Ligand control in thiol stabilized Au38 clusters†
Daniel
Stellwagen
ab,
Andrew
Weber
a,
Gudrun Lisa
Bovenkamp
a,
Rongchao
Jin
c,
J. H.
Bitter
b and
Challa S. S. R.
Kumar
*ad
aCenter for Advanced Microstructures and Devices, Louisiana State University, Baton Rouge, LA 70806, USA
bUtrecht University, P. O. Box 80083, 3508 TB Utrecht, The Netherlands
cDepartment of Chemistry, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA. E-mail: ckumar1@lsu.edu
dCenter for Atomic Level Catalyst Design (CALC-D), South Stadium Road, Baton Rouge, LA 70803
First published on 13th February 2012
Abstract
Alkyl thiols (CnH2n + 1SH) with chain lengths (n = 4, 6, 8, 12, 16) were investigated for etching the polydisperse Aun(SG)m mixture in order to obtain nearly monodisperse Au38(SR)24 clusters. FT-IR studies demonstrate that all thiol ligands bound to Au38 clusters are highly ordered and in an all-trans state. X-ray absorption studies at the S K-edge as well as at the Au L3 edge show similarities between Au(I)S species and Au38(SR)24 clusters, in good agreement with the ‘S-Au-S’ capping motifs in the Au38(SR)24 structure and confirming charge transfer from gold to sulfur atoms. We report for the first time the use of Wide-angle X-ray scattering (WAXS) analysis as a more reliable tool than TEM to evaluate particle size and size distributions Au38(SR)24 clusters.
In recent years a new field in gold nanoparticle research has emerged, focusing on ultra-small (<2 nm) clusters of gold, also called quantum-sized gold clusters, protected by thiolate (–SR) ligands. Unlike larger gold colloids, whose electronic properties resemble the bulk state, quantum-sized gold clusters are characterized by distinct quantum confinement effects where one can observe discrete electronic structure (as opposed to quasi-continuous band structure in Au nanocrystals) and molecular type properties such as HOMO–LUMO transitions and intrinsic magnetism. Such gold clusters also provide a unique opportunity to bridge the gap between experimental and computational efforts in nanoparticle research. They are small enough to allow for DFT calculations on the entire system, but big enough to allow experimental control of size and shape. Besides being of theoretical interest, ultra-small gold clusters have also found practical applications in the field of catalysis. There have been several publications on the catalytic activity of ultra-small gold-thiolate clusters, showing both improved activity and selectivity compared to larger gold nanoparticles in several oxidation and hydrogenation reactions.1–3 In addition to catalysis, these clusters have potential opportunities in various applications including biological labeling, and nano-electronics4 due to their excellent chemical stability and elegant optical properties.
A major challenge lies in achieving synthetic control over the size distribution of the gold clusters. Research has mainly focused on the synthesis of thiolate (–SR) stabilized clusters, and promising results have been achieved. Several wet-chemistry methods have recently become available for obtaining atomically monodisperse Aun(SR)m clusters. Well defined synthesis protocols for Au25(SR)18, Au38(SR)24 and Au144(SR)60 are now available.5–8 Post synthesis purification is performed by simple solvent extraction procedures, instead of the more complicated low-yield methods previously used, such as gel electrophoresis and size exclusion chromatography.
While almost all studies focus on cluster size control, studies on ligand control in gold cluster synthesis are largely non-existent. This is surprising, since the thiol capping agent has a large influence on the behavior of the Aun(SR)m clusters.9 For example, both the HOMO and LUMO bands of Au25(SR)18 have relatively large degrees of S(3p) character, indicating that ligands significantly influence the electronic structure and possibly catalytic behavior of such gold clusters.3,5 Well established procedures for ligand control could be a major asset for the practical application of gold clusters. For instance, choosing the proper ligand can improve solubility or facilitate interaction between the Aun(SR)m clusters and a catalyst support. Although Au38 clusters are the subject of many experimental and theoretical studies, only a few different Au38(SR)24 cluster syntheses have been reported thus far.6,10–14 Early work by Tsukuda and co-workers explored polyacrylamide gel electrophoresis to separate a polydisperse mixture of glutathione (SG)-capped gold nanoclusters. Amongst others, a 10.4 kDa fraction was identified, consisting of high-purity Au25(SG)18 clusters.13
Other work has utilized thiol etching procedures to achieve size focusing of polydisperse gold clusters. During thiol etching a polydisperse Au:SR mixture, obtained via a two-step reduction of the gold salt precursor, is heated to high temperature in the presence of an excess of neat thiol over a period of several hours. In this process, the original capping ligand is replaced by a monolayer of the excess neat thiol present in the reaction mixture. Simultaneously, a size focusing effect is observed in which the polydisperse mixture of gold clusters is converted to a set of highly monodisperse gold clusters. Although the thiol etching process is pivotal to the preparation of monodisperse Au38(SR)24 and Au144(SR)60 clusters, its size focusing and ligand exchange mechanisms remain poorly understood.
Previous work involving thiol etching6,10,14 has yielded Au38 clusters stabilized by various alkyl and aryl thiols. However, the differences in thiol functionality were either introduced prior to the etching step, resulting in low yields, or they were introduced while varying the conditions of the thiol etching step. Thus it is currently not clear what range of thiol ligands can be introduced during the etching step when using a single set of experimental conditions. Also, one of the challenges in nanocluster synthesis in general and gold cluster synthesis in particular, using wet chemistry, is the ability to synthesize the same cluster size with different stabilizers. The obvious reason for this problem is that the nature of the stabilizer influences the nucleation and growth processes and therefore influences the size of the cluster. In order to develop a synthetic strategy to prepare a wide variety of Au38(SCnH2n+1)24 clusters using a single experimental methodology, a polydisperse Aun(SG)m mixture was etched with aliphatic thiols under experimental conditions known to yield high purity Au38(SC12H25)24 clusters.10 The influences of changes in the thiol chain length on the degree of size focusing and ligand exchange, were investigated and will be discussed below. Experimental details are as follows.
Chemicals. The following chemicals used in this work are of highest purity, which were obtained from Sigma Aldrich and used as received. Tetrachloroauric(III) acid (99.99%); L-glutathione reduced (>98%); sodium borohydride (99.99%); acetone (HPLC-grade); ethanol (HPLC-grade); methanol (anhydrous); toluene (HPLC-grade); 1-butanethiol (99%); 1-hexanethiol (95%); 1-octanethiol (>98.5%); 1-dodecanethiol (>98%); 1-hexadecanethiol (99%).
