A facile approach to prepare Ni, Co, and Fe nanoarrays inside a native porous alumina template via a redox reaction†
Ren
Li
,
Qianwang
Chen
*,
Xianyi
Hu
,
Mingsheng
Wang
,
Chengwei
Wang
and
Hao
Zhong
Hefei National Laboratory for Physical Science at Microscale and Department of Materials of Science and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: cqw@ustc.edu.cn
First published on 7th February 2012
Abstract
We report a novel approach to prepare magnetic metal (e.g.Ni, Co and Fe) nanowire arrays by reduction of metal chloride salts in the pores of an anodic aluminum oxide (AAO) template with a backside Al sheet via a hydrothermal process. When a magnetic field was applied during the redox reaction, the Ni nanoarrays exhibit an enhanced magnetic anisotropy behavior. It is shown that the coercivity field and squareness are distinct for different directions, especially the coercivity of the AF// (300 Oe) is much larger than the AF⊥ (20 Oe).
The study of metallic nanoarrays has always been the focus of intensive research because of its attractive electrical, optical, magnetic, and thermal properties, which leads to potential applications in microelectronics, optoelectronics, magnetic manipulation, biotechnology, catalysis, and sensors, especially in the field of high density magnetic storage medium.1–19 Including self-assembly, a number of methods have been reported to prepare magnetic nanoarrays. For instance, Sun et al. reported the synthesis and two-dimensional ordered assembly of 4 nm FePt nanoparticles by reduction of platinum acetylacetonate and decomposition of iron pentacarbonyl in the presence of oleic acid and indicated that these assemblies are promising as new-style storage medium.20 Sun et al. reported that monodisperse Fe3O4 nanoparticles of a controlled size from 3 to 20 nm have been synthesized by a high-temperature solution-phase reaction of Fe(acac)3 in phenyl ether with alcohol, oleic acid, and oleylamine.21 Hyeon et al. reported that highly crystalline and monodisperse γ–Fe2O3 nanocrystallites controlled size from 4 to 16 nm have been synthesized by high-temperature by high-temperature solution-phase reaction Fe(CO)5 in octyl ether and oleic acid at 100 °C ,then dehydrated (CH3)3NO was added and heated to 130 °C.22Unfortunately, most methods are not suitable for large scale device fabrication because they are multistep, expensive, or time-consuming procedures, especially the size and shape of assembling units obtained by chemical synthesis are not completely identical. Comparatively, the synthesis of solid templates (e.g., anodized aluminum oxide (AAO)) is simple, of low-cost and scalable. In addition, the as-prepared templates can easily achieve excellent long-range order. The AAO membrane is one of the templates that are generally used to produce nanoarrays. Previous studies have already shown the formation of arrays of magnetic nanowires and magnetic alloys in AAO templates by direct pore filling of the AAO template via methods of vapor–liquid–solid (VLS),23pulsed laser deposition (PLD),24 and electro-deposition.25–27 The electroless synthesis of large scale magnetic nanowire arrays and their magnetic behaviors have been extensively reported recently. For instance Xia et al. reported a novel method to prepare CoPt nanodot arrays via a thermal decomposition precursor composed by CoCl2, H2PtCl6 and citric acid monohydrate, which was easily integrated with a widely used spin-coating process to form a hydrophilic polymer template for fabricating uniform nanostructure arrays over a large area.28 Yuan et al. reported a novel method to prepare Co–P alloy nanowire arrays via an electroless deposition in the AAO template with Pd nanoparticles on the pore walls.29 Xu et al. reported a novel method of preparation by merely infiltrating aqueous solutions of metal chloride salts into a Au-coated native porous anodic aluminum oxide template with Al foil on its outside edge.30 Comparatively, porous-template-assisted electrodeposition and electroless deposition are the most popular techniques. However, for the porous-template-assisted electrodeposition, electric power is required, whereas for the conventional porous-template-assisted electroless deposition, metallic ions are typically reduced in the presence of organic surfactants, and the pore walls of the template are usually modified via a complex sensitization–preactivation process. Here, neither electric power nor organic surfactants are required, only infiltrating aqueous solutions of metal chloride salts into the native AAO template and reduction by Al sheet on the backside of AAO template is required. High density single crystalline Ni (as well as other metals, such as Co, Fe, etc.) nanowires with an excellent surface coverage can be prepared via a novel simple synthesis route, in which Ni2+ ions were reduced by the Al substrate of the AAO (Fig. 1). By this novel approach, the problem of large area preparation and magnetic anisotropy for high density magnetic data storage media (HDMDS) media could be solved effectively.
