Continuous hydrogen production by immobilized cultures of Thermotoga neapolitana on an acrylic hydrogel with pH-buffering properties†
Maria Assunta
Basile
a,
Cosimo
Carfagna
b,
Pierfrancesco
Cerruti
*b,
Giovanna
Gomez d'Ayala
b,
Angelo
Fontana
c,
Agata
Gambacorta
c,
Mario
Malinconico
b and
Laura
Dipasquale
*c
aDepartment of Biochemistry and Biophysics, Second University of Naples, Via L. De Crecchio, 7 - 80100, Naples, Italy
bInstitute of Polymer Chemistry and Technology (CNR), Via Campi Flegrei, 34 - 80078, Pozzuoli (Na), Italy. E-mail: cerruti@ictp.cnr.it; Fax: +39-081-8675230; Tel: +39 081-8675214
cInstitute of Biomolecular Chemistry (CNR), Via Campi Flegrei, 34 - 80078, Pozzuoli (Na), Italy. E-mail: ldipasquale@icb.cnr.it; Fax: +39-081-8041770; Tel: +39 081-8675096
First published on 15th March 2012
Abstract
This communication reports on the continuous biohydrogen production by Thermotoga neapolitana cells immobilized on a stable cationic hydrogel bearing amine groups. This hydrogel was designed to perform two functional activities: to promote adhesion of T. neapolitana cells, and to buffer pH changes in the bacterial cultures. Repeated fed-batch cultures showed an average hydrogen production rate and yield of 50.6 mL L−1 h−1 and 3.3 mol H2/mol glucose, respectively. To the best of our knowledge, this is the first report detailing the immobilization of this bacterial strain on a polymeric support.
In recent years there has been a considerable effort to increase the use of renewable sources of energy with an aim to limit economic dependence on conventional fossil fuel sources, and to face environmental problems caused by the consequent increase of anthropogenic CO2 in the atmosphere. Renewable energies come from natural sources that can be naturally replenished, such as sunlight, wind, rain, tides, geothermal heat and biomass. The 93 billion litres of biofuel produced worldwide in 2009 displaced the equivalent of an estimated 68 billion litres of gasoline, equal to about 5% of world gasoline production.1
In this scenario there is an important role for biohydrogen (bioH2) as a clean, CO2-neutral energy vector, because it can be converted to electricity very efficiently, producing only water as a waste product. In order to produce biohydrogen sustainably, bacterial fermentation of organic substrates in the absence of light, defined as dark fermentation, is a promising way in consideration of high biogas yields and wide variety of substrates that can be used to feed the cultures.2–5
Thermotoga neapolitana is an extreme thermophilic, anaerobic, heterotrophic, Gram-negative bacterium isolated from a marine hot-spring near Lucrino (Gulf of Pozzuoli, Italy). It belongs to the Thermotogales order, which includes thermophilic bacteria showing an external thick peptidoglycan membrane called toga.6 We have recently shown that T. neapolitana reaches hydrogen yield values close to the theoretical 4 molecules of H2 per molecule of glucose that is fermented by the classic Embden–Meyerhoff pathway.7 This result suggests that T. neapolitana could be of great interest for biohydrogen production. However, the process is currently hampered by a number of biochemical and biotechnological drawbacks, including stability of culture conditions, difficulty to set up continuous reactors and inhibition of hydrogen production as result of pH lowering due to the accumulation of acidic catabolites (e.g. acetic acid).5,8,9 Indeed hydrogen production by fermentative processes is associated with the release of organic acids as fermentation end products in a culture medium; the accumulation of these metabolites inhibits hydrogen production as the pH drops to values lower than those suitable for cell growth. It was demonstrated that when the pH is adjusted with a buffer solution, cultures completely metabolize the carbon source, e.g. glucose.7,10 Another possible way to achieve pH buffering of the culture medium for optimizing H2 production through dark fermentation is by the use of a suitable pH-responsive immobilization matrix.11
Therefore, cell immobilization by means of a properly designed matrix is envisaged as a possible solution to the technical hitches mentioned above,12,13 as well as the propaedeutic step to the achievement of long-term operation.