Synthesis of Au 38(SR)24. All glassware used was thoroughly washed and rinsed with concentrated nitric acid before use. 0.5 g (1.25 mmol) HAuCl4·3H2O was dissolved in 80 ml MeOH. 1.55 g (5 mmol) glutathione was dissolved in 40 ml nanopure water. The two solutions were mixed, upon which a white suspension (Au(I)SG polymer) instantly formed. The white suspension was cooled to 0 °C, and a cold NaBH4 solution (0.47 g, 12.5 mmol in 24 ml nanopure water) was added with vigorous stirring. The black suspension was kept at 0 °C for 1 h, after which the reaction was stopped. The black product (a mixture of Aun(SG)m clusters) was isolated by centrifugation and washed with 100 ml MeOH. The black product was then dissolved in 12 ml nanopure water and transferred to a reflux setup. 15 ml acetone and 20 ml thiol (see Table 1) were added to the solution, yielding a two phase system in which the black aqueous layer was topped by the transparent thiol layer. The mixture was heated to the reaction temperature (see Table 1) and left overnight (16 h) while stirring. Phase transfer of the black gold clusters was observed, indicating successful thiol exchange. The reaction mixture was transferred to a separating funnel and the aqueous phase was removed. The target product was precipitated from the remaining solution by adding a non-solvent (see Table 1, wash 1), and washed several times with copious amounts of EtOH and acetone (see Table 1, washes 1–3) to remove by-products and excess free thiol. The Au38(SR)24 product was isolated from the remaining fraction by extraction with toluene. The pitch black toluene solution was centrifuged (5000 rpm, 5 min) to remove by-products. The remaining Au38(SR)24 solution was stored as a 1 mg ml−1 solution in a refrigerator. Additional remarks. Butanethioletching: since the hydrocarbon chain of the ligand used in this synthesis is short, the target product is slightly soluble in acetone, complicating the acetone washing step. Hexadecanethioletching: the excess free thiol present after the etching procedure tends to solidify around room temperature, complicating the washing procedure. To prevent this, the mixture was heated slightly to ∼35 °C during the washing step.
| Sample | Au–S–C4 | Au–S–C6 | Au–S–C8 | Au–S–C12 | Au–S–C16 |
|---|---|---|---|---|---|
| a Au38(SC4H9)24 is soluble in acetone. | |||||
| Thiol | Butanethiol | Hexanethiol | Octanethiol | Dodecanethiol | Hexadecanethiol |
| T/°C | 80 | 80 | 80 | 80 | 80 |
| Wash 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 H2O/MeOH 10 H2O/MeOH |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/EtOH 1 MeOH/EtOH |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/EtOH 1 MeOH/EtOH |
EtOH | EtOH |
| Wash 2 | EtOH | EtOH | EtOH | Acetone | Acetone |
| Wash 3 | (Acetone)a | Acetone | Acetone | — | — |
| Extract 1 | Toluene | Toluene | Toluene | Toluene | Toluene |
| Extract 2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 Tol/Ac 4 Tol/Ac |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 Tol/Ac 4 Tol/Ac |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 Tol/Ac 5 Tol/Ac |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 Tol/Ac 5 Tol/Ac |
Characterization. Matrix assisted laser desorption ionization (MALDI) mass spectrometry was performed with a PerSeptive-Biosystems Voyager DE super-STR time-of-flight (TOF) spectrometer at Carnegie Mellon University in Pittsburgh and at LSU's chemistry department. Trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene] malononitrile (DCTB) was used as the matrix for MALDI. 1 mg of matrix and 0.1 mg of stock solution (1 mg ml−1) were mixed in either 100 μl CH2Cl2 or 100 μl toluene. A 10 μl portion was applied to the steel sample plate and then air dried.
The UV-Vis absorption spectra of toluene solutions of the samples were measured at LSU's Chemistry Department and the FT-IR spectra were recorded at CAMD using a Thermo-Nicolet 6700 FT-IR spectrophotometer. For FT-IR studies, samples were dissolved in toluene (∼1 mg ml−1), applied to a KBr disk, and then air dried. TEM images were recorded at the University of Texas in Arlington (UTA) using a Hitachi H-9500 high-resolution TEM. To minimize cluster fusion, TEM imaging was also conducted on a Hitachi 7100 microscope operated at 75 kV. Sulfur K edge (2472 eV) XANES (X-ray absorption near edge spectroscopy) measurements were performed at the DCM (double crystal monochromator) beam line at CAMD under 10 Torr nitrogen atmosphere. Samples were prepared by adding a concentrated sample solution dropwise to adhesive sulfur-free Kapton® tape. The toluene solvent was evaporated in air to concentrate the gold thiolate clusters in a small area that was roughly equivalent to the beam size. The procedure was repeated until approximately 2 mg of gold thiolate clusters was added to the Kapton® tape. XANES spectra were recorded in the fluorescence mode, and averaged over multiple (typically five) scans. Gold L3-edge (11919 eV) and XANES measurements were performed at the WDCM (wiggler double crystal monochromator) beam line at CAMD under 760 Torr argon atmosphere. The Au–S–C12 sample solution was concentrated and added dropwise to Kapton® tape in a similar way as described above. Approximately 200 mg of gold clusters was added to a 1 cm2 area of Kapton®. The XANES spectrum was recorded in transmission mode and averaged over multiple scans.
The WAXS measurements were performed at the SAXS/WAXS beam line at CAMD. Samples were measured as a toluene solution (1 mg ml−1) in a 1 mm diameter glass capillary. WAXS patterns were collected using an imaging plate (Molecular Dynamics storage phosphor screen) in a Q-range between 0.1 and 2 Å−1. Data analysis was conducted using ‘Igor Pro’ and ‘Fit2D’ software.