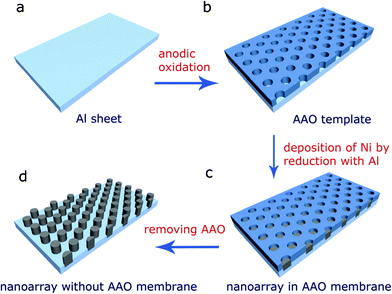 | ||
| Fig. 1 A scheme of the preparation of the high density magnetic nanoarray in the AAO template, (a) Al sheet; (b) AAO template; (c) Ni nanoarray with AAO membrane; (d) Ni nanoarray after dissolving the AAO membrane. | ||
The phase of the nanowires obtained by dissolving AAO templates and Al substrates in a 3 M NaOH aqueous solution was characterized by X-ray (Cu-Kα) powder diffraction (XRD). As shown in Fig. 2a, all the peaks can be indexed to a face-centered cubic Ni (JCPDS card No. 87-0712 space groupFm3m). The crystallite size is calculated to be 30 nm from the (111) peak according to the Scherrer equation. The preparations of Co and Fe nanoarrys via this process were also achieved (see ESI†). The morphology of the product released by dissolving the AAO templates in a 3 M NaOH aqueous solution was observed by a field emission scanning election microscopy (FESEM). Fig. 2b shows the typical FESEM image of a sample, which reveals that Ni nanowires grow within the pores of AAO to produce a 2D Ni array. The nanowires vary in size from 30 to 60 nm in the diameter and up to several micrometres in length (Fig. 2c). The FESEM image of the Ni nanoarrays without any disposal is shown in the ESI (Fig. 3).† The Ni nanoarrays in AAO template with chromic acid disposal is shown in the ESI (Fig. 4).† A representative TEM image of the sample derived by wet etching of AAO template was shown in Fig. 2d, where wire-like products can be observed. The high resolution transmission electron microscopy (HRTEM) image shown in Fig. 2e reveals the single-crystalline nature of the Ni nanowire. The lattice fringe observed in this image is 0.34 nm, which agrees well with the lattice spacing between two adjacent (100) planes of Ni.
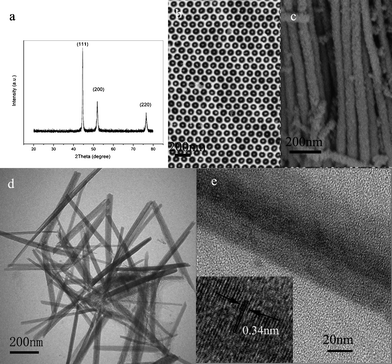 | ||
| Fig. 2 (a) XRD pattern of Ni nanowires collected after dissolving AAO templates. (b) SEM top image of Ni nanowires array grown in the pores of AAO. (c) SEM image of Ni nanowires without AAO template. (d) Representative TEM images of typical Ni nanowires formed in the hole of AAO. (e) A HRTEM image of a Ni single nanowire, the inset in the lower left corner shows the HRTEM lattice image of the nanowire. | ||
It is suggested that the deposition of Ni nanowires in the pores of the AAO is the result of electron transfer from the Al plate to the Ni2+ ions according to the principle relating to battery technology (Al(s) | AlCl3 (a1) | NiCl2 (a2) | Ni (s)). When it meets the metallic ions with the higher redox potential, the Al sheet plays the role of reducing agent, which offers the possibility of spontaneous electron transfer from Al to the metallic ions (for example Mn+) in solution. As a result, the metallic ions reduced by Al are deposited on the top of the surface of the Al sheet as metal atoms. This process can be considered as galvanic cell reduction, in which some locations on the Al foil surface serve as anodes and are oxidized into Al3+, which subsequently enters the solution.
| Al →Al3+ + 3e |
Then the generated electrons transfer to other parts of the Al sheet, which serve as the cathodes, across the Al sheet, and the metallic ions in solution gain electrons and are reduced to metal atoms right at the cathodes.
