Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets†
Xiguang
Han
,
Xue
Wang
,
Shuifen
Xie
,
Qin
Kuang
*,
Junjie
Ouyang
,
Zhaoxiong
Xie
* and
Lansun
Zheng
State Key Laboratory for Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry Engineering, Xiamen University, Xiamen, 361005, P.R. China. E-mail: qkuang@xmu.edu.cn, zxxie@xmu.edu.cn; Fax: +86-592-2183047; Tel: +86-592-2187879
First published on 2nd March 2012
Abstract
Anatase TiO2 nanoparticles exposed with a remarkable amount (60%) of high-energy (001) facets were synthesized via a fluorine-free hydrothermal route, using K-titanate nanowires as a precursor and urea as a surface regulator. The carbonate ions were found to play a key role in the formation of the exposed (001) facets.
TiO2 is an important, wide-bandgap semiconductor and has been widely applied as a pigment and in photovoltaic devices, sensors, batteries and photocatalysts due to its chemical stability, nontoxicity, low cost, and many other advantageous properties. Surface science has demonstrated that the order of the average surface energies of anatase TiO2 is 0.90 J m−2 for (001) > 0.53 J m−2 for (100) > 0.44 J m−2 for (101) facets.1 Therefore, most of the synthetic and natural anatase TiO2 crystal surfaces are dominated by the low energy (101) facets, which constitute more than 94% of total exposed surface according to the Wolff construction.2 Recently, an important breakthrough in preparation of anatase TiO2 single crystals with exposed (001) facets by binding with fluoride ions was achieved by Lu and co-workers.3 Soon after, we used a similar strategy to synthesize anatase TiO2 nanosheets with 89% exposed (001) facets, and demonstrated their excellent photocatalytic efficiency.4 From then on, many groups in succession reported syntheses of anatase TiO2 with exposed (001) facets.5–8 These studies have demonstrated that such highly exposed (001) facets are favorable for enhancing the performance of TiO2 nanoparticles in related applications, such as photodegradation of organic pollutants,4,6 dye-sensitized solar cells7b,9 and lithium ion batteries.10 In most of the reported methods, however, hydrofluoric acid or other F-containing compounds were usually used as capping agents to control the morphology of anatase TiO2 nanoparticles.3–8 As we know, these fluorine containing compounds, such as hydrofluoric acid, may generate many unexpected toxic and corrosive products at high temperature. Furthermore, the crystal planes are apt to be drastically passivated by fluoride ions, which are difficult to remove from the crystal surface.3,7a Therefore, it is highly desirable to develop some new synthetic strategies with fluorine-free compounds as the capping agent.11 Recently, a few papers concerning fluorine-free syntheses were reported. Ohtani and co-workers reported the preparation of TiO2 nanoparticles enclosed with (001) facets via a gas-phase reaction process with rapid heating and quenching.12 The Zheng13 and Lou groups14 reported the synthesis of ultrathin anatase TiO2 nanosheets with exposed (001) facets via amine-mediated methods. The Demopoulos group reported the synthesis of TiO2 nanoflakes by an alcohol-mediated method.15 On the whole, fluorine-free synthesis of TiO2 nanosheets with exposed (001) facets is comparatively rare until now. In this communication, we reported a carbonate ions-assisted synthetic strategy for fabricating anatase TiO2 nanoparticles exposed with a remarkable amount (60%) of high-energy (001) facets. Our experimental results demonstrated that the carbonate ions produced by the decomposition of urea in a basic environment can effectively lower the surface energy of (001) facets, resulting in the formation of the exposed (001) facets.