Recently, Qian et al. significantly improved known thiol etching procedures for gold clusters by using a polydisperse starting mixture of glutathione-capped gold clusters.10 The water soluble, polydisperse, Aun(SG)m mixture was etched with excess neat dodecanethiol (C12H25SH) in a two phase system. The thiol etching procedure and further washing steps give monodisperse Au38(SC12H25)24 in high yield (10%, Au atom basis). The stability of Au38 clusters at high temperatures was thought to account for the conversion of the polydisperse Aun(SG)m mixture to monodisperse Au38(SC12H25)24. Interestingly, Au25 clusters, known to be highly stable under ambient conditions, were not observed among the reaction products. A significant advantage of this method is that it is possible to scale up the reaction to obtain the desired product on a gram scale. The method was later modified by the same authors.6,11 The synthesis of Au38(SCH2CH2Ph)24 reached higher yields (∼25%, Au atom basis) by adjusting the size distribution of the initial Aun(SG)m mixture. The thiol etching method has also been adapted to yield different gold cluster sizes. Monodisperse Au144(SCH2CH2Ph)607 and Au40(SCH2CH2Ph)2415 were later synthesized in good yield, although the latter was found to co-exist with Au38(SCH2CH2Ph)24, thus requiring separation with SEC (size exclusion chromatography) to isolate the Au40(SCH2CH2Ph)24 cluster. Of note, Au40(SCH2CH2Ph)24 is slightly less stable than Au38(SCH2CH2Ph)24 and is an intermediate product during the course of thiol etching for the synthesis of Au38(SCH2CH2Ph)2415 hence, pure Au38(SCH2CH2Ph)24 can be obtained after thorough thiol etching,6 but Au40(SCH2CH2Ph)24 cannot be obtained without SEC separation of the intermediate product.15
Using the high yield thiol etching method reported by Qian et al., alkyl thiols (CnH2n+1SH) with chain lengths (n = 4, 6, 8, 12, 16) were tested for etching the polydisperse Aun(SG)m mixture, while leaving the other reaction parameters (temperature, concentration of reactants, etc.) unchanged. The effects on the yield and purity of the target product, monodisperse Au38(SR)24 clusters, were investigated using various techniques. An overview of the experimental conditions for the synthesis of various thiol stabilized Au38 clusters is provided in the experimental details above.
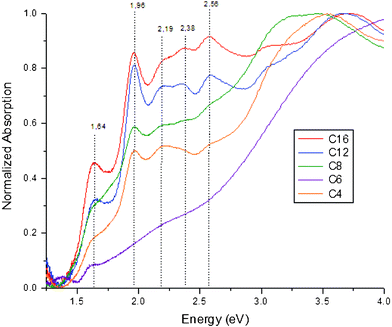
UV-Vis spectroscopy is a convenient way to evaluate gold cluster synthesis. The method does not require sample preparation and the shape of the spectrum gives a good indication if monodisperse gold clusters have been obtained. The step-like features typical for gold clusters are generally not observed when the sample is not monodisperse. The absorption spectrum and crystal structure of Au38(SR)24 clusters have also been reported and theoretically analyzed.10,11,16,17 Several strong peaks in the absorption spectrum at 1.17 eV, 1.64 eV, and 2.0 eV have also been reported in earlier work, together with less intense peaks at 2.2, 2.4 and 2.6 eV.
Fig. 1 shows the UV-Vis absorption spectra for Au38 clusters stabilized with various alkyl-thiol ligands. Contrary to the essentially featureless decay curves observed in UV-Vis spectra of polydisperse gold nanoparticle mixtures, the UV-Vis spectra of all samples show a prominent stepwise absorption spectrum characteristic of the discrete electronic transitions of the Au38(SR)24 clusters. The absorption spectrum of the dodecanethiol etched sample (C12, blue line) matches well with previous reports,6 indicating the synthesis was successfully reproduced. Samples etched with other alkyl thiols (C16, C8, C4) show similar features in their absorption profiles. One can see characteristic sharp peaks at 1.64 and 1.96 eV, indicating that highly monodisperse Au38(SR)24 clusters were obtained.
 | ||
| Fig. 1 UV-Vis absorption spectra. Abs(E) = Abs(w)*w2. | ||
These characteristic features are obscured in the Au–S–C6 sample, where only a weak transition at 1.64 eV is visible. Similar absorption spectra have been previously reported for purified Au38 fractions.18 While the peak at 1.64 eV indicates that the synthesis has yielded a large fraction of Au38(SC6H13)24 clusters, the superimposed broad absorption band is characteristic for larger gold nanocrystals (>2 nm). Possibly, these are still present due to an incomplete washing step. Overall, it is clear from the UV-Vis studies that the thiol etching resulted in size focusing of the gold thiolate clusters in all samples, obtaining highly monodisperse Au38 clusters.
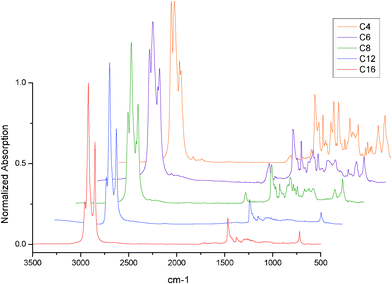
The IR spectra of Au38(SR)24 clusters are provided in Fig. 2. Most of the IR data on the Au38(SR)24 clusters are in very good agreement with earlier work by Hostetler et al. on larger gold nanoparticles with various alkyl-thiolate monolayer cappings.19–20 The most prominent peaks in the 3300–2500 cm−1 region of the spectra are attributed to symmetric (d+, ∼2850 cm−1) and antisymmetric (d−, ∼2920 cm−1) methylene stretching. The peak values of these vibrations can be correlated to the degree of ordering in the methylene chain. Typically, alkylthiol monolayers are highly ordered with a low number of gauche defects. In previous work on gold surfaces, Porteret al. found that thiolate monolayers with chains longer than hexanethiol were highly ordered, whereas the smaller molecules mostly resembled the liquid state.21
 | ||
| Fig. 2 The FTIR spectra of gold cluster samples. | ||
For all samples, the energies of the vibrations are shifted compared to the free alkane thiol samples, indicating a higher degree of ordering (i.e. crystallinity) is present in the ligands of the gold clusters. The methyl stretching peaks (antisymmetric, r−, 2955 cm−1 ; symmetric, r+, 2871 cm−1) increase in relative intensity upon decrease of the methylene chain length. For the long alkyl chains (C12 and C16), the symmetric stretching peak is largely obscured. The possibility of creating a ‘near-crystalline’ area around the nanoparticle core gives large energy and entropic gains. This increase for longer chained ligands is likely to cause a high degree of ordering typical for ligand protected nanoclusters.