| Ni2+ + 2e− → Ni |
The detailed formation process of the nanaoarrys in the nanopores is illustrated in Fig. 3. Reduction reactions occurred in the pores of the AAO, while the Al sheet at the bottom of pores plays an essential role as a reductant in reducing Ni2+ ions attracted into the pores under hydrothermal conditions. With the proceeding of the reaction, the Ni nanopillars formed, and the Ni2+ ions cannot touch the Al sheet. However, the Ni2+ can still be reduced to a Ni atom at the top of the nanopillar due to the electron transfer from the Al sheet through the deposited Ni, while holes were generated and moved to the bottom end of the Ni nanopillars to oxide Al sheet; as a result, the nanowires were produced. This special electron transfer-related reaction results in epitaxy growth of Ni on the Ni nanopillars, which is suggested to be responsible for the growth of single crystalline nanowires. Theoretically, nanoarrays of any metal could be achieved if their corresponding water-soluble chloride salts are employed and their metallic ions have a higher redox potential than that of Al. Herein, we shall describe Co and nanowires (NWs) achieved via simply infiltrating AAO template with sheet in aqueous solution of CoCl2 and FeCl2 (see ESI, Figs. 1 and 2). We can easily extend this approach to nanoarrays of other metals (e.g., Pt, Ag, Cu, Ni, and Co). Compared to the self-assembly process, large scale nanoarrays can be easily produced via this approach. The single crystal nanowire arrays formed can not be gained in the electro-deposition method. Magnetic anisotropy behavior can also be enhanced due to the novel approach allowing a magnetic field to be applied during the redox reaction.
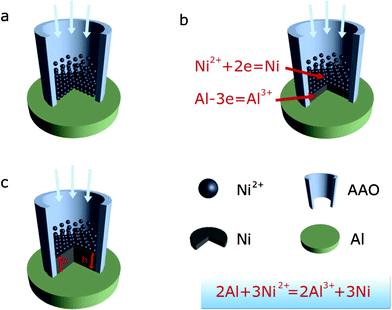 | ||
| Fig. 3 Schematic diagram of the growth process of Ni nanowires by chemical reduction with an Al substrate in the pores of the AAO. | ||
For HDMDS, the enhanced magnetic anisotropy behavior is most important, although it is believed that the magnetic properties of materials are highly dependent on the sample morphology, crystallinity, magnetization direction, etc. Here, a magnetic field was applied during the redox reaction in order to enhance the magnetic anisotropy behavior of the nanoarrays. The hysteresis loops of the nanoarray, with the applied magnetic field parallel (//) and perpendicular (⊥) to the film surface, were measured at room temperature (Fig. 4). It is obviously shown that the coercivity field and squareness are distinct for different directions, especially the coercivity of AF//(300 Oe) is much larger than AF⊥(20 Oe). The different magnetic properties between the AF// and AF⊥ demonstrates a large perpendicular anisotropy typical of high aspect ratio magnetic nanopillars ascribed to the (100)-preferred growth in the AAO membrane under magnetic fields.
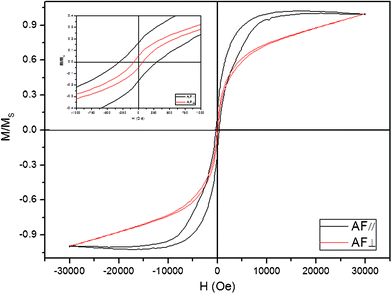 | ||
| Fig. 4 Magnetic hysteresis loops of the as-prepared products at room temperature with the applied field parallel (red line) and perpendicular (black line) to the film surface. The upper left inset shows the hysteresis loops between −1000 and 1000 Oe. | ||
Magnetic fields not only affect the movement of the metal ions but also control the growth of the metal nanowires along the direction of the magnetic lines of force,31,32 which produces the magnetic anisotropy behavior of nanoarrays.33 Although nickel is not a satisfied material for HDMDS due to its lower coercivity and large superparamagnetic critical size, our method provides a way to prepare HDMDS based on metal alloys, such as FePt, CoPt.
According to previous results, the anodization process for preparing AAO templates is a simple and efficient method. The size of the pore diameter is adjustable from 20 to 200 nm, and the pore periodicity is also adjustable in the nm range from 50 to 400 nm. Therefore, the highest storage density could be 1011 cm−2via this novel method. This process has its advantages as follows: (1) metal nanowires whose electrode potential is greater than that of aluminum can be prepared; (2) the system is simple to set up, easy to operate, easy to repair, and durable for industrialization; (3) it is a one-pot reaction process for the preparation of large-area uniform nanostructure arrays; (4) single crystalline metal nanowires with high magnetic anisotropy can be prepared.