Bacteria attachment on immobilizing supports is influenced by cell surface charge, along with matrix structure and hydrophobicity. Generally, bacterial cells have a net negative charge on the cell wall,14 however the magnitude of this charge varies from strain to strain, and for this reason cationic supports are expected to significantly enhance cell adhesion.15
Here we report on the continuous biohydrogen production by T. neapolitana cells immobilized on a stable cationic polymeric hydrogel bearing amine groups, namely poly(hydroxyethylmethacrylate-co-methacryloxyethyl)trimethylammonium chloride-co-dimethylaminoethyl acrylate (HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). This hydrogel performed two functional activities: to promote adhesion of T. neapolitana cells, and to buffer pH changes of the bacterial cultures, making an external pH correction unnecessary.
3). This hydrogel performed two functional activities: to promote adhesion of T. neapolitana cells, and to buffer pH changes of the bacterial cultures, making an external pH correction unnecessary.
Scheme 1 shows the chemical structure of the copolymer HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, evidencing the presence of the three comonomer unities: 2-hydroxyethylmethacrylate (HEMA) provides hydrophilicity and also ensures mechanical stability, [2-(Methacryloyloxy)ethyl]trimethylammonium chloride (METAC) gives cationic character to the hydrogel, while the pH-buffering activity is related to 2-(dimethylamino)ethyl acrylate (DMAEA).
3, evidencing the presence of the three comonomer unities: 2-hydroxyethylmethacrylate (HEMA) provides hydrophilicity and also ensures mechanical stability, [2-(Methacryloyloxy)ethyl]trimethylammonium chloride (METAC) gives cationic character to the hydrogel, while the pH-buffering activity is related to 2-(dimethylamino)ethyl acrylate (DMAEA).
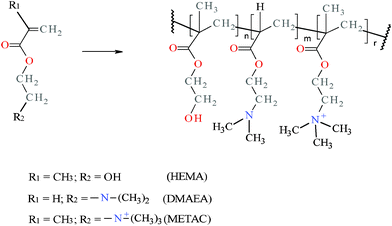 | ||
| Scheme 1 Chemical structures of the acrylic monomers HEMA, METAC, DMAEA, and the HMD copolymer hydrogels. | ||
The presence of the constituent comonomers in the hydrogel was evaluated by NMR and FTIR spectroscopy, since it has been reported that the reactivity of acrylic monomers depends on the kind of pendant groups.16 Solid-state 13C-NMR spectroscopy was employed, as the hydrogel was not soluble due to its crosslinked structure. 13C-NMR spectra of pure poly(2-hydroxyethyl methacrylate) (PHEMA) and poly(2-hydroxyethyl methacrylate-co-[2-(methacryloyloxy)ethyl]trimethylammonium chloride), in a HEMA to METAC molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (HM 8
3 (HM 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), were also recorded in order to facilitate peak attribution (Fig. S1, ESI†). Due to severe peak overlapping, direct quantification of the respective mole fraction in HMD 5
3), were also recorded in order to facilitate peak attribution (Fig. S1, ESI†). Due to severe peak overlapping, direct quantification of the respective mole fraction in HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was not possible.
3 was not possible.
However, it was found through peak integration that METAC reacted quantitatively in the polymerization of HM 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, that is, in the experimental conditions used, the reactivity of the two monomers was comparable.17 Taking into account this result, and comparing the values of the ratio between the peak area of saturated carbons (80 to 40 ppm) and that of methyls bound to the polymer backbone (30 to 10 ppm) (Fig. S2, ESI†) for all the spectra, it was estimated that about 65% of the reactant DMAEA was incorporated into the polymer. However, further studies are required to better elucidate this aspect.
3, that is, in the experimental conditions used, the reactivity of the two monomers was comparable.17 Taking into account this result, and comparing the values of the ratio between the peak area of saturated carbons (80 to 40 ppm) and that of methyls bound to the polymer backbone (30 to 10 ppm) (Fig. S2, ESI†) for all the spectra, it was estimated that about 65% of the reactant DMAEA was incorporated into the polymer. However, further studies are required to better elucidate this aspect.
Since immobilization capacity of the supporting carrier and diffusion of medium nutrients towards the matrix are related to its hydrophilicity,18 the ability of the hydrogel HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to absorb and desorb water was specifically evaluated. The presence of trimethylammonium ions on METAC allowed the HMD copolymer to absorb a large amount of water (450%) with a net five-fold increase in comparison with PHEMA.11 In addition, no hysteresis in the swelling properties was found after repeated water adsorption–desorption cycles.