In the hexanethiol capped gold cluster IR spectrum an additional very broad peak seems to be superimposed in the 3500–2500 cm−1 region, indicating the presence of carboxylic acid moieties in the sample. This is consistent with the UV-Vis results, which indicates the presence of other larger gold particles in the sample, possibly due to incomplete ligand exchange in the etching procedure. The presence of a carboxyl group is further confirmed by the presence of a large peak at 1712 cm−1, a region where the carboxylic acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration is found. Although this seems to suggest that glutathione still remains as a gold cluster ligand, the strong C
O stretching vibration is found. Although this seems to suggest that glutathione still remains as a gold cluster ligand, the strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations typical for amide bonds in glutathione (∼1600 cm−1) are not observed. It seems logical to assume that the tripeptide glutathione undergoes degradation during the harsh thiol etching conditions, retaining only a cysteine-like functionality attached to the gold clusters.22 A similar carboxylic C
O vibrations typical for amide bonds in glutathione (∼1600 cm−1) are not observed. It seems logical to assume that the tripeptide glutathione undergoes degradation during the harsh thiol etching conditions, retaining only a cysteine-like functionality attached to the gold clusters.22 A similar carboxylic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak can be found in the C8 and C4 capped samples, although the intensity of the peak is much smaller in these cases. The carbonyl stretch vibration is not observed in the C16 or C12 capped samples. This correlates well with UV-Vis, since the Au–S–C6 sample, and the Au–S–C8 and Au–S–C4 samples to a lesser extent, show UV-Vis spectra with obscured features compared to Au–S–C16 and Au–S–C12.
O peak can be found in the C8 and C4 capped samples, although the intensity of the peak is much smaller in these cases. The carbonyl stretch vibration is not observed in the C16 or C12 capped samples. This correlates well with UV-Vis, since the Au–S–C6 sample, and the Au–S–C8 and Au–S–C4 samples to a lesser extent, show UV-Vis spectra with obscured features compared to Au–S–C16 and Au–S–C12.
In the IR spectra of the respective free thiols (not shown) an additional peak is observed at 2561 cm−1, which can be assigned to the S–H stretching vibration. Such a peak is not observed in any of the gold cluster spectra, indicating that no free thiol is present in any of the samples. Several peaks are observed in the 1500–1400 cm−1 range. The most prominent peaks are located at 1465 and 1458 cm−1. The 1465 cm−1 peak is commonly assigned to scissoring of an all-transmethyl chain, while the 1458 cm−1 peak can be assigned to an anti-symmetric methyl bending vibration. The 1465 cm−1 peak is more intense for all gold cluster samples compared to their respective free thiols, which confirms the high degree of ordering of the alkyl chains in the gold clusters.
The presence of a series of progression bands in the 1400–1200 cm−1 range as well-resolved peaks is also a strong indicator of crystallinity. Several defect-related peaks are also observed in this range of the spectrum. A peak characteristic for a chain-end gauche defect is observed in all samples. This peak is observed at 1334 and 1336 cm−1 for the respective C4 and C6 samples, while the peak position stabilizes at 1342 cm−1 for longer alkyl chains (C8–C16). In the 1000–500 cm−1 range, the most prominent peak in all spectra is the all-transmethylene rocking at 721 cm−1. This band is located at higher energy for the butanethiol sample, where the major peak (all-trans) is located at 730 cm−1. Further methylene rocking progression bands can be found between 720 and 1065 cm−1. An additional peak is observed at 802 cm−1 for all short chained (C4–C8) samples. This peak is not observed in previous work on gold thiolate monolayer systems. It can be readily assigned to methylene rocking adjacent to a carboxyl group. This is also consistent with the presence of a weak peak at 640 cm−1, which can be assigned to O–C–O deformation vibration.
Of further interest is the C–S bond, an important characteristic of the gold-thiolate clusters. In the IR spectra of the gold thiolate clusters, the gauche C–S vibration decreases significantly in intensity with decreasing chain length. The peak position shifts to 659 cm−1 for chain lengths smaller than C12, when the peak is already barely observed. The trans C–S vibration peak shifts to higher energy (713 cm−1), causing it to fully overlap with the methylene rocking vibration. The trans C–S vibration is only visible in the butanethiol capped sample due to the shift of the methylene rocking peak to 730 cm−1.
There is no evidence for the presence of an S–S bond, usually found around 575 cm−1, in any of the samples. The Au–S related peaks lie at even lower energies (220 cm−1), and fall outside the range of our FT-IR spectrometer. In general the Au–S vibrations are very weak and difficult to observe, although there have been reports on FTIR investigations of these vibrations on gold thiolate clusters.19,23
It can be concluded from the IR data that all thiol ligands bound to Au38 clusters are highly ordered, with no internal kink defects present. Peaks related to chain end gauche defects were detected, although the major fraction of the alkylthiol ligands appears to be in an all-trans state. In several samples (C4–C8) the presence of a carboxylic acid moiety was confirmed, most likely part of a glutathione decomposition product remaining as a ligand on the gold cluster. The intensity of the COOH related peaks was low, especially considering the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch is usually a very strong peak dominating the IR spectrum. In the C12, and C16 stabilized clusters no evidence for carboxyl groups was observed, confirming that total ligand exchange was achieved during the thiol etching process. Comparing these results with UV-Vis, WAXS and MALDI data (vide infra) indicates that incomplete ligand exchange, confirmed by the presence of a C
O stretch is usually a very strong peak dominating the IR spectrum. In the C12, and C16 stabilized clusters no evidence for carboxyl groups was observed, confirming that total ligand exchange was achieved during the thiol etching process. Comparing these results with UV-Vis, WAXS and MALDI data (vide infra) indicates that incomplete ligand exchange, confirmed by the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, is directly correlated to the presence of impurities of different cluster sizes due to incomplete size focusing.
O stretching vibration, is directly correlated to the presence of impurities of different cluster sizes due to incomplete size focusing.
To evaluate the effects of different ligands on S–Au bonds in gold thiolate clusters, the samples were probed at both the sulfur K-edge as well as the gold L3-edge using X-ray absorption near edge spectroscopy (XANES). In Fig. 3A the XANES spectra of the six gold cluster samples are shown, together with the spectra for free dodecanethiol and gold(I) sulfide (Au2S, Sigma Aldrich, 99,9%, trace metals). It should be noted that the peak at 2482 eV in the gold(I) sulfide sample is due to the presence of sulfate groups caused by oxidation. Contrary to results on 2D SAMs, the gold thiolate clusters do not show signs of oxidative degradation at ambient conditions in their XANES spectra.24
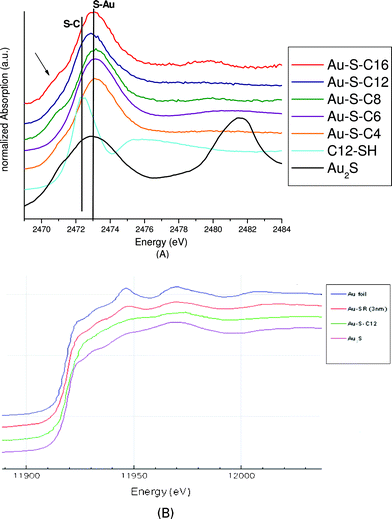 | ||
| Fig. 3 A: Sulfur K-edge XANES spectra of Au38(SR)24 and reference compounds gold sulfide and dodecanethiol, B: gold L3-edge XANES spectra of Au38(SR)24 and reference compounds gold metal, gold sulfide. | ||
Both the samples as well as the gold(I) sulfide exhibit a pre-edge shoulder around 2470.8 eV. The pre-edge shoulder is attributed to the antibonding state of the Au–S interaction.25 This shoulder is absent in the free thiol. It also appears to be absent in the hexanethiol capped sample, although reasons for this are not immediately apparent. All gold clusters show a broadening of the white line compared to the free thiol similar to the gold(I) sulfide. The S K-edge of all gold thiolate clusters is shifted compared to the free thiol, to 2472.9 eV. The large similarity of the S K-edge between Au(I)S species and Au38(SR)24 clusters is in good agreement with the ‘S–Au–S’ capping motifs in the Au38(SR)24 structure.
