Designable fabrication of flower-like SnS2 aggregates with excellent performance in lithium-ion batteries†
Jianmin
Ma
a,
Danni
Lei
a,
Xiaochuan
Duan
b,
Qiuhong
Li
a,
Taihong
Wang
a,
Anmin
Cao
*c,
Yuhua
Mao
d and
Wenjun
Zheng
*b
aKey Laboratory for Micro-Nano Optoelectronic Devices of Ministry of Education, State Key Laboratory for Chemo/Biosensing and Chemometrics, Hunan University, Changsha, P. R. China
bDepartment of Materials Chemistry, Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin, P. R. China. E-mail: zhwj@nankai.edu.cn
cKey Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, Beijing, P. R. China. E-mail: caoanmin@iccas.ac.cn
dShenzhen Capchem Technology Co., LTD, Shenzhen, P. R. China
First published on 14th March 2012
Abstract
Flower-like SnS2 aggregates have been prepared and show better electrochemical performances than nanoplates, which could be attributed to their structural matrix with the functions of facilitating the Li+ diffusion and electron transfer as well as reducing the crumbling and cracking of the electrode.
As one of the most important tin-based materials, tin disulfide (SnS2) has attracted more and more attention in lithium-ion batteries. SnS2 has a layered CdI2-type structure, which can facilitate the intercalation of lithium ions and also tolerate the volume change during the lithiation–delithiation process. Thus, much effort has been made to synthesize SnS2 with an improved electrochemical performance for their use as alternative anode materials.1–5 In particular, nanostructured SnS2 with controlled morphologies has become favorable due to their unique properties such as larger surface area, greater accessibility to electrolyte, faster transportation of Li+, and accelerated phase transitions.6,7 However, compared to those well-reported simple SnS2 nanostructures including nanoplates, nanowires, nanorods, nanobelts and nanotubes,4,5,9–13 there are only limited reports on the fabrication of SnS2 electrodes with hierarchical structures, which are known to have the advantage of not only facilitating the Li+ diffusion and electron transfer, but also effectively reducing the crumbling and cracking of electrodes2,3,6–8 Thus, it is necessary to explore novel protocols for the synthesis of SnS2 with hierarchical structures.
In this work, we have successfully developed a method to synthesize hierarchical SnS2 structures (i.e., flower-like aggregates) under hydrothermal conditions without any additives. By controlling the reaction conditions, we can also fabricate SnS2 nanomaterials from pure nanoplates to flower-like aggregates. Our method has two excellent merits: i) it is designable for this synthetic method, based on our knowledge of the principle of the lowest energy in our synthetic system; ii) it is a simple synthetical protocol with no need for any organic additive, compared with some other reported technologies.2,3,14,15 Our design of flower-like SnS2 aggregates is also promising for the synthesis of other inorganic nanomaterials with hierarchical structures. Moreover, electrochemical experiments demonstrate that the flower-like SnS2 aggregates have an excellent electrochemical performance in lithium storage and can act as an alternative anode material in lithium-ion batteries.
Fig. 1a and b display typical SEM images of the as-synthesized products. It's clear that such as-prepared SnS2 samples (Fig. 1a) were mainly nanoplates with a regular shape. A magnified SEM image in Fig. 1b reveals that most of the SnS2 nanoplates are hexagons with an angle of 120° between adjacent sides. Also, the thickness of each nanosheet is around 10 to 15 nm, as revealed from the cross section of those nanoplates standing up on their edges. The SnS2 nanoplates are further studied by HRTEM for further structural information. As shown in Fig. 1c, the lattice spacing of the surface of a representative nanoplate is measured to be 0. 31 nm, which reveals that the surfaces are composed of (100) planes.14 The XRD pattern in Fig. 1d is in good agreement with the standard SnS2 one (JSPDF: 23-0677), implying a high purity of the final product.
