Synthesis of highly crystalline In2Ge2O7(En) hybrid sub-nanowires with ultraviolet photoluminescence emissions and their selective photocatalytic reduction of CO2 into renewable fuel†
Qi
Liu
acf,
Yong
Zhou
*abd,
Yue
Ma
e and
Zhigang
Zou
*abcd
aEco-Materials and Renewable Energy Research Center (ERERC), Nanjing, 210093, P. R. China. E-mail: zgzou@nju.edu.cn (Z. G. Zou); Fax: +86-25-83686632; Tel: +86-25-83686630
bSchool of Physics, Nanjing University, Nanjing, 210093, P. R. China. E-mail: zhouyong1999@nju.edu.cn (Y. Zhou)
cDepartment of Materials Science and Engineering, Nanjing University, Nanjing, 210093, P. R. China
dNational Laboratory of Solid State Microstructures, Nanjing University, Nanjing, 210093, P. R. China
eKuang Yaming Honors School, Nanjing University, Nanjing, 210093, P. R. China
fAnhui Polytechnic University, Nanjing, 210093, P. R. China
First published on 3rd February 2012
Abstract
A novel, highly crystalline indium germinate hybrid sub-nanowire, which we denote as In2Ge2O7(En) (En = ethylenediamine), with general diameters of 2–3 nm and lengths up to hundreds of nanometres was synthesized using a solvothermal route in a binary En/water solvent system. The hybrid ultrathin nanowire exhibits an ultraviolet photoluminescence emission, a dramatic blue shift by more than 100 nm relative to pure inorganic In2Ge2O7 nanowire and microtubes. The In2Ge2O7(En) ultrathin nanowire performs selectively the photocatalytic reduction of carbon dioxide (CO2) into carbon monoxide (CO) in the presence of water vapor. With reference to our Zn2GeO4 nanoribbon photocatalyst, which was recently used to produce CH4 under the same photocatalytic conditions, this work is a significant sign that the greenhouse gas, CO2, can be ameliorated into desirable kinds of renewable fuels using different germanate catalysts.
Nanowires (NWs) with sub-nanometre diameters below 2–3 nm possess pronounced quantum and surface effects, and dramatic changes in their electronic structures, and thus in their physical and chemical properties, which are anticipated to inspire new fundamental and technological research.1 While very few metal, metal oxide and chalcogenide ultrathin nanowires have so far been synthesized,2–9 the production of complex compounds such as ternary oxides and the related organic–inorganic hybrids were limited due to the increased difficulty in controlling the growth of the multi components at such an atomic level.
Germanates have attracted attention in the areas of catalysis, adsorption, ion exchange, and as porous materials for humidity sensors.10,11 Utilization of the flexible coordination behavior of germanium with indium enhances the structural diversity of the microporous indium–germanium compounds.12 In2Ge2O7 one-dimensional (1D) nanostructures including microtubes,13 nanobelts,14 and nanowires15 were synthesized via vapor–solid mechanisms.
Herein we report the synthesis of novel, highly crystalline indium germinate ultrathin nanowires, which we denote as In2Ge2O7(En) (En = ethylenediamine), with general diameters of 2–3 nm and lengths up to hundreds of nanometres using a solvothermal route in a binary En/water solvent system. The hybrid ultrathin nanowire exhibits an ultraviolet photoluminescence emission, a dramatic blue shift by more than 100 nm relative to pure inorganic In2Ge2O7 nanowire and microtubes, showing a potential application in laser diodes, free-space quantum communications and calibration purposes.16 The In2Ge2O7(En) ultrathin nanowire also performs selectively the photocatalytic reduction of carbon dioxide (CO2) into carbon monoxide (CO) in the presence of water vapor. With reference to our Zn2GeO4 nanoribbon photocatalysts which were recently used to produce CH4 under the same photocatalysis conditions,17 this work gives a meaningful sign that the greenhouse gas CO2 can be meliorated into desirable kinds of renewable fuels using different germinate catalysts.