The gold L3XANES spectra also show a large similarity between gold(I) sulfide and Au38(SC12H25)24 clusters. Larger gold thiolate nanoparticles (unpublished results) instead show large similarity with gold foil, indicating that their relatively large nanoparticle gold core (∼3 nm) dominates their Au L3 edge spectra. The Au L3 edge probes the electronic transitions from 2p to unoccupied 5d states. A more intense white line corresponds to more unoccupied 5d states (d-hole) or less 5d electrons. In Fig. 3B, the Au38(SC12H25)24 clusters are found to have a more intense white line than the bulk gold foil, indicating a d-electron depletion of the Au atoms in the Au38 clusters, caused by charge transfer from gold to sulfur atoms. This is in agreement with a theoretical prediction of a d-electron depletion of 0.05 e− in Au38(SCH3)24 and Au25(SR)18 systems.26,27
Mass spectrometry has become an essential tool in determining the exact composition of gold thiolate clusters. By using the MALDI protocol reported by Dass et al., using low laser intensities and a DCTB matrix, it was possible to limit fragmentation of the gold thiolate clusters to a large extent.28 All samples were measured up to 50 kDa (not shown). In all cases, there were no peaks detected beyond 20 kDa. It was not possible to completely suppress fragmentation of the intact cluster ion as reported by Dass in his work on Au25 clusters, even at threshold laser intensities.
The MALDI spectrum of Au38(SC12H25)24 is shown in Fig. 4A. The intact cluster ion (M+) can be seen at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316.7 Da (theoretical: 12
316.7 Da (theoretical: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317.9) with the major peak at 10
317.9) with the major peak at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556.1 Da, identified as a cluster fragment corresponding to the loss of four [Au–S–L] and one [L] unit (theoretical: 10
556.1 Da, identified as a cluster fragment corresponding to the loss of four [Au–S–L] and one [L] unit (theoretical: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556.2), where L = ligand = [C12H25]. The minor peaks in between can be identified as either loss of [Au–S–L] units, or loss of [L] units. Fragmentation via Au–S bond breaking does not seem to occur with the chosen experimental conditions. A similar pattern of fragmentation is found for all samples identified as highly monodisperse Au38 clusters. The MALDI spectrum for Au38(SC16H33)24 (Fig. 4B) shows additional peaks compared to the dodecanethiol capped cluster, although the main peaks can still be identified as cluster fragments. For example, the peak at 8002.3 Da can be assigned to loss of 7 [Au–S–L] units and 11 [L] units (theoretical: 8002.2). It is not clear why the elongated alkyl chain prompts increased fragmentation of the gold clusters under the chosen MALDI conditions.
556.2), where L = ligand = [C12H25]. The minor peaks in between can be identified as either loss of [Au–S–L] units, or loss of [L] units. Fragmentation via Au–S bond breaking does not seem to occur with the chosen experimental conditions. A similar pattern of fragmentation is found for all samples identified as highly monodisperse Au38 clusters. The MALDI spectrum for Au38(SC16H33)24 (Fig. 4B) shows additional peaks compared to the dodecanethiol capped cluster, although the main peaks can still be identified as cluster fragments. For example, the peak at 8002.3 Da can be assigned to loss of 7 [Au–S–L] units and 11 [L] units (theoretical: 8002.2). It is not clear why the elongated alkyl chain prompts increased fragmentation of the gold clusters under the chosen MALDI conditions.
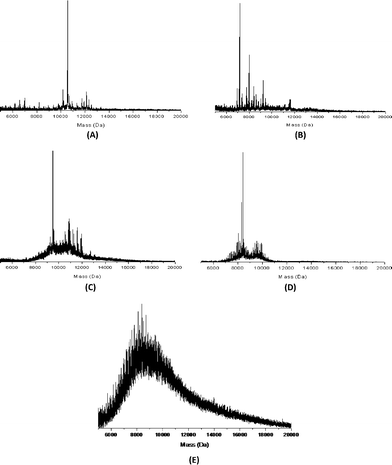 | ||
| Fig. 4 MALDI spectra of (A) Au38(SC12H25)24, (B) Au38(SC16H33)24, (C) Au38(SC8H17)24, (D) Au38(SC4H9)24, and (E) Au38(SC6H13)24. | ||
The octanethiol capped gold cluster Au38(SC8H17)24 (Fig. 4C) shows the ‘–4[Au–S–L]–L’ fragment as the most prominent peak in the MALDI spectrum at 9488.3 Da (theoretical: 9490.3), similar to the dodecanethiol capped cluster. The ion of the intact cluster is visible at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969.5 Da (theoretical: 10
969.5 Da (theoretical: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971.5), although it is difficult to distinguish from other prominent peaks in the same area. There are several major peaks at a molecular weight between 11 kDa (the intact Au38(SC8H17)24 cluster) and 13 kDa. Clearly, these cannot be related to the Au38 cluster. These additional peaks at higher mass indicate that there is a fraction of larger gold clusters present in the sample, with core sizes between 45 and 50 gold atoms. It is not possible to unambiguously determine the cluster size due to the large fragmentation of the intact cluster ions. In previous work on Au38(SR)24 clusters, Au40(SR)24 has been observed as a side product in the synthesis.15 However, a peak for the Au40(SR)24 cluster could not be identified in the MALDI spectrum of the Au–S–C8 sample.