In summary, a novel method that allowed the large-area preparation of high density magnetic nanoarrays in an AAO membrane by the direct chemical reduction of Ni2+ ions with an Al substrate of AAO was achieved. The Ni nanoarrays exhibit an enhanced magnetic anisotropy behavior due to an applied magnetic field which was applied during the chemical reduction deposition, which makes them possible to be used as high density magnetic storage media. This approach provides a direct and cost-effective method to produce not only magnetic metal nanoarrays but also metal alloy nanoarrays (e.g., FePt).
Experiments
The detailed process for preparing Ni nanoarrays was schematically shown in Fig. 1. A high-purity aluminum sheet (99.999%, 0.25 mm thickness, 1.2 cm diameter) was used as a substrate material. It was electropolished in a solution of perchloric acid (10 wt%) and ethanol (90 wt%) to a mirror finish. Clean aluminum sheets were anodized in a 0.3 mol L−1oxalic acid solution at 8 °C at a constant applied voltage of 40 V for 24 h. The resultant aluminum oxide film was subsequently removed by dipping the anodized sheet into an aqueous mixture of phosphoric acid (6 wt%) and chromic acid (1.8 wt%) at 60 °C. A second anodization was performed for 2 h under the same conditions as the first one. A voltage drop from 40 V to 0 V by 5 V steps was done at the end of the second anodization.34 The pores of the template were widened by dipping the material into an aqueous 0.1 mol L−1phosphoric acid solution for 40 min at 40 °C. In a typical procedure, the AAO templates with aluminum substrates which enveloped by Teflon bracket was sealed into a 50 ml Teflon autoclave filled with NiCl2 (1 mol L−1) solution, followed by the hydrothermal treatment at 150 °C for 6 h and then cooled to room temperature naturally. The AAO template with Ni nanowires was collected after washing with deionized water several times and subsequently dried in vacuum at 60 °C for 3 h. The magnetic field was applied with a permanent magnet under the Teflon vessel as described in ref. 35. The strengths of the magnetic fields on the inner lower surface of the cells were 0.25 T.Acknowledgements
This work was supported by the National Synchrotron Radiation Laboratory of China (KY206014002) and the National Natural Science Foundation of China (no. 20932002, 20972144, 90813008, 20772188 and J1030412).References
- J. Byun, J. I. Lee, S. Kwon, G. Jeon and J. K. Kim, Adv. Mater., 2010, 22, 2028–32 CrossRef CAS
.
- Z. L. Huang, G. W. Meng, Q. Huang, Y. J. Yang, C. H. Zhu and C. L. Tang, Adv. Mater., 2010, 22, 4136–4139 CrossRef CAS
.
- S. H. Kwon, J. H. Kang, C. Seassal, S. K. Kim, P. Regreny, Y. H. Lee, C. M. Lieber and H. G. Park, Nano Lett., 2010, 10, 3679–3683 CrossRef CAS
.
- L. F. Liu, E. Pippel, R. Scholz and U. Gosele, Nano Lett., 2009, 9, 4352–4358 CrossRef CAS
.
- W. L. Zhou, J. B. He, J. Y. Fang, T. A. Huynh, T. J. Kennedy, K. L. Stokes and C. J. O'Connor, J. Appl. Phys., 2003, 93, 7340–7342 CrossRef CAS
.
- C. X. Wang, W. D. Ruan, N. Ji, W. Ji, S. Lv, C. Zhao and B. Zhao, J. Phys. Chem. C, 2010, 114, 2886–2890 CAS
.
- V. Guieu, F. Lagugne-Labarthet, L. Servant, D. Talaga and N. Sojic, Small, 2008, 4, 96–99 CrossRef CAS
.
- J. B. C. Engelen, M. Delalande, A. J. le Febre, T. Bolhuis, T. Shimatsu, N. Kikuchi, L. Abelmann and J. C. Lodder, Nanotechnology, 2010, 21, 035703 CrossRef CAS
.
- Y. J. Liu, Z. Y. Zhang, Q. Zhao, R. A. Dluhy and Y. P. Zhao, J. Phys. Chem. C, 2009, 113, 9664–9669 CAS
.
- Y. J. Liu, H. Y. Chu and Y. P. Zhao, J. Phys. Chem. C, 2010, 114, 8176–8183 CAS
.
- V. Parekh, E. Chunsheng, D. Smith, A. Ruiz, J. C. Wolfe, P. Ruchhoeft, E. Svedberg, S. Khizroev and D. Litvinov, Nanotechnology, 2006, 17, 2079–2082 CrossRef CAS
.