In the proposed synthetic route, K-titanate nanowires (KTNWs) were used as precursor (Fig. S1, ESI†), which transformed into truncated bipyramidal nanoparticles of anatase TiO2via a hydrothermal process at 200 °C for 16 h in the presence of urea (experimental details are given in ESI†). Fig. 1a shows a typical powder X-ray diffraction (XRD) pattern of the product, which can be indexed to anatase TiO2 (JCPDS No. 01-0562). The sharp peaks indicate good crystallinity of the sample. Scanning electron microscopic (SEM) and transmission electron microscopy (TEM) images show that the product consists of high-purity truncated bipyramidal nanoparticles with square or rectangle bases, the average size of which is about 250 nm (Fig. 1b and c). The selected-area electron diffraction (SEAD) pattern of a representative TiO2 nanoparticle is indexed as the diffraction from the [001] zone axis (Fig. 1d), indicating that the top and bottom of the bipyramidal truncated structures are enclosed by the (001) facets, the percentage of which reaches 60%.
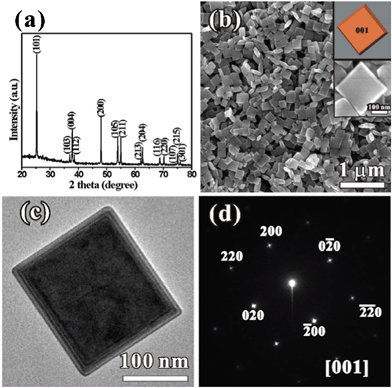 | ||
| Fig. 1 (a) XRD pattern and (b) SEM image of the TiO2 truncated bipyramids. Insets in (b) shows magnified SEM image and corresponding model, respectively; (c) low-magnification TEM image and (d) corresponding SEAD pattern of a representative TiO2 nanoparticle. | ||
Urea is crucial for the formation of truncated bipyramidal nanoparticles with exposed (001) facets. The percentage of (001) facets were demonstrated to increase with the amount of urea (Fig. S2, ESI†). The product composed of tetragonal bipyramidal TiO2 nanoparticles in the absence of urea (Fig. S2a, ESI†). TEM characterization indicated that these tetragonal bipyramidal TiO2 nanoparticles were fully enclosed by (101) facets (Fig. S3, ESI†). With the addition of urea from 5 mmol to 20 mmol, tetragonal bipyramidal TiO2 nanoparticles were gradually truncated, and the percentage of the (001) facets increased from 5% to the maximum value, 60% (Fig. S2b–d, ESI†).
Usually, anatase TiO2 prefers to grow into a tetragonal bipyramidal shape with exposed low energy (101) facets. As reported by the Xu and Amano groups,7a,16 a hydrothermal reaction of alkali metal contained titanate nanowires usually resulted in the formation of anatase nanoparticles enclosed by (101) or (100) facets with lower surface energy. The formation of truncated bipyramidal nanoparticles exposed with high energy (001) facets indicates that urea or its decomposed products could lower the surface energy of (001) facets. As we know, urea can be decomposed into carbon dioxide and ammonia at high temperature, which is converted to carbonate and ammonium ions in the alkaline environment. To investigate the role of carbonate and ammonium in the formation of the exposed (001) surfaces, a series of control experiments were carried out by replacing urea with NH4Cl, (NH4)2SO4, K2CO3 and (NH4)2CO3 (Fig. 2). When either NH4Cl or (NH4)2SO4 was used, pure bipyramidal TiO2 nanoparticles with exposed (101) facets, being the same as those obtained in the absence of urea, were obtained (Fig. 2a, b). This indicates that ammonium ions are not a key for the formation of the (001) surface of the truncated bipyramidal nanoparticles. When K2CO3 was used, some truncated bipyramidal nanoparticles with exposed (001) facets were observed (Fig. 2c), suggesting that carbonate ions might stabilize the (001) facets and thus result in the formation of high-energy (001) facets. However, KTNWs were not fully transformed into anatase TiO2, which could be due to the addition of K2CO3 increasing the basicity of solution and thus preventing the decomposition of K-titanate. To avoid the increase of the pH value of the solution, (NH4)2CO3 was then used instead of K2CO3, well truncated bipyramidal nanoparticles were obtained, as expected. The percentage of (001) facets can also be tuned by changing the amount of the (NH4)2CO3 (Fig. S4, ESI†). From the above results, we concluded the carbonate ions can lower the surface energy of (001) surfaces. On the other hand, urea or (NH4)2CO3 could also act as a pH buffer, which provides a stable environment for the growth of well shaped TiO2. Our experiments demonstrated that the pH value of the reaction solution is about 11.0 in the absence of urea while it is stabilized at 9.7 in the presence of urea (Fig. S5a, ESI†), and just fluctuates in a narrow range after reaction for 4 h (Fig. S5b, ESI†).