The FE-SEM image the cotton-like feature of the product (Fig. S1a†), implies a very tiny 1D nanostructure. The corresponding EDX confirms the presence of the In, Ge, N, and O moieties (Fig. S1b†). The TEM and HRTEM image reveals a high-yield production of the ultrathin nanowires of 2–3 nm in diameter and with lengths up to several hundreds of nanometres (Fig. 1 and Fig. S2†). These ultrathin nanowires tend to twist each other together, showing flexibility compared with bulk materials. Both the bright dots of the selected-area electron diffraction (SAED) pattern (inset of Fig. 1a) and the well-resolved lattice fringes prove that the ultrathin nanowires possess high crystallinity. It is noteworthy that the ultrathin nanowires possess multiple V- and Z-shaped structures (Fig. S3†), which often appear in II–VI semiconductors (e.g., In2O3, ZnO, ZnSe, ZnS, CdS, CdSe, CdTe)18 and noble metals (e.g., Ag, Pd, Pt, Au),19 but rarely in ternary oxides. The two main reasons, which are responsible for such nonlinear structures, are that the twinned plannes and crystal lattice match (end-to-end and end-to-side approaches) to minimize the surface energy. Development of nonlinear junctions is important for greatly increased complication and flexibility in the device design.20
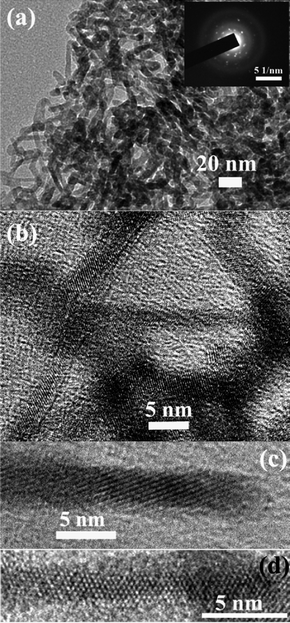 | ||
| Fig. 1 (a) TEM image and SAED pattern (inset), (b, c, and d) HRTEM images of the In2Ge2O7(En) ultrathin nanowires; (c) and (d) viewed along different crystal planes. | ||
The broadening profile of the XRD peaks is consistent with the ultrathin nature of the nanowires (Fig. S4a†). For determination of the crystal phase of the nanowires, the solvothermal time was prolonged to 7 days, which allowed us to obtain a relatively crystalline powder.21 The corresponding XRD pattern (Fig. S4b†) could not be indexed as two known indium–germanium compounds, i.e. In2Ge2O7 (Fig. S4c†) and In2Ge6O15(En)2 (Fig. S4d†), which indicates this material is possibly a new inorganic–organic hybrid phase.
XPS spectra demonstrate the presence of In 3d, Ge 3d, O 1s and N 1s peaks (Fig. S5†). The In 3d peaks appeared at 444.08 eV and 451.58 eV, representing 3d5/2 and 3d3/2, respectively. The Ge 3d at 31.78 eV is consistent with that of Ge(IV) (31.8 eV) in germinate rather than Ge (32.5 eV) in GeO2,22 indicating no existence of GeOx-En. The O 1s peak centered at 530.18 eV is assignable to the lattice oxygen, and not to the hydroxyl groups located at 531.4 eV, indicating that the nanowire contains no –OH group. The N 1s peak at 399.38 eV is attributed to the –NH2 of alkylammonium (399.6 eV), which is distinct from that in InN (396.4 eV)23 and –NH3+ (402.4 eV) of alkylammonium, implying that the En intercalated in the nanowire exists neither in the In-N bond form, nor the –NH3+, but possibly by being located in the channel of the frameworks as a neutral form,11 which also occurs in In2Ge6O5(OH)2(H2dien) (dien = diethylenetriamine).12c The inductive coupled plasma (ICP) emission spectrometer confirms the atomic ratios as approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of In/Ge.
1 of In/Ge.
The FTIR spectra reveal the N–H stretching band (3355 cm−1 and 3278 cm−1) of the ultrathin nanowire, which further suggests the existence of En in the nanowire (Fig. S6†). A slight red shift of the –N–H band of the hybrid nanowire relative to pure En indicates that the intercalated En interacts with the framework oxygen atoms through hydrogen bonding.24
Differential thermoanalysis (DTA) and thermogravimetry (TG) revealed that the ultrathin nanowire decomposes in four steps with a total weight loss of ∼15% in the measured temperature range (Fig. S7†). Surface water desorbs below 150 °C. Weight loss of intercalated En in the channel follows two steps with total mass loss of 9.6%, covering the temperature from ∼150 °C up to ∼500 °C, which is in good agreement with that calculated for the removal of one En molecule (calcd 9.21%). Calcination at 1000 °C led the nanowire to decompose into In2Ge2O7 and trace GeO2 (Fig. S4e†).
