Base recognition by L-nucleotides in heterochiral DNA†
Shuji
Ogawa
,
Shun-ichi
Wada
and
Hidehito
Urata
*
Osaka University of Pharmaceutical Sciences, 4-20-1 Nasahara, Takatsuki, Osaka, 569-1094, Japan. E-mail: urata@gly.oups.ac.jp; Fax: +81 (72) 690 1089; Tel: +81 (72) 690 1089
First published on 6th February 2012
Abstract
Thermal stabilities of homo- and heterochiral DNA duplexes with and without a base pair mismatch at the chiral modification site were evaluated. The results indicated that the L-nucleotide residue in heterochiral duplexes retains its selectivity toward the complementary D-nucleotide residue.
An L-nucleotide consisting of L-deoxyribose as the chiral unit is the enantiomer of natural D-nucleotide. The nucleic acids which construct living bodies are only composed of D-nucleotides. Therefore, the D-homochirality is essential for the formation of higher-order structures and the biological functions of nucleic acid. L-Homochiral DNA forms left-handed duplexes with complementary L-homochiral sequences,1 whereas they cannot hybridize with complementary D-homochiral sequences.2 The unnatural L-DNA is not recognized by natural enzymes consisting of L-amino acids and thus show excellent resistance to enzymatic degradation. By using such characteristics, L-DNA has been utilized for the development of various tools, such as anti-HIV (human immunodeficiency virus) agents,3aptamers,4 molecular beacons,5 and microarrays.6
The effect of the substitution of D-nucleotides with their L-counterparts on the duplex stability has been also investigated. Heterochiral DNAs that contain an L-nucleotide within natural D-sequences were found to retain the duplex structure, in which the L-nucleotide residue forms a stable Watson–Crick type base pair with its natural complementary residue.7 These results suggest that the L-nucleotide residue in heterochiral DNA may possess base-discriminating ability, although the duplex stability is somewhat decreased compared to that of parental D-homochiral DNA.8 Similar results showing that chiral modification decreases the thermal stability of duplexes were also reported by other groups.9 However, those reports simply described the duplex stability of heterochiral oligodeoxynucleotides (ODNs) containing L-nucleotides at a specific site, and the base-discriminating ability of L-nucleotides in heterochiral DNA has not been reported.
Here we report the duplex stability of heterochiral DNA, each of which has an L-nucleotide at a different site, and the base pairing selectivity of the L-nucleotides in the heterochiral DNA.
We synthesized homochiral and some heterochiral template strands (Table 1) and investigated their duplex forming ability with the complementary strands by conducting UV-melting experiments (Fig. 1). Each heterochiral duplex showed a cooperative transition as well as a D-homochiral duplex. Table 1 summarizes the effects of the substitution of D-nucleotide with L-nucleotide at different sites of the D-homochiral template strand on the duplex stability. The substitution of D-nucleotide with L-nucleotide resulted in a decrease of Tm values in the range of 3.9 to 9.5 °C. It was clearly shown that the heterochiral duplexes have lower Tm values than the parental ones, and the decrease of Tm values shows the sequence dependence as reported previously.8
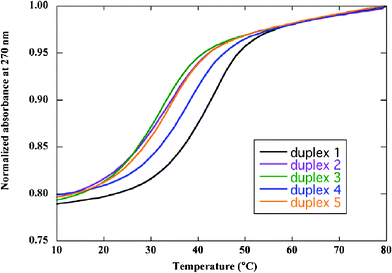 | ||
| Fig. 1 UV-melting profiles of homo- (duplex 1) and heterochiral duplexes (duplexes 2–5). Samples contained 6 μM duplex in 10 mM MgCl2, 100 mM NaCl, and 70 mM MOPS (pH 7.1). | ||
| a Samples contained 6 μM duplex in 10 mM MgCl2, 100 mM NaCl, and 70 mM MOPS (pH 7.1). b Melting temperature difference from the homochiral duplex. | ||||