3 to absorb and desorb water was specifically evaluated. The presence of trimethylammonium ions on METAC allowed the HMD copolymer to absorb a large amount of water (450%) with a net five-fold increase in comparison with PHEMA.11 In addition, no hysteresis in the swelling properties was found after repeated water adsorption–desorption cycles.
According to literature reports, polymeric materials containing tertiary amines can be predicted as effective buffering agents, capable of neutralizing the pH drop due to the release of organic acids during the fermentation process.19,20
The buffering effect of HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was evaluated by a titration assay with acetic acid at room temperature (Fig. 1). The medium acidification was reduced in the presence of the hydrogel that buffered the pH to around 4.5, even after addition of high amounts of the organic acid, and was shown to be suitable as an active matrix for buffering culture medium acidity due to fermentative acidic metabolites.
3 was evaluated by a titration assay with acetic acid at room temperature (Fig. 1). The medium acidification was reduced in the presence of the hydrogel that buffered the pH to around 4.5, even after addition of high amounts of the organic acid, and was shown to be suitable as an active matrix for buffering culture medium acidity due to fermentative acidic metabolites.
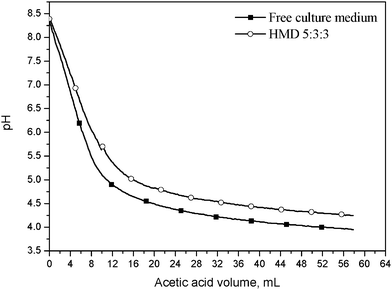 | ||
Fig. 1 Titration curves of the standard culture medium, with and without the immobilizing hydrogel HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3. | ||
The stability of the hydrogel in the culture conditions was evaluated by incubating the material for 20 days at 80 °C, in a medium depleted of yeast extract, tryptone and glucose, and then analyzing the chemical changes that occurred by FTIR spectroscopy (Fig. S3, ESI†).
Based on the assumptions that different carbonyl species (i.e. carboxylic acids and esters) have the same molar absorptivity and the carbonyl content did not change upon incubation, changes in absorption of the other functional groups were ratioed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak area (at 1720 cm−1) that was taken as reference. After incubation, the total –O–H absorption (broad band at 3600–3000 cm−1) decreased by about 20%, whereas the carboxylate groups absorption (band at 1580 cm−1), which was also detected in the freshly prepared HMD 5
O absorption peak area (at 1720 cm−1) that was taken as reference. After incubation, the total –O–H absorption (broad band at 3600–3000 cm−1) decreased by about 20%, whereas the carboxylate groups absorption (band at 1580 cm−1), which was also detected in the freshly prepared HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, increased by about 50%. The areas of the absorption peaks related to alkylammonium, –C–H and –C–N– bonds (peaked at 1460, 2950 and 950 cm−1, respectively)21,22 remained unchanged. These experimental findings suggest that the polymer hydrogel can undergo hydrolysis, resulting in a partial removal of the side chains bearing hydroxyl groups in HEMA. The net result is an increase in the concentration of carboxylic acids, which are stable as carboxylate salts in the buffered solution. The presence of carboxylate groups in the as-prepared hydrogel are likely to be explained by the presence of acrylic acid as an impurity in the reactants. It is noteworthy that the DMAEA and METAC units were shown to be less susceptible to hydrolysis, suggesting that cationic character and pH-buffering activity can be maintained, even after prolonged incubation times.
3, increased by about 50%. The areas of the absorption peaks related to alkylammonium, –C–H and –C–N– bonds (peaked at 1460, 2950 and 950 cm−1, respectively)21,22 remained unchanged. These experimental findings suggest that the polymer hydrogel can undergo hydrolysis, resulting in a partial removal of the side chains bearing hydroxyl groups in HEMA. The net result is an increase in the concentration of carboxylic acids, which are stable as carboxylate salts in the buffered solution. The presence of carboxylate groups in the as-prepared hydrogel are likely to be explained by the presence of acrylic acid as an impurity in the reactants. It is noteworthy that the DMAEA and METAC units were shown to be less susceptible to hydrolysis, suggesting that cationic character and pH-buffering activity can be maintained, even after prolonged incubation times.
Based on the above reported results, the HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 hydrogel was utilized as a pH-buffering immobilization matrix in a fed-batch culture bioreactor with T. neapolitana cells; control tests were performed without supporting material.