Accordingly, based on XRD, XPS, ICP, FTIR and TG analysis, the valences of Ge, In, and O were determined to be +4, +3, and −2, respectively. The formulae of the hybrid materials can be proposed as In2Ge2O7(En), and the structure models were tentatively simulated as shown in Fig. 2. Each In atom is coordinated to six O atoms to form an {InO6} octahedron. These {InO6} octahedra lie in a nearly hexagonal arrangement in the (001) plane and share edges to form a distorted honeycomb ensemble with the composition {[In2O6]6−}n. Each Ge atom is coordinated to four O atoms to form a {GeO4} tetrahedron. Two tetrahedra share one corner to form a [Ge2O7]6− group. The In2Ge2O7 (En) can therefore be described as a stacking of alternating parallel sheets of InO6 octahedra and isolated [Ge2O7]6− groups (Fig. 2d). The En molecules are described to locate within the channels and interact via terminal N atoms with the framework oxygen atoms through hydrogen bonding (Fig. 2b). The failure of attempts to exchange En with methanol, ethanol, and ethylene glycol implies high strength of the H-bonding. An endeavor is underway to synthesize the single crystal sample to obtain a more precise crystal structure of this novel hybrid nanowire.
![The proposed framework structure of the In2Ge2O7(En) ultrathin nanowires viewed along a) [100], c) [001], and d) [010] direction. b) Detail of the channel location of the intercatated En in a cell unit, which interact with framework oxygen atoms through H-bonding.](/image/article/2012/RA/c2ra20186k/c2ra20186k-f2.gif) | ||
| Fig. 2 The proposed framework structure of the In2Ge2O7(En) ultrathin nanowires viewed along a) [100], c) [001], and d) [010] direction. b) Detail of the channel location of the intercatated En in a cell unit, which interact with framework oxygen atoms through H-bonding. | ||
The UV-vis adsorption spectrum of the ultrathin nanowire exhibits a distinct peak at 260 nm with the adsorption edges at 312 nm (Fig. S8†), the corresponding band gap is about 3.98 eV. The room-temperature photoluminescence spectrum shows an ultraviolet emission peak at about 358 with a shoulder at 378 nm (Fig. 3). Since the {Ge2O7} group does not behave as a luminescent centre in indium germinate, the luminescence may originate from the honeycomb sheets of the {InO6} octahedra,25 in which the associated optical transitions can be considered as charge transfer processes between ions within the {InO6} octahedral. The present emission shows a dramatic blue shift relative to 496 nm for the prevenient inorganic In2Ge2O7 nanowires15 and 560 nm for the microtubes.13 It may result from very strong two-dimensional quantum confinement effects and the different framework structures between the hybrid and pure inorganic phase. Moreover, the feature of the dual emission peaks of the In2Ge2O7(En) hybrid nanowire is in contrast to the singlet of the pure inorganic In2Ge2O7 nanowire and microtube, and is most possibly assigned to intercalation of the amine molecules in the framework.
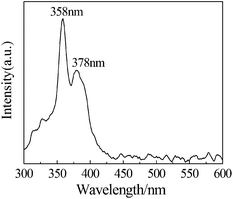 | ||
| Fig. 3 Photoluminescence spectra of In2Ge2O7(En) ultrathin nanowires at room temperature, excited at 260 nm. | ||
It is well-known that alkylamines display bifunctionalities that mediate the crystal structure growth and control the 1D morphology of II–VI semiconductor and inorganic–organic hybrids because they can adsorb on solid surfaces and selectively bind to some specific panels to control the velocity and direction of crystal growth; this has been called the solvent-coordination molecular template mechanism.26,27 The present En/H2O composition plays a important role on both morphology and phase of the product. Employment of pure En as a single solvent produced monodisperse In2O3 beads with a diameter of (30 ± 2) nm consisting of tiny nanocrystals (5 ± 0.5) nm (Fig. S4f† for XRD and Fig. S9† for FE-SEM and TEM). Use of pure water as the solvent enabled us to obtain aggregation of pure In2Ge2O7 inorganic short nanorods with 20–30 nm diameter and 40–50 nm length (Fig. S4g† for XRD and Fig. S10† for FE-SEM). Thus, we believe that the In2Ge2O7(En) ultrathin nanowire in the En/H2O binary solvent obviously originates from the synergistic effect between two solvents. H2O acted as structure-directing role to regulate the nucleating indium germinate to 1D grow; En strictly confined other 2D evolution through coordination and H-bonding interactions between the amino groups of En and the In, Ge, and O moieties of the framework. While alkylamine/H2O mixed-solvent systems were developed early for the synthesis of micrometre-size single crystals In2Ge6O15(En)212a,12b and In2Ge6O15(OH)2(H2dien),12c the novelty of our work is to extend this system to the successful one-step synthesis of hybrid ultrathin nanowires.