|---|---|---|---|---|
| Duplex | Template strand | Complementary strand | T m (°C) | ΔTm (°C)b |
| Homochiral strand | ||||
| 1 | d(GCGTCTAAA) | d(TTTAGACGC) | 42.1 | — |
| Heterochiral strand | ||||
| 2 | d(GCGTCTLAAA) | d(TTTAGACGC) | 33.6 | −8.5 |
| 3 | d(GCLGTCTAAA) | 32.6 | −9.5 | |
| 4 | d(GCGTLCTAAA) | 38.2 | −3.9 | |
| 5 | d(GCGTCLTAAA) | 33.9 | −8.2 | |
To investigate the base pairing selectivity of L-nucleotides in heterochiral duplexes, we examined the thermal stability of the homo- and heterochiral duplexes containing a base pair mismatch at the chiral modification site. In the case of homochiral duplexes (Fig. 2a–d), the Tm values were considerably decreased by the introduction of a base pair mismatch (ΔTm>10 °C), which means that D-nucleotides in D-homochiral strands have high base-discriminating ability (Fig. 2a–d). Notably, the decrease of the Tm value by substituting D-nucleotide with L-nucleotide is smaller (Table 1) than that by introducing a base pair mismatch (Table S1†). This result suggests that L-nucleotide residues in heterochiral duplexes form hydrogen bonds with their complementary bases. All of the fully matched heterochiral duplexes gave the highest Tm values among the four duplexes (Fig. 2e–h; yellow bars) in each series, similarly to the fully matched homochiral ones. Particularly, the fully matched heterochiral duplex containing L-dC (Fig. 2f; yellow bars) showed the similar base-discriminating ability to the fully matched homochiral one (Fig. 2b; yellow bars). Furthermore, the fully matched heterochiral duplexes containing L-dA and L-T also showed higher Tm values than the corresponding mismatched duplexes (Fig. 2e and h; yellow bars), although their respective selectivities to the complementary T and dA are somewhat lower than those of the corresponding homochiral duplexes. (Fig. 2a and d). In the case of chiral modification of dG, L-dG was unable to discriminate between dC and dG. These results suggest that L-nucleotides in heterochiral duplexes generally retain their base-discriminating ability, which is probably based on Watson–Crick type hydrogen bonding in a manner similar to D-nucleotides in D-homochiral duplexes, although the base pairing selectivity may be affected by the kind of L-nucleotide and its neighboring bases. Heterochiral duplexes containing L-dA, L-dG, and L-T (Fig. 2e, g, and h, respectively) have lower base pairing selectivity than their corresponding homochiral duplexes. On the other hand, the L-dC residue in the heterochiral strand (Fig. 2f) shows similar base-discriminating ability to the D-dC residue in the corresponding homochiral strand (Fig. 2b), and their ΔTm values by base pair mismatch are similar (Table S1†). Considering the quite small ΔTm value of duplex 4 (Table 1), the L-nucleotide in a heterochiral duplex may have the strong base-discriminating ability when the chiral modification does not cause marked duplex destabilization.
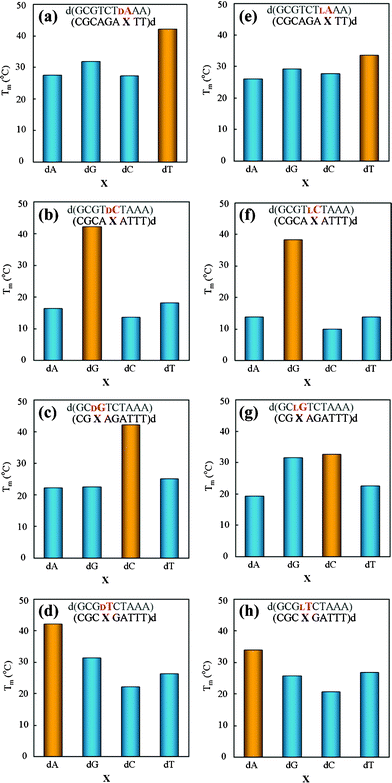 | ||
| Fig. 2 Effects of base pair mismatch of D- (a–d) and L-nucleotide (e–h) on duplex stability. Samples contained 6 μM duplex in 10 mM MgCl2, 100 mM NaCl, and 70 mM MOPS (pH 7.1). Yellow bars denote Tm values of fully matched duplexes, and blue bars denote Tm values of mismatched duplexes. | ||
In conclusion, we found that heterochiral duplexes have generally lower stability than parental homochiral duplexes and L-nucleotides in heterochiral ODNs possess the base pairing selectivity although the selectivity depends on the kind of L-nucleotide and its neighboring bases. The base-discriminating ability of L-nucleotide in heterochiral ODNs may shed light on their potential use as a tool for molecular recognition.