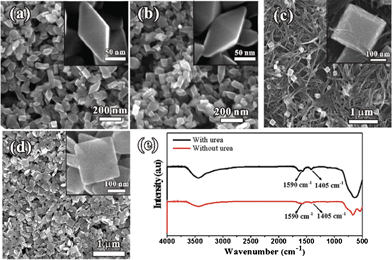 | ||
| Fig. 2 Typical SEM images of TiO2 nanoparticles synthesized by using KTNWs (0.01 g) as a precursor in the presence of (a) 40 mmol NH4Cl, (b) 20 mmol (NH4)2SO4, (c) 20 mmol K2CO3, and (d) 20 mmol (NH4)2CO3. (e) FT-IR spectrum of the TiO2 nanoparticles prepared with and without urea. | ||
Furthermore, to use titanate nanowires as a precursor is also crucial for the formation of the (001) facets. Under hydrothermal conditions, the transformation into anatase from titanate nanowires is relatively slow compared to the hydrolysis process of other precursors (such as TiCl4 or tetrabutyl titanate).7a Such a slow transformation process kinetically provides a chance for carbonate ions to change the surface energy of the (001) facets by specific adsorption during the crystal growth. It should be pointed out that the transformation process from KTNWs to anatase is factually a heterogeneous reaction where species diffusion is not homogeneous during the growth of nanoparticles. Such limited diffusion has certain influences on the growth rate of anatase in different directions, which may result in the truncated facets of some nanoparticles present being rectangle rather than square (see Fig. 1c).
To reveal how carbonate ions bind with the TiO2 surface, the Fourier transform infrared (FT-IR) spectra of TiO2 particles synthesized with and without adding 20 mmol urea were measured (Fig. 2e). Usually, the unidentate, bidentate-chelating and bidentate-bridging coordination modes are observed in the carbonate complexes.17 For the product obtained with urea, the strong bands at 1405 cm−1 and 1590 cm−1 (Fig. 2e) can be observed, which are attributed to symmetric and asymmetric stretching vibrations of bidentate-chelating carbonates bound on the TiO2 surface.18 By contrast, the intensities of these bands are very weak in the product without urea, which is due to the unavoidable reaction of carbon dioxides in the basic solution. The increase in the FT-IR intensity indicates that more bidentate-chelating carbonates are bound to the TiO2 surface by decomposition of urea. Therefore, we proposed a bidentate-chelating mode of carbonate on the TiO2 surface. On the bare (001) surface, all Ti atoms are five fold-coordinated and O atoms are two fold-coordinated (Fig. S6, ESI†). Such (001) facets are not stable due to excess charges and unsaturated coordination. Consequently, the vacant coordination sites of Ti atoms on the (001) surfaces could be fully occupied by the carbonate ions by the bidentate-chelating mode. Such a carbonate binding surface is neutralized, and therefore becomes the low energy surface.