971.5), although it is difficult to distinguish from other prominent peaks in the same area. There are several major peaks at a molecular weight between 11 kDa (the intact Au38(SC8H17)24 cluster) and 13 kDa. Clearly, these cannot be related to the Au38 cluster. These additional peaks at higher mass indicate that there is a fraction of larger gold clusters present in the sample, with core sizes between 45 and 50 gold atoms. It is not possible to unambiguously determine the cluster size due to the large fragmentation of the intact cluster ions. In previous work on Au38(SR)24 clusters, Au40(SR)24 has been observed as a side product in the synthesis.15 However, a peak for the Au40(SR)24 cluster could not be identified in the MALDI spectrum of the Au–S–C8 sample.
In the MALDI spectrum of the butanethiol capped clusters Au38(SC4H9)24 (Fig. 4D), the ‘–4[Au–S–L]–L’ fragment is the most prominent peak at 8421.2 Da (theoretical: 8424.3). The intact cluster peak (theoretical: 9624.9) is buried between several major other peaks in the 9.5–10 kDa area and is difficult to observe. This second set of peaks in the 9.5–10 kDa area is most likely related to the presence of Au40(SC4H9)24 clusters and fragments thereof. The peak of the intact Au40 cluster is observed at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016.9 Da (theoretical: 10
016.9 Da (theoretical: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020.1 Da).
020.1 Da).
The MALDI spectrum of the hexanethiol capped gold clusters (Fig. 4E) does not show peaks that can be assigned to intact Au38 clusters, nor does it show the characteristic –4[Au–S–L]–L peak present in the other Au38 samples. The MALDI spectrum of the hexanethiol capped sample shows severe fragmentation centered on 8.4 kDa. For LDI conditions, this would indicate the presence of an Au38 core. However, with the chosen conditions for this measurement (low laser intensity, DCTB matrix) there is no reason to assume full dissociation of all ligands in the ionization process. The peak of the intact Au38(SC6H13)24 cluster should be visible at 10.3 kDa, while the ‘–4[Au–S–L]–L’ peak should show up at 9 kDa. Since neither is clearly distinguishable, the MALDI data indicate that the major species in the sample is not Au38. Instead, the sample appears to be a mixture of several larger gold cluster species as evidenced by the broad shoulder of the fragmentation peak up to 20 kDa. No significant fragmentation peaks above 20 kDa are observed (not shown).
The HR-TEM images (Fig. 5) show all samples are relatively monodisperse, except for the butanethiol etched sample where particle size distribution could not be determined due to the formation of large aggregates. Although the hexanethiol capped sample has a reasonably narrow size distribution, a small fraction of larger (3–3.5 nm diameter) gold clusters appears to be present as a contamination. A clear trend is observed when comparing the average particle diameter obtained from TEM with the amount of carbon atoms in the ligand. Average particle size appears to decrease with increasing carbon chain length, which is a well-known observation for dependence of colloid size on the chain length of stabilizing ligand.29 However, the results from TEM suggesting variation in size contradict the results from the UV-Vis and MALDI analysis, which indicate the presence of Au38 clusters as the major species in all samples. Also, the particle size obtained by TEM is not consistent with theoretical values for Au38 cluster core size (∼1.1 nm).11
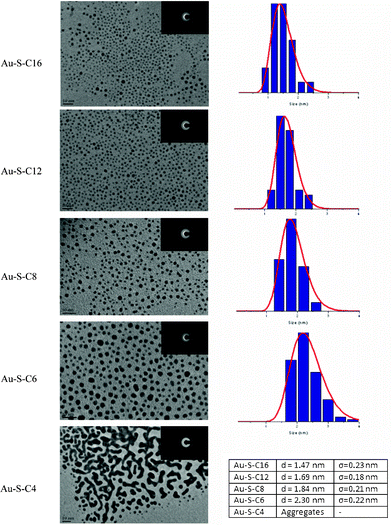 | ||
| Fig. 5 HR-TEM images of Au38(SR)24 clusters. The insets show the EDX diffraction patterns. Particle size distributions fitted with a log normal distribution are shown on the right side of the HR-TEM images. | ||
A more likely explanation for the TEM results is related to damage caused to the sample by the electron beam during beam focusing and the TEM measurements. As reported in earlier work, exposure of gold clusters to an intense electron beam will destroy the organic ligands in the sample and cause severe particle aggregation.30 Assuming the carbonaceous material will remain on the TEM grid and thus hinder complete particle aggregation, the observed trends in the TEM results can be readily explained.
The electron diffraction (ED) patterns show fcc crystallinity in all samples. The brightest diffraction pattern is observed in the butanethiol capped sample, where the [111] (bright), [200], [220], [311/222] (bright), and the [400] lattices can be distinguished. Although Au38 clusters are not fcc crystalline, such a structure is typical for larger gold nanoparticles.
To minimize cluster aggregation/fusion under high energy electron beam irradiation (e.g. 200 keV), we have performed TEM imaging of Au38 clusters under low acceleration voltage (75 kV) at the cost of resolution (Fig. 6). Cluster coalescence is minimized under such conditions. The measured particle size of ∼1.2 nm is consistent with the size determined by X-ray crystallography.11
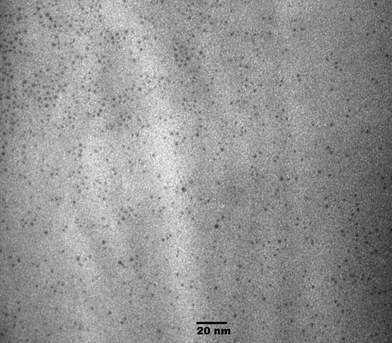 | ||
| Fig. 6 TEM image of Au38 clusters under low acceleration voltage (75 kV). | ||
While MALDI provides an alternative method to study the size and purity of small clusters, the size and nature of larger nanoparticle impurities often cannot be unambiguously determined. To get a better view of the particle size distribution in the samples we employed wide-angle X-ray scattering (WAXS). Wide-angle X-ray scattering (WAXS) provides a relatively easy way to obtain information on particle size distribution of the gold nanocluster samples, since samples can be measured as dilute solutions. This circumvents many of the experimental challenges that are faced when trying to obtain XRD spectra of such materials. X-Ray scattering patterns are usually represented as scattered intensity as a function of the scattering vector q (= 4πsin(θ)/λ). A typical q vs. I plot for the gold thiolate clusters discussed in this work is shown in Fig. 7A (ligand = C12H25SH).