Reduction of the greenhouse gas CO2 to a valuable high energy fuel using solar energy is one of the best solutions to both the global warming and energy shortage problems.28 Many studies have demonstrated that CH4 and CO were detected as the major redox product of the CO2 photocatalytic reduction of CO2.29 The In2Ge2O7 (En) ultrathin nanwire proves to possess photocatalytic activity towards the conversion of CO2 to CO and O2 in the present water vapour under Xe arc lamp irradiation (Fig. 4a and 4b). The generation rate of CO could be significantly enhanced by loading 1 wt% Pt as a co-catalyst, as Pt is of high work function which is beneficial for collecting photogenerated electrons. The In2Ge2O7(En) nanowire shows a higher activity than the bulk In2Ge2O7 inorganic phase obtained by the conventional solid-state reaction (SSR) of In2O3 and GeO2 at 1200 °C for 16 h (Fig. 4c). The higher photocatalytic activity of the In2Ge2O7(En) nanowire, compared to the SSR sample, can be contributed to the following two reasons: (1) reducing the lateral dimension to the nanometre length scale with regards to the ultrathin nanowires offers a high specific surface area of 132.5 m2 g−1, which is over 55 times larger than the 2.4 m2 g−1 of SSR materials. (2) The ultra-thin geometry of the nanowire allows the charge carriers to have faster mobilities, as they are able to move quickly from the interior onto the surface to participate in the photoreduction reaction. With reference to our Zn2GeO4 nanoribbon photocatalysts, which was recentlt used to produce CH4 under the same photocatalysis conditions,17 this work gives a significant sign that CO2 can be selectively turned into desirable kinds of renewable fuels using different germanate catalysts, despite the relatively low conversion rate, which may be further greatly improved through the building of appropriate heterostructures. The CO2 reduction experiment performed in the dark or in the absence of H2O showed no detection of CO. This photoreduction process is believed to be a single-photon 2-electron process, with water as the stoichiometric electron donor.30,31 The FTIR band of the N–H stretching region of the In2Ge2O7(En) remains unchanged before and after absorption of CO2 (Fig. S6d†), indicating that there are no interactions between the absorbed CO2 and the intercalated En. The FTIR spectra of the hybrid nanowires before and after the photocatalysis reaction also remains almost constant (Fig. S6c†), demonstrating that (1) the formation of CO derives from the CO2 reduction rather than En decomposition; (2) the CO2 reduction process was guided by photo-generated electrons from the inorganic moiety of the nanowire but not by the amine groups of the En as a reductant. Furthermore, the hybrid nanowires, as a photocatalyst, also possess high photo-stability against light irradiation that no variation in both the FTIR and the XRD being observed after irradiation for several days.
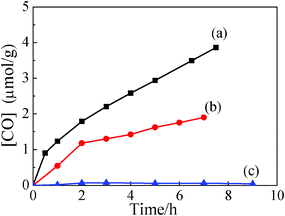 | ||
| Fig. 4 CO generation over (a) 1 wt% Pt-loaded In2Ge2O7(En) ultrathin nanowires, (b) In2Ge2O7(En) ultrathin nanowires and (c) SSR sample as a function of light irradiation time. | ||
In summary, novel indium germinate ultrathin nanowires were synthesized using a solvothermal route in a binary En/water solvent system. The ultrathin nanowires exhibit ultraviolet photoluminescence emissions, very strong two-dimensional quantum confinement effects and photocatalytic performance in the conversion of CO2 to CO.
