M. Ikuno, K. Takeshita, A. Tashiro and M. Fujii, Lab Chip, 2002, 2, 15 RSC.
- A. Kirschning, H. Monenschein and R. Wittenberg, Angew. Chem., Int. Ed., 2001, 40, 650 CrossRef CAS.
- J. Krenkova and F. Foret, Electrophoresis, 2004, 25, 3550 CrossRef CAS.
- P. Fernandes, Int. J. Mol. Sci., 2010, 11, 858 CrossRef CAS.
- B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef CAS.
- P. W. Miller, N. J. Long, A. J. Mello, R. Vilar, J. Passchier and A. Gee, Chem. Commun., 2006, 546 RSC.
- M. Miyazaki and H. Maeda, Trends Biotechnol., 2006, 24, 463 CrossRef CAS.
- A. Nagaki, N. Takabayashi, Y. Tomida and J. Yoshida, Org. Lett., 2008, 10, 3937 CrossRef CAS.
- A. Nagaki, H. Kim and J. Yoshida, Angew. Chem., Int. Ed., 2008, 47, 7833 CrossRef CAS.
- D. R. Patil, D. G. Rethwisch and J. S. Dordick, Biotechnol. Bioeng., 1991, 37, 639 CrossRef CAS.
- H. E. Schoemaker, D. Mink and M. G. Wubbolts, Science, 2003, 299, 1694 CrossRef CAS.
- P. L. Urban, D. M. Goodall and N. C. Bruce, Biotechnol. Adv., 2006, 24, 42 CrossRef CAS.
- A. H. Haines, Adv. Carbohydr. Chem. Biochem., 1976, 33, 11 CrossRef CAS.
- P. Watts and C. Wiles, Chem. Commun., 2007, 443 RSC.
- J. Yoshida, A. Nagaki, T. Iwasaki and S. Suga, Chem. Eng. Technol., 2005, 28, 259 CrossRef CAS.
- (a) M. A. Ku and Y. D. Hang, Biotechnol. Lett., 1995, 17, 1081 CrossRef CAS; (b) M. Woudenberg, F. Van Rantwijk and R. A. Sheldon, Biotechnol. Bioeng., 1996, 49, 328 CrossRef.
- M. Ferrer, M. A. Cruces, M. Bernabé, A. Ballesteros and F. J. Plou, Biotechnol. Bioeng., 1999, 65, 10 CrossRef CAS.
- F. Ganske and U.T. Bornscheuer, J. Mol. Catal. B: Enzym., 2005, 36, 40 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c2ra01112c |
| This journal is © The Royal Society of Chemistry 2012 |