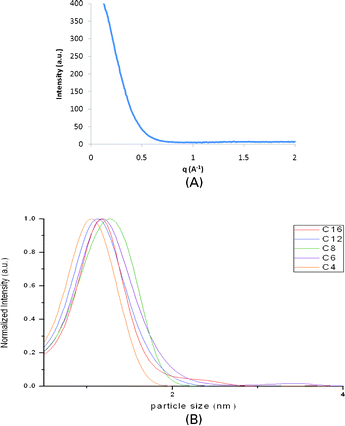 | ||
| Fig. 7 A: q vs. I plot of Au–S–C12. B: Best-fit WAXS particle size distribution of the gold thiolate clusters. | ||
For a nanoparticle system, the scattering intensity I(q) is given by I(q) = c∫n(r)f(qr)S(qr)dr, where c is a constant, n(r) is the particle size distribution, f(qr) is the individual nanoparticle form factor and S(qr) is the structure factor. When a diluted nanoparticle solution is used, allowing inter-particle scattering to be ignored in data analysis, a structure factor of S(qr) ≈1 can be assumed. Assuming a spherical particle size to estimate the form factor f(qr), the experimental data can be fitted using a Schultz particle size distribution model.31 This yields a best-fit particle size distribution, with an error margin of approximately 0.5 Å in this work. It should be noted that the Au38(SR)24 structure is not completely spherical, leading to additional inaccuracy in the fitting process. The obtained particle size distributions of the gold thiolate clusters are shown in Fig. 7B. The peak shapes that are observed are heavily affected by size-induced peak broadening, typical for WAXS measurements. However, the size distribution maxima derived from the WAXS data are in excellent agreement with theoretical values for the size of an Au38 core (1.1 nm) (Table 2).11
| Sample | Au–S–C16 | Au–S–C12 | Au–S–C8 | Au–S–C6 | Au–S–C4 |
|---|---|---|---|---|---|
| Maximum/nm | 1.16 | 1.12 | 1.26 | 1.18 | 1.05 |
| 2.4 | 3.4 |
Besides this main peak at 1.1 nm, several other features are present in Fig. 7B. For Au–S–C16 a weak shoulder is observed at 2.4 nm, possibly a contamination of larger gold nanoparticles present in the sample. However, such a contamination is not observed with other characterization methods. The Au–S–C8 sample has a maximum shifted to a larger particle size at 1.26 nm. This shift can be explained by the presence of a fraction of larger gold clusters (Au40–Au45), in good agreement with the MALDI data of the octanethiol-capped sample. The Au–S–C6 sample has a maximum at 1.18 nm, a value corresponding with the presence of Au38(SC6H13)24 clusters. A second maximum is observed at 3.4 nm, suggesting a contamination with larger gold clusters. The presence of such a fraction of larger gold nanoparticles is confirmed by the UV-Vis measurements. Due to this contamination, the presence of Au38(SC6H13)24 clusters could not be unambiguously confirmed by either UV-Vis or MALDI without additional WAXS data. The Au–S–C4 sample has a maximum at 1.05 nm, a value lower than expected for Au38 clusters. This shift indicates the presence of smaller gold clusters (Au30–Au35). Although UV-Vis data suggest the sample consists of highly monodisperse Au38(SC4H9)24 clusters, MALDI data do indicate the presence of a fraction of smaller clusters.
In conclusion, we have demonstrated a simple strategy to yield monodisperse Au38(SR)24 clustersvia the thiol etching method. Several alkyl thiols (CnH2n+1SH), with n ranging from 4 to 16 were tested in this protocol. Synthesis with all alkyl thiols was successful, with the major product after purification being Au38(SR)24 in all cases, as confirmed by UV-Vis, MS and WAXS measurements. This demonstrates for the first time that the (alkylthiol) surface functionality of an Au38(SR)24 cluster can be tuned simply by altering the thiol used in the etching procedure. Although one of the samples (Au–S–C6) contained a minor fraction of larger gold nanoparticles as an impurity, its analysis illustrated the strengths and weaknesses of the employed characterization techniques in their ability to identify the nature of the impurity in the sample.
The infrared spectroscopy results indicate that ligand exchange did not go to full completion for the shorter (C8, C6, C4) alkyl thiol ligands. Carboxylic acid moieties from a cysteine-like decomposition product of glutathione are observed in the IR spectra of these samples, although the intensity of the related carbonyl stretch was negligible for the Au–S–C8 and Au–S–C4 samples. A carbonyl stretch vibration was not observed in the Au–S–C16 and Au–S–C12 samples, indicating full ligand exchange was achieved. Comparing IR results with size distribution data obtained from other techniques indicates a direct correlation between the degree of ligand exchange and the degree of size focusing. This provides a novel way to identify impurities in gold cluster syntheses.
The synchrotron radiation-based spectroscopy tools appear to be the most suitable for unequivocal characterization of small metal clusters. We demonstrate for the first time that WAXS is a powerful tool for analyzing ultra-small gold clusters as it provides a way to evaluate particle size distributions of samples containing Au38(SR)24 clusters. Sulfur K-edge and gold L3-edge XANES measurements provided data on the structure of the Au38(SR)24 clusters, confirming the presence of the theoretically predicted ‘S–Au–S’ surface staple motifs. The HR-TEM, which is routinely used in nanomaterial characterization, appears to be unreliable for ultra-small gold clusters due to severe aggregation caused by electron beam damage.
Acknowledgements
This material is based upon work supported as part of the Center for Atomic Level Catalyst Design, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001058.References
- Y. Zhu, H. F. Qian, B. A. Drake and R. Jin, Atomically precise Au25(SR)18 nanoparticles as catalysts for the selective hydrogenation of α,β-unsaturated ketones and aldehydes, Angew. Chem., Int. Ed., 2010, 49, 1295–1298 CrossRef CAS
.
- Y. Zhu, H. F. Qian, M. Zhu and R. Jin, Thiolate-protected Au nanoclusters as catalysts for selective oxidation and hydrogenation processes, Adv. Mater., 2010, 22(17), 1915–1920 CAS
.
- O. Lopez-Acevedo, K. A. Kacprzak, J. Akola and H. Hakkinen, Quantum size effects in ambient CO oxidation catalysed by ligand-protected gold clusters, Nat. Chem., 2010, 2(4), 329–334 CrossRef CAS
.
- R. Jin, Quantum sized, thiolate-protected gold nanoclusters, Nanoscale, 2010, 2(3), 343–362 RSC
.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS
.
- H. F. Qian, Y. Zhu and R. Jin, Size-focusing synthesis, optical and electrochemical properties of monodisperse Au38(SC2H4Ph)24 nanoclusters, ACS Nano, 2009, 3, 3795–3803 CrossRef CAS
.