Acknowledgements
This work was supported by 973 Programs (no. 2011CB933303 and 2007CB613305), JST-MOST (no. 2009DFA61090), JSPS-NSFC (no. 20811140087), NSFC (no. 20971048 and 50732004), Fundamental Research Funds for the Central Universities (no. 1113020401).References
- O. Gulseren, F. Ercolessi and E. Tosatti, Phys. Rev. Lett., 1998, 80, 3775 CrossRef CAS.
- See review: L. Cademartiri and G. A. Ozin, Adv. Mater., 2009, 21, 1013 CrossRef CAS.
- Z. T. Deng, H. Yan and Y. Liu, Angew. Chem., Int. Ed., 2010, 49, 8695 CrossRef CAS.
- (a) X. M. Lu, M. S. Yavuz, H. Y. Tuan, B. A. Korgel and Y. N. Xia, J. Am. Chem. Soc., 2008, 130, 8900 CrossRef CAS; (b) Z. Li, J. Tao, X. M. Lu, Y. Zhu and Y. N. Xia, Nano Lett., 2008, 8, 3052 CrossRef CAS.
- (a) Z. Huo, C. K. Tsung, W. Huang, X. Zhang and P. Yang, Nano Lett., 2008, 8, 2041 CrossRef CAS; (b) Z. Huo, C. K. Tsung, W. Huang, M. Fardy, R. Yan, X. Zhang, Y. Li and P. Yang, Nano Lett., 2009, 9, 1260 CrossRef CAS.
- (a) C. Wang, Y. Hu, C. M. Lieber and S. Sun, J. Am. Chem. Soc., 2008, 130, 8902 CrossRef CAS; (b) Y. H. Luo, J. G. Huang and I. Ichinose, J. Am. Chem. Soc., 2005, 127, 8296 CrossRef CAS.
- S. Palchoudhury, W. An, Y. Xu, Y. Qin, Z. Zhang, N. Chopra, R. A. Holler, C. H. Turner and Y. Bao, Nano Lett., 2011, 11, 1141 CrossRef CAS.
- C. Liu and S. Yang, ACS Nano, 2009, 3, 1025 CrossRef CAS.
- Y. P. Du, Y. W. Zhang, Z. G. Yan, L. D. Sun and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 16364 CrossRef CAS.
- T. E. Gier, X. Bu, P. Feng and G. D. Stucky, Nature, 1998, 395, 154 CrossRef CAS.
- See review: Z. E. Lin and G. Y. Yang, Eur. J. Inorg. Chem., 2010, 2895 CrossRef CAS.
- (a) D. Pitzschke and W. Bensch, Angew. Chem., Int. Ed., 2003, 42, 4389 CrossRef CAS; (b) C. M. Wang, C. W. Yang, C. H. Lin and K. H. Lii, Inorg. Chem., 2010, 49, 10229 CrossRef CAS; (c) G. Liu, S. Zheng and G. Yang, Angew. Chem., Int. Ed., 2007, 46, 2827 CrossRef CAS.
- (a) J. Zhan, Y. Bando, J. Hu, L. Yin, X. Yuan, T. Sekiguchi and D. Golberg, Angew. Chem., Int. Ed., 2006, 45, 228 CrossRef CAS; (b) C. Yan, T. Zhang and P. S. Lee, Crys. Growth Des., 2008, 8, 3145 Search PubMed.
- (a) L. Li, P. S. Lee, C. Yan, T. Zhai, X. Fang, M. Liao, Y. Koide, Y. Bando and D. Golberg, Adv. Mater., 2010, 22, 5145 CrossRef CAS; (b) Y. Su, S. Li, L. Xu, Y. Chen, Q. Zhou, B. Peng, S. Yin, X. Meng, X. Liang and Y. Feng, Nanotechnology, 2006, 17, 6007 CrossRef CAS; (c) C. Yan, N. Singh and P. S. Lee, Cryst. Growth Des., 2009, 9, 3697 CrossRef CAS.
- S. S. Kim, J. Y. Park, H. S. Kim, H. G. Na, J. C. Yang, S. H. Shim, C. Lee, D. Park, D. Nam, H. Cheong and H. W. Kim, J. Phys. D: Appl. Phys., 2011, 44, 025502 CrossRef.
- G. Sun, Y. Ding, J. G. Liu, G. S. Huang, H. Zhao, N. Tansu and J. B. Khurgin, Appl. Phys. Lett., 2010, 97, 021904 CrossRef.