- C. L. Haynes, A. D. McFarland, L. L. Zhao, R. P. Van Duyne, G. C. Schatz, L. Gunnarsson, J. Prikulis, B. Kasemo and M. Kall, J. Phys. Chem. B, 2003, 107, 7337–7342 CrossRef CAS
.
- B. L. Broglin, A. Andreu, N. Dhussa, J. A. Heath, J. Gerst, B. Dudley, D. Holland and M. El-Kouedi, Langmuir, 2007, 23, 4563–4568 CrossRef CAS
.
- X. S. Gao, L. F. Liu, B. Birajdar, M. Ziese, W. Lee, M. Alexe and D. Hesse, Adv. Funct. Mater., 2009, 19, 3450–3455 CrossRef CAS
.
- X. R. Li, Y. Q. Wang, G. J. Song, Z. Peng, Y. M. Yu, X. L. She, J. Sun, J. J. Li, P. D. Li, Z. F. Wang and X. F. Duan, J. Phys. Chem. C, 2010, 114, 6914–6916 CAS
.
- L. Liu, S. H. Yoo and S. Park, Chem. Mater., 2010, 22, 2681–2684 CrossRef CAS
.
- D. F. Gu, H. Baumgart, T. M. Abdel-Fattah and G. Namkoong, ACS Nano, 2010, 4, 753–758 CrossRef CAS
.
- C. J. Choi, Z. D. Xu, H. Y. Wu, G. L. Liu and B. T. Cunningham, Nanotechnology, 21, 415301 CrossRef
.
- Y. G. Guo, L. J. Wan, C. F. Zhu, D. L. Yang, D. M. Chen and C. L. Bai, Chem. Mater., 2003, 15, 664–667 CrossRef CAS
.
- S. H. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 CrossRef CAS
.
- S. H. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS
.
- T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. Bin Na, J. Am. Chem. Soc., 2001, 123, 12798–12801 CrossRef CAS
.
- H. Chik, J. Liang, S. G. Cloutier, N. Kouklin and J. M. Xu, Appl. Phys. Lett., 2004, 84, 3376–3378 CrossRef CAS
.
- C. H. Bae, S. M. Park, S. C. Park and J. S. Ha, Nanotechnology, 2006, 17, 381–384 CrossRef CAS
.
- G. W. Meng, A. Y. Cao, J. Y. Cheng, A. Vijayaraghavan, Y. J. Jung, M. Shima and P. M. Ajayan, J. Appl. Phys., 2005, 97, 064303 CrossRef
.
- D. D. Li, R. S. Thompson, G. Bergmann and J. G. Lu, Adv. Mater., 2008, 20, 4575–4578 CrossRef CAS
.
- W. C. Yoo and J. K. Lee, Adv. Mater., 2004, 16, 1097–1101 CrossRef CAS
.
- G. Xia, S. Wang and S.-J. Jeong, Nanotechnology, 2010, 21, 485302 CrossRef
.
- X. Y. Yuan, G. S. Wu, T. Xie, Y. Lin and L. D. Zhang, Nanotechnology, 2004, 15, 59–61 CrossRef CAS
.
- Q. L. Xu, G. W. Meng, X. B. Wu, Q. Wei, M. G. Kong, X. G. Zhu and Z. Q. Chu, Chem. Mater., 2009, 21, 2397–2402 CrossRef CAS
.
- K. Chie, M. Fujiwara, Y. Fujiwara and Y. Tanimoto, J. Phys. Chem. B, 2003, 107, 14374–14377 CrossRef CAS
.
- L. X. Sun, Q. W. Chen, Y. Tang and Y. Xiong, Chem. Commun., 2007, 2844–2846 RSC
.
- L. X. Sun and Q. W. Chen, J. Phys. Chem. C, 2009, 113, 2710–2714 CAS
.
- M. L. Tian, S. Y. Xu, J. G. Wang, N. Kumar, E. Wertz, Q. Li, P. M. Campbell, M. H. W. Chan and T. E. Mallouk, Nano Lett., 2005, 5, 697–703 CrossRef CAS
.
- J. Wang, Q. W. Chen, C. Zeng and B. Y. Hou, Adv. Mater., 2004, 16, 137–140 CrossRef CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: details of any supplementary information available should be included here. See DOI: 10.1039/c2ra00915c/ |
| This journal is © The Royal Society of Chemistry 2012 |