- H. F. Qian and R. Jin, Controlling nanoparticles with atomic precision: the case of Au144(SCH2CH2Ph)60, Nano Lett., 2009, 9, 4083–4087 CrossRef CAS
.
- R. Jin, H. Qian, Z. Wu, Y. Zhu, M. Zhu, A. Mohanty and N. Garg, Size focusing: a methodology for synthesizing atomically precise gold nanoclusters, J. Phys. Chem. Lett., 2010, 1, 2903 CrossRef CAS
.
- M. D. Rohini, V. Palshin, D. Nalin, L. L. Henry and C. S. S. R. Kumar, A new role for surfactants in the formation of cobalt nanoparticles, J. Mater. Chem., 2008, 18(7), 738–747 RSC
.
- H. F. Qian, M. Zhu, U. N. Andersen and R. Jin, Facile, large-scale synthesis of dodecanethiol-stabilized Au38 clusters, J. Phys. Chem. A, 2009, 113, 4281–4284 CrossRef CAS
.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, Total structure determination of thiolate-protected Au38 nanoparticles, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS
.
- Y. Negishi, Y. Takasugi, S. Sato, H. Yao, K. Kimura and T. Tsukuda, Magic-numbered Aun clusters protected by glutathione monolayers (n = 18, 21, 25, 28, 32, 39): isolation and spectroscopic characterization, J. Am. Chem. Soc., 2004, 126, 6518–6519 CrossRef CAS
.
- Y. Negishi, K. Nobusada and T. Tsukuda, Glutathione-protected gold clusters revisited: bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals, J. Am. Chem. Soc., 2005, 127, 5261–5270 CrossRef CAS
.
- N. K. Chaki, Y. Negishi, H. Tsunoyama, Y. Shichibu and T. Tsukuda, Ubiquitous 8 and 29 kDa gold:alkanethiolate cluster compounds: mass-spectrometric determination of molecular formulas and structural implications, J. Am. Chem. Soc., 2008, 130, 8608–8610 CrossRef CAS
.
- H. F. Qian, Y. Zhu and R. Jin, Isolation of ubiquitous Au40(SR)24 clusters from the 8 kDa gold clusters, J. Am. Chem. Soc., 2010, 132(13), 4583–4585 CrossRef CAS
.
- Y. Pei, Y. Gao and X. C. Zeng, Structural prediction of thiolate-protected Au38: a face-fused bi-icosahedral Au core, J. Am. Chem. Soc., 2008, 130, 7830–7833 CrossRef CAS
.
- O. Lopez-Acevedo, H. Tsunoyama, T. Tsukuda, H. Hakkinen and C. M. Aikens, Chirality and electronic structure of the thiolate-protected Au38 nanocluster, J. Am. Chem. Soc., 2010, 132, 8210–8218 CrossRef CAS
.
- O. Toikkanen, V. Ruiz, G. Ronnholm, N. Kalkinnen, P. Liljeroth and B. M. Quinn, Synthesis and stability
of monolayer-protected Au38 clusters, J. Am. Chem. Soc., 2008, 130, 11049–11055 CrossRef CAS
.
- M. J. Hostetler, J. J. Stokes and R. W. Murray, Infrared spectroscopy of three-dimensional self-assembled monolayers: N-alkanethiolate monolayers on gold cluster compounds, Langmuir, 1996, 12, 3604–3612 CrossRef CAS
.
- M. J. Hostetler, J. E. Wingate, C. J. Zhong, J. E. Harris, R. W. Vachet, M. R. Clark, J. D. Londono, S. J. Green, J. J. Stokes, G. D. Wignall, G. L. Glish, M. D. Porter, N. D. Evans and R. W. Murray, Alkanethiolate gold cluster molecules with core diameters from 1.4 to 5.2 nanometers: core and monolayer properties as a function of core size, Langmuir, 1998, 14, 17–30 CrossRef CAS
.
- M. D. Porter, T. B. Bright, D. L. Allara and C. E. D Chidsey, Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry, J. Am. Chem. Soc., 1987, 109, 3559–3568 CrossRef CAS
.
- A. Fialaire, E. Postaire, P. Prognon, F. Pradier and D. Pradeau, Thermal decomposition of reduced glutathione in solution for organ preservation, J. Pharm. Biomed. Anal., 1992, 10, 457–460 CrossRef CAS
.
- J. Petroski, M. Chou and C. Creutz, The coordination chemistry of gold surfaces: Formation and far-infrared spectra of alkanethiolate-capped gold nanoparticles, J. Organomet. Chem., 2009, 694, 1138–1143 CrossRef CAS
.
- S. Chen and R. W. Murray, Arene thiolate monolayer-protected gold clusters, Langmuir, 1999, 15, 682–689 CrossRef CAS
.
- T. M. Wiley, A. L. Vance, T. Van Buuren, C. Bostedt and C. S. Fadley, Rapid degradation of alkanethiol-based self-assembled monolayers on gold in ambient laboratory conditions, Surf. Sci., 2005, 576, 188–196 CrossRef
.
- H. Hakkinen, R. Barnett and U. Landman, Electronic structure of passivated Au38(SCH3)24 nanocrystal, Phys. Rev. Lett., 1999, 82, 3264 CrossRef CAS
.
- G. A. Simms, J. D. Padmos and P. Zhang, Structural and electronic properties of protein/thiolate-protected gold nanocluster with “staple” motif: a XAS, L-DOS, and XPS study, J. Chem. Phys., 2009, 131(21), 214703 CrossRef
.
- A. Dass, A. Stevenson, G. R. Dubay, J. B. Tracy and R. W. Murray, Nanoparticle MALDI-TOF mass spectrometry without fragmentation: Au25(SCH2CH2Ph)18 and mixed monolayer Au25(SCH2CH2Ph)18−x(L)x, J. Am. Chem. Soc., 2008, 130, 5940–5946 CrossRef CAS
.
- B. Veisz and Z. Kiraly, Size-selective synthesis of cubooctahedral palladium particles mediated by metallomicelles, Langmuir, 2003, 19(11), 4817–4824 CrossRef CAS
.
- W. D. Pyrz and D. J. Buttrey, Particle size determination using TEM: a discussion of image acquisition and analysis for the novice microscopist, Langmuir, 2008, 24, 11350–11360 CrossRef CAS
.
- B. Gilbert, F. Huang, H. Zhang, G. A. Waychunas and J. F. Banfield, Nanoparticles: strained and stiff, Science, 2005, 305, 651–652 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image of the glutathione coated gold clusters prior to thiol etching. See DOI: 10.1039/c2ra00747a |
| This journal is © The Royal Society of Chemistry 2012 |