References
- (a) H. Urata, K. Shinohara, E. Ogura, Y. Ueda and M. Akagi, J. Am. Chem. Soc., 1991, 113, 8174 CrossRef CAS; (b) H. Urata, E. Ogura, K. Shinohara, Y. Ueda and M. Akagi, Nucleic Acids Res., 1992, 20, 3325 CrossRef CAS.
- A. Garbesi, M. L. Capobianco, F. P. Colonna, L. Tondelli, F. Arcamone, G. Manzini, C. W. Hilbers, J. M. E. Aelen and M. J. J. Blommers, Nucleic Acids Res., 1993, 21, 4159–4165 CrossRef CAS.
- H. Urata, T. Kumashiro, T. Kawahata, T. Otake and M. Akagi, Biochem. Biophys. Res. Commun., 2004, 313, 55 CrossRef CAS.
- (a) K. P. Williams, X. H. Liu, T. N. M. Schumacher, H. Y. Lin, D. A. Ausiello, P. S. Kim and D. P. Bartel, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 11285 CrossRef CAS; (b) W. G. Purschke, F Radtke, F. Kleinjung and S. Klussmann, Nucleic Acids Res., 2003, 31, 3027 CrossRef CAS.
- Y. Kim, C. J. Yang and W. Tan, Nucleic Acids Res., 2007, 35, 7279 CrossRef CAS.
- N. C. Hauser, R. Martinez, A. Jacob, S. Rupp, J. D. Hoheisel and S. Matysiak, Nucleic Acids Res., 2006, 34, 5101 CrossRef CAS.
- (a) H. Urata, Y. Ueda, H. Suhara, E. Nishioka and M. Akagi, J. Am. Chem. Soc., 1993, 115, 9852 CrossRef CAS; (b) M. J. J. Blommers, L. Tondelli and A. Garbesi, Biochemistry, 1994, 33, 7886 CrossRef CAS; (c) M. J. Damha, P. A. Giannaris and P. Marfey, Biochemistry, 1994, 33, 7877 CrossRef CAS.
- (a) H. Urata and M. Akagi, Tetrahedron Lett., 1996, 37, 5551 CrossRef CAS; (b) H Urata, H. Shimizu, H. Hiroaki, D. Koda and M. Akagi, Biochem. Biophys. Res. Commun., 2003, 309, 79 CrossRef CAS.
- (a) Y. Hashimoto, N. Iwanami, S. Fujimori and K. Shudo, J. Am. Chem. Soc., 1993, 115, 9883 CrossRef CAS; (b) S. Vichier-Guerre, F. Morvan, G. Fulcrand and B. Rayner, Tetrahedron Lett., 1997, 38, 93 CrossRef CAS; (c) S. Vichier-Guerre, F. Santamaria and B. Rayner, Tetrahedron Lett., 2000, 41, 2101 CrossRef CAS; (d) J. Kawakami, K. Tsujita and N. Sugimoto, Anal. Sci., 2005, 21, 77 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: MALDI-TOF mass data of oligodeoxynucleotides and melting points of the duplexes. See DOI: 10.1039/c2ra01013e |
| This journal is © The Royal Society of Chemistry 2012 |