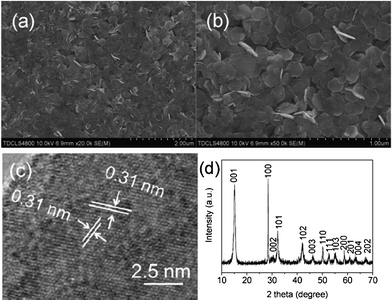 | ||
| Fig. 1 (a and b) low and high magnification SEM images; (c) HRTEM image and (d) XRD pattern of spherical SnS2 microstructures. | ||
SnS2 is built on a layered CdI2-type structure,16 which in turn governs its growth and facilitates the formation of nanoplates. Therefore, it is easy to grow SnS2 into plate-like shapes under proper conditions, as shown in the above-mentioned synthesis. Our effort also shows the possibility to prepare a nanoplate-based structural matrix, which has been favored for improved electrochemical performance.2,3,6–8 Thus, one important task for our synthetical effort is to focus on the controlled formation of SnS2 into a well-organized hierarchical structure. Herein, we employed the strategy of concentration control to facilitate the assembly of preformed building blocks of nanoplates. This strategy is based on our knowledge of the principle of the lowest energy in a synthetic system: the preformed nanoplates will tend to aggregate together through heterogeneous nucleation. Fig. 2 shows the SEM images of the as-prepared flower-like aggregates. It's clear that an increase of concentration will lead to the formation of a large amount of flower-like SnS2 aggregates assembled by nanoplates. In an enlarged SEM image, as shown in Fig. 2c, the thickness of the nanoplates from these SnS2 aggregates is in the range from 10 to 15 nm. The XRD pattern in Fig. 2d identifies such SnS2 aggregates as pure SnS2 (JSPDF: 23-0677).
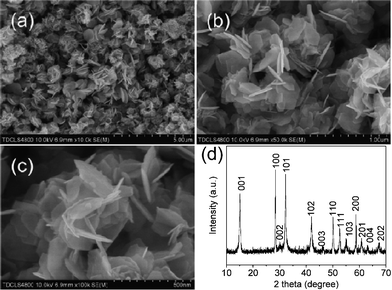 | ||
| Fig. 2 (a) low magnification SEM image; (b and c) high magnification SEM images and (d) XRD pattern of flower-like SnS2 aggregates. | ||
To further understand the growth mechanism of the flower-like SnS2 aggregates, we carried out the time-dependent synthesis and investigated the products at different stages by SEM and XRD techniques. From the XRD patterns in Fig. S1,† the SnS2 phase will form pretty fast, after reacting for just 2 h, which is especially different from the reaction system with thiourea as the sulfur source.16 This can be attributed to the difference in the hydrolysis speed of the different sulfur sources.17 Fig. S2a–c shows the morphological evolution from the nanoparticles formed at the beginning, and to the final flower-like aggregates.† Usually two different processes are involved during this morphological transformation: nucleation and Ostwald ripening. The nanoparticles (Fig. S2a for 2 h) firstly grew into aggregates with irregular shapes (Fig. S2b for 5 h) through anisotropic nucleation following its crystal growth habit,4 and then evolved into flower-like SnS2 aggregates (Fig. S2c for 20 h) via Ostwald ripening.† In addition, two possible reasons can be explained why the higher precursor concentration favored the formation of flower-like SnS2 aggregates. Firstly, when the precursor concentration is increased, the nucleation rate increases and it may result in different particle sizes as shown by Fig. 1a and b and 2a–c. It seems that the flower-like sample exhibits a wider particle size distribution and varied surface energy for individual nanoplate particles. For particles with a high aspect ratio, the atoms on the surface and edges are not fully coordinated. In larger particles, the lack of coordination has a greater effect at the edges, thus results in edge-to-surface interactions, which produces the as-obtained flower-like structure.18 Secondly, at higher precursor concentration, the presence of more electrolytes could promote coagulation and three-dimensional aggregation effects,19 thus facilitating the formation of flower-like SnS2 aggregates. In summary, the result is in accordance with our designed SnS2. The whole process is illustrated in Scheme 1 for a better understanding.