The high-energy (001) surface of anatase TiO2 has been demonstrated to exhibit a much higher photodegradation activity than other low energy facets,4,6 which could be due to a high density of unsaturated coordination Ti atoms and active surface oxygen atoms with the large Ti–O–Ti bond angles at the surface.19 In this study, methylene blue (MB) as a model organic pollutant was used, which can be completely degraded in the presence of TiO2 photocatalysts through an oxidative process under UV irradiation (Fig. S7, ESI†), to demonstrate the photodegradation ability of different crystal facets. Herein, four anatase TiO2 nanoparticles obtained with different amounts of urea were used as photocatalysts (Fig. S2, ESI†), which are denoted as Sample A, Sample B, Sample C, and Sample D for convenience. Compared with the naturally slow degradation process, the degradation rate of MB was remarkably accelerated by adding TiO2 catalysts with exposed different percentages of (001) facets (Fig. 3). After irradiation for 20 min, only 46% MB was degraded in the presence of Sample A, the tetragonal bipyramidal nanoparticles dominantly surrounded with the lowest-energy (101) facets. Due to the appearance of (001) facets, the degradation ratio of MB increased to 59% and 73% for Sample B and Sample C with 5% and 20% (001) facets, respectively. Within the same time, however, almost all MB (98%) was degraded for Sample D, the truncated tetragonal bipyramidal nanoparticles with the maximum percentage of (001) facets. Considering the four anatase nanoparticles have a close size, it can be concluded from the above results that the photocatalytic efficiency increases with the increase of percentage of (001) facets. In other words, the photodegradation ability of (001) facets is better than that of (101) facets with low surface energy. It should be pointed out that the photocatalytic activity order of the exposed surface of anatase nanoparticles has a close relation to the type of concrete photo-redox reactions. Previous studies have demonstrated that photogenerated electrons prefer to migrate towards the (101) facets while photogenerated holes towards the (001) facets.20,21 Therefore the change of (101) and (001) percentages exposed have a distinct influence on the photocatalytic activity of anatase nanoparticles with regard to different photo-redox processes. For example, Liu and co-workers found that the photo-reactivity of (101) facets is greater than that of anatase (001) facets in H2 evolution, which is a photo-reduction process.22
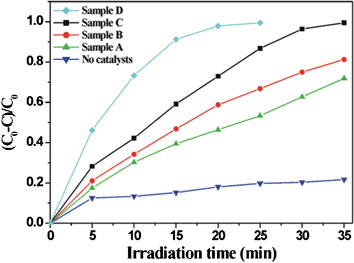 | ||
| Fig. 3 Photodegradation curves of MB as a function of irradiation time with different shaped anatase TiO2 nanoparticles as photocatalysts. | ||
In summary, the truncated tetragonal bipyramidal TiO2 nanoparticles exposed with tunable (001) facets were successfully synthesized. The percentage of (001) facets can be controlled by changing the amount of urea. The carbonate ions decomposed from urea plays the crucial role to reduce the surface energy of (001) facets. It has been demonstrated that a high energy (001) surface exhibits much better degradation ability than that of (101) facets with low surface energy.
This work was supported by the National Basic Research Program of China (Grant No. 2011CBA00508), and the National Natural Science Foundation of China (Grants No. 21021061, 21073145 and 21131005) and the Fundamental Research Funds for the Central Universities (Grant No. 2010121017).
References
- (a) M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter, 2002, 65, 119901 CrossRef; (b) U. Diebold, Surf. Sci. Rep., 2003, 48, 53 CrossRef CAS.
- (a) M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter, 2001, 63, 155409 CrossRef; (b) A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- X. G. Han, Q. Kuang, M. S. Jin, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2009, 131, 3152 CrossRef CAS.
- (a) H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS; (b) G. Liu, H. G. Yang, X. W. Wang, L. N. Cheng, H. F. Lu, L. Z. Wang, G. Q. Lu and H. M. Cheng, J. Phys. Chem. C, 2009, 113, 21784 CrossRef CAS; (c) G. Liu, C. Sun, H. G. Yang, S. C. Smith, L. Wang, G. Q. Lu and H. M. Cheng, Chem. Commun., 2010, 46, 755 RSC; (d) C. Z. Wen, J. Z. Zhou, H. B. Jiang, Q. H. Lu, S. Z. Qiao and H. G. Yang, Chem. Commun., 2011, 47, 4400 RSC.