- Q. Liu, Y. Zhou, J. H. Kou, X. Y. Chen, Z. P. Tian, J. Gao, S. C. Yan and Z. G. Zou, J. Am. Chem. Soc., 2010, 132, 14385 CrossRef CAS.
- G. Z. Shen, J. Xu, X. F. Wang, H. T. Huang and D. Chen, Adv. Mater., 2011, 23, 771 CrossRef CAS.
- X. C. Jiang, S. X. Xiong, Z. A. Tian, C. Y. Chen, W. M. Chen and A. B. Yu, J. Phys. Chem. C, 2011, 115, 1800 CAS.
- K. Keren, M. Krueger, R. Gilad, G. Ben-Yoseph, U. Sivan and E. Braun, Science, 2002, 297, 72 CrossRef CAS.
- The size of the powder is not large enough for characterization of the 3D molecular structure based on single-crystal X-ray diffraction.
- Q. S. Gao, P. Chen, Y. H.Zhang and Y. Tang, Adv. Mater., 2008, 20, 1837 CrossRef CAS.
- (a) I. J. Lee, J. Y. Kim and H. J. Shin, J. Appl. Phys., 2004, 95, 5540 CrossRef CAS; (b) K. R. Reyes-Gil, Y. Sun, E. Reyes-Garcı' and D. Raftery, J. Phys. Chem. C, 2009, 113, 12558 CrossRef CAS.
- T. Ogoshi and Y. Chujo, Polymer, 2006, 47, 4036 CrossRef CAS.
- I. Tanaka, M. Mizuno and H. Adachi, Phys. Rev. B, 1997, 56, 3356 Search PubMed.
- (a) For reviews, see: W. T. Yao and S. H. Yu, Adv. Funct. Mater., 2008, 18, 3357 CrossRef CAS; (b) H. B. Yao, M. R. Gao and S. H. Yu, Nanoscale, 2010, 2, 323 RSC; (c) J. Li, Z. Chen, R. J. Wang and D. M. Proserpio, Coord. Chem. Rev., 1999, 190–192, 707 CrossRef CAS; (d) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef.
- Y. D. Li, H. W. Liao, Y. Ding, Y. Fan, Y. Zhang and Y. T. Qian, Inorg. Chem., 1999, 38, 1382 CrossRef CAS.
- (a) V. P. Indrakanti, J. D. Kubicki and H. H. Schobert, Energy Environ. Sci., 2009, 2, 745 RSC; (b) S. C. Roy, O. K. Varghese, M. Paulose and C. A. Grimes, ACS Nano, 2010, 4, 1259 CrossRef CAS; (c) U. P. Apfel and W. Weigand, Angew. Chem., Int. Ed., 2011, 50, 4262 CrossRef CAS; (d) T. W. Woolerton, S. Sheard, E. Reisner, E. Pierce, S. W. Ragsdale and F. A. Armstrong, J. Am. Chem. Soc., 2010, 132, 2132 CrossRef CAS; (e) O. Lazarus, T. W. Woolerton, A. Parkin, M. J. Lukey, E. Reisner, J. Seravalli, E. Pierce, S.W. Ragsdale, F. Sargent and F. A. Armstrong, J. Am. Chem. Soc., 2009, 131, 14154 CrossRef CAS.
- (a) Y. Zhou, Z. P. Tian, Z. Y. Zhao, Q. Liu, J. H. Kou, X. Y. Chen, J. Gao, S. C. Yan and Z. G. Zou, ACS Appl. Mater. Interfaces, 2011, 3, 3594 CrossRef CAS; (b) T. Yui, A. Kan, C. Saitoh, K. Koike, T. Ibusuki and O. Ishitani, ACS Appl. Mater. Interfaces, 2011, 3, 2594–2600 CrossRef CAS; (c) G. Dey, J. Nat. Gas Chem., 2007, 16, 217–226 CrossRef CAS.
- M. Anpo and M. Takeuchi, J. Catal., 2003, 216, 505 CrossRef CAS.
- W. Lin, H. Han and H. Frei, J. Phys. Chem. B, 2004, 108, 18269 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Details of experimental procedures, characterizations, and supporting images. See: DOI: 10.1039/c2ra20186k/ |
| This journal is © The Royal Society of Chemistry 2012 |