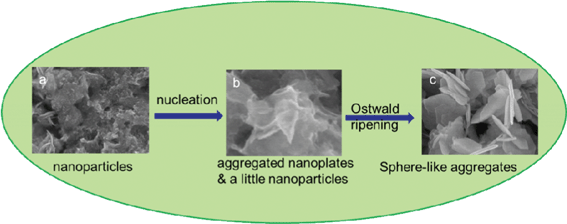 | ||
| Scheme 1 Schematic formation process of stacked SnS2 nanoplates. | ||
As reported by many groups,2,3,6–8 assembled structures have better electrochemical performance than simple nanostructures. After the preparation of different SnS2 structures, we also test the performances of the as-prepared samples with different morphologies to understand the structure-performance relationship of our SnS2 nanomaterials. Fig. 3a shows the charge–discharge curves of the two electrodes made from the SnS2 nanoplates and flower-like SnS2 aggregates. All the data are collected between 0.05 V and 1.2 V vs. Li+/Li at a current density of 100 mA g−1. In the first discharge process, the initial charge–discharge curves display a similar pattern, which implies that they have the same electrochemical processes,1,17 expressed by the following:
| SnS2 + 4Li+ + 4e− →Sn + 2Li2S | (1) |
| Sn + xLi+ + xe− ↔ LixSn | (2) |
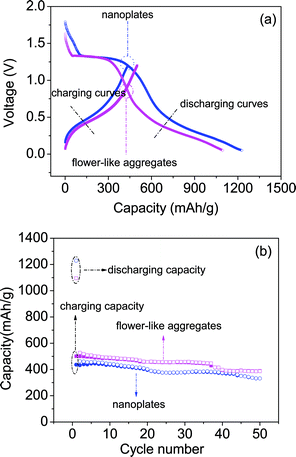 | ||
| Fig. 3 (a) initial charge–discharge curves and (b) cycling performances for the two SnS2 samples tested at a current density of 100 mA g−1 in the potential range of 0.05–1.2 V. | ||
It's interesting that both SnS2 electrodes show high initial discharging capacities (1228 mAh g−1 for nanoplates and 1095 mAh g−1 for flower-like aggregates). This might be due to the high surface area of the SnS2 nanoplates, which could promote side-reactions with the electrolytes and the lithium intercalation of the SnS2 layers without phase decomposition.20
The effects of different morphologies on the electrochemical performance can be further manifested through the further cycling of the prepared electrodes.21,22 Herein, we investigated the charge–discharge cycling performance for the two electrodes. In Fig. 3b, it is clear that the aggregated SnS2 has a higher capacity. The initial capacity of the flower-like SnS2 aggregates is 1095 mAh g−1, and decreases gradually to 387 mAh g−1 after the 50th cycle, while for the pure SnS2 nanoplates, their capacity decreases from 1288.5 to 331.7 mAh g−1. Moreover, it can be seen that the efficiency was kept at a value of about 95% from the 2nd cycle. In conclusion, our flower-like SnS2 aggregates could be potentially used as an anode material of lithium-ion batteries which is obviously superior to other reported chalcogenides (Sb2Se3 and Bi2S3).23,24
In summary, we have successfully controlled the formation of flower-like SnS2 aggregates based on the reaction system for preparing simple nanoplates. The mechanism of such a shape transformation has been discussed in detail. The electrochemical results shows that the electrochemical properties of SnS2 electrodes are morphologically dependent and flower-like SnS2 aggregates show a high discharge capacity and improved cyclability, which endows them with great potential in lithium-ion batteries.
This work was financially supported by the National Natural Science Foundation of China (No. 20971070, 21073095 and 21003041) and MOE (No. RT0927).
References
- C. X. Zhai, N. Du, H. Zhang and D. R. Yang, Chem. Commun., 2011, 47, 1270–1272 RSC.
- J. T. Zai, K. X. Wang, Y. Z. Su, X. F. Qian and J. S. Chen, J. Power Sources, 2011, 196, 3650–3654 CrossRef CAS.