- (a) D. Q. Zhang, G. S. Li, X. F. Yang and J. C. Yu, Chem. Commun., 2009, 4381 RSC; (b) D. Q. Zhang, G. S. Li, H. B. Wang, K. M. Chan and J. C. Yu, Cryst. Growth Des., 2010, 10, 1130 CrossRef CAS.
- (a) W. G. Yang, J. M. Li, Y. L. Wang, F. Zhu, W. M. Shi, F. R. Wan and D. S. Xu, Chem. Commun., 2011, 47, 1809 RSC; (b) J. M. Li, Y. X. Yu, Q. W. Chen, J. J. Li and D. S. Xu, Cryst. Growth Des., 2010, 10, 2111 CrossRef CAS.
- (a) S. F. Xie, X. G. Han, Q. Kuang, J. Fu, L. Zhang, Z. X. Xie and L. S. Zheng, Chem. Commun., 2011, 47, 6722 RSC; (b) M. Liu, L. Piao, L. Zhao, S. Ju, Z. Yan, T. He, C. Zhou and W. Wang, Chem. Commun., 2010, 46, 1664 RSC; (c) H. Yu, B. Z. Tian and J. L. Zhang, Chem.–Eur. J., 2011, 17, 5499 CrossRef CAS.
- X. Wu, Z. G. Chen, G. Q. Lu and L. Z. Wang, Adv. Funct. Mater., 2011, 21, 4167 CrossRef CAS.
- J. H. Liu, J. S. Chen, X. F. Wei, X. W. Lou and X. W. Liu, Adv. Mater., 2011, 23, 998 CrossRef CAS.
- C. Z. Wen, H. B. Jiang, S. Z. Qiao, H. G. Yang and G. Q. Lu, J. Mater. Chem., 2011, 21, 7052 RSC.
- F. Amano, O. O. Prieto-Mahaney, Y. Terada, T. Yasumoto, T. Shibayama and B. Ohtani, Chem. Mater., 2009, 21, 2601 CrossRef CAS.
- B. H. Wu, C. Y. Guo, N. F. Zheng, Z. X. Xie and G. D. Stucky, J. Am. Chem. Soc., 2008, 130, 17563 CrossRef CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Y. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124 CrossRef CAS.
- G. B. Shan and G. P. Demopoulos, Nanotechnology, 2010, 21, 025604 CrossRef.
- (a) F. Amano, T. Yasumoto, O. Prieto-Mahaney, S. Uchida, T. Shibayama and B. Ohtani, Chem. Commun., 2009, 2311 RSC; (b) J. M. Li and D. S. Xu, Chem. Commun., 2010, 46, 2301 RSC.
- K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds, Wiley, New York, 5th edn, 1997 Search PubMed.
- L. F. Liao, C. F. Lien, D. L. Shieh, M. T. Chen and J. L. Lin, J. Phys. Chem. B, 2002, 106, 11240 CrossRef CAS.
- A. Selloni, Nat. Mater., 2008, 7, 613 CrossRef CAS.
- T. Ohno, K. Sarukawa and M. Matsumura, New J. Chem., 2002, 26, 1167 RSC.
- (a) T. Tachikawa, N. Wang, S. Yamashita, S. C. Cui and T. Majima, Angew. Chem., Int. Ed., 2010, 49, 8593 CrossRef CAS; (b) T. Tachikawa, S. Yamashita and T. Majima, J. Am. Chem. Soc., 2011, 133, 7197 CrossRef CAS.
- J. Pan, G. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details, XRD pattern and TEM image of KTNWs precursor, SEM images of samples obtained with urea, TEM of Sample A, SEM images of the products obtained with (NH4)2CO3, urea- and time-dependent pH changes of reaction solution, surface atom arrangement of (001) facets, and UV-Vis data/discussion of MB degradation. See DOI: 10.1039/c2ra00834c/. |
| This journal is © The Royal Society of Chemistry 2012 |