3 hydrogel was utilized as a pH-buffering immobilization matrix in a fed-batch culture bioreactor with T. neapolitana cells; control tests were performed without supporting material.
Several bio-chemical parameters of cultures with immobilized and freely suspended cells were evaluated to assess the influence of the presence of HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 on bacterial growth.
3 on bacterial growth.
When a T. neapolitana suspended culture was run without pH buffering, about 50% of the initial glucose was still present even after 48 h of incubation, with a hydrogen production rate of 23.9 mL L−1 h−1. On the other hand, pH buffering at a value of 7.5, achieved by a periodic addition of a sodium hydroxide solution, led to complete glucose consumption in the same time range.
Table 1 shows the comparison of growth parameters between the pH-buffered suspended and immobilized T. neapolitana cultures after 21 h of incubation.
| Culture method | ΔpH | Glucose consumption (%) | Hydrogen production rate, mL L−1 h−1 | Hydrogen production yield, mol H2/mol glu |
|---|---|---|---|---|
| a The pH value change was determined prior to pH correction after 16 h of incubation. | ||||
| Suspended | 4.2 ± 0.3 (2.9 ± 0.2a) | 50.0 ± 1.5 | 49.5 ± 2.5 | 3.6 ± 0.2 |
| Immobilized | 2.3 ± 0.2 | 75.0 ± 2.1 | 39.8 ± 2.1 | 2.6 ± 0.2 |
It can be observed that in the presence of the supporting hydrogel the change in the pH value (ΔpH) was 2.3. For the pH-buffered suspended culture a direct comparison of pH change with the immobilized culture was not possible, due to NaOH addition after 16 h. Nevertheless, the sum of the pH changes in the time ranges 0 to 16 h (before NaOH addition) and 16 to 21 h (after NaOH addition) was 4.2. It is also noteworthy to note that the observed pH change prior to the NaOH addition was 2.9, which was higher than that measured in the immobilized culture after 21 h. These results confirm that the tertiary amine groups on the hydrogel backbone were able to buffer the acidity of the culture medium due to the release of fermentative metabolites.
It can be also noted that in the buffered suspended culture, 50.0% glucose was metabolized in 21 h, with a hydrogen production rate of 49.5 mL L−1 h−1. On the other hand, in the case of the immobilized cells, about 75.0% of the substrate was consumed in 21 h, even if lower hydrogen production was measured. Nevertheless, it is likely that part of the glucose diffused into the polymeric amine-branched support, thus determining an apparently lower concentration of the sugar in solution. Moreover, it can be hypothesized that the presence of the immobilizing hydrogel could influence the T. neapolitana cells’ metabolism, probably shifting it towards cell biomass synthesis as well as metabolic pathways independent from H2 production. In order to support this hypothesis, the acetate–lactate ratio was measured for the buffered suspended and the immobilized cultures. Indeed, acetate production is directly related to hydrogen synthesis, while production of end-products, such as lactate, affect the efficiency of glucose conversion to hydrogen by dark fermentation.3,7,23,24 It was found that this ratio was equal to 3.0 and 2.8 for the buffered suspended and immobilized cells system, respectively. This result is in agreement with the observed lower hydrogen production in the immobilized culture.
To get an insight into the stability and recyclability of the supporting hydrogel, preliminary tests on repeated fed-batch operations were carried out on the immobilized system. In Table 2, the comparison of growth parameters between the repeated fed-batch runs of immobilized T. neapolitana cultures after 21 h of incubation is shown.
| Number of repeated fed-batch runs | ΔpH | Glucose consumption (%) | Hydrogen production rate, mL L−1 h−1 | Hydrogen production yield, mol H2/mol glu |
|---|---|---|---|---|
| I cycle | 2.3 ± 0.1 | 58.5 ± 0.9 | 51.1 ± 2.0 | 3.0 ± 0.3 |
| II cycle | 1.8 ± 0.4 | 56.1 ± 2.2 | 50.7 ± 1.7 | 3.4 ± 0.1 |
| III cycle | 2.1 ± 0.3 | 53.3 ± 1.6 | 50.0 ± 1.9 | 3.4 ± 0.2 |
From the table, it can be noted that the values of the growth parameters are fairly constant upon hydrogel recycling, thus suggesting that stability and durability