- S. Liu, X. M. Yin, L. B. Chen, Q. H. Li and T. H. Wang, Solid State Sci., 2010, 12, 712–718 CrossRef CAS.
- J. Seo, J. Jang, S. Park, C. Kim, B. Park and J. Cheon, Adv. Mater., 2008, 20, 4269–4273 CrossRef CAS.
- T. J. Kim, C. Kirn, D. Son, M. Choi and B. Park, J. Power Sources, 2007, 167, 529–535 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- Y. G. Guo, J. S. Hu and L. J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS.
- J. M. Ma, X. C. Duan, J. B. Lian, T. I. Kim, P. Peng, X. D. Liu, Z. F. Liu, H. B. Li and W. J. Zheng, Chem.–Eur. J., 2010, 16, 13210–13217 CrossRef CAS.
- W. M. Du, D. H. Deng, Z. T. Han, W. Xiao, C. Bian and X. F. Qian, CrystEngComm, 2011, 13, 2071–2076 RSC.
- B. Ludi, I. Olliges-Stadler, M. D. Rossell and M. Niederberger, Chem. Commun., 2011, 47, 5280–5282 RSC.
- Y. C. Zhang, Z. N. Du, S. Y. Li and M. Zhang, Appl. Catal., B, 2010, 95, 153–159 CrossRef CAS.
- A. Yella, E. Mugnaioli, M. Panthoefer, H. A. Therese, U. Kolb and W. Tremel, Angew. Chem., Int. Ed., 2009, 48, 6426–6430 CrossRef CAS.
- D. K. Ma, W. Zhang, Q. Tang, R. Zhang, W. C. Yu and Y. T. Qian, J. Nanosci. Nanotechnol., 2005, 5, 806–809 CrossRef CAS.
- J. T. Zai, X. F. Qian, K. X. Wang, C. Yu, L. Q. Tao, Y. L. Xiao and J. S. Chen, CrystEngComm, 2012, 14, 1364 RSC.
- H. Ke, W. Luo, G. Cheng, X. Tian and Z. Pi, Micro Nano Lett., 2009, 4, 177–180 CAS.
- J. M. Ma, D. N. Lei, L. Mei, X. C. Duan, Q. H. Li, T. H. Wang and W. J. Zheng, CrystEngComm, 2012, 14, 832–836 RSC.
- J. M. Ma, J. Q. Yang, L. F. Jiao, T. H. Wang, J. B. Lian, X. C. Duan and W. J. Zheng, Dalton Trans., 2011, 40, 10100–10108 RSC.
- J. A. Gursky, S. D. Blough, C. Luna, C. Gomez, A. N. Luevano and E. A. Gardner, J. Am. Chem. Soc., 2006, 128, 8376–8377 CrossRef CAS.
- Z. P. Xu, G. Stevenson, C. Q. Lu and G. Q. Lu, J. Phys. Chem. B, 2006, 110, 16923–16929 CrossRef CAS.
- C. Julien and C. Perez-Vicente, Solid State Ionics, 2006, 89, 337–343 CrossRef.
- J. M. Ma, J. B. Lian, X. C. Duan, X. D. Liu and W. J. Zheng, J. Phys. Chem. C, 2010, 114, 10671–10676 CAS.
- Z. Y. Wang, D. Y. Luan, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 4738–4741 CrossRef CAS.
- J. M. Ma, Y. P. Wang, Y. J. Wang, P. Peng, J. B. Lian, X. C. Duan, Z. F. Liu, X. D. Liu, Q. Chen, T. I. Kim, G. Yao and W. J. Zheng, CrystEngComm, 2011, 13, 2369–2374 RSC.
- J. M. Ma, Z. F. Liu, J. B. Lian, X. C. Duan, T. I. Kim, P. Peng, X. D. Liu, Q. Chen, G. Yao and W. J. Zheng, CrystEngComm, 2011, 13, 3072–3079 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra00965j |
| This journal is © The Royal Society of Chemistry 2012 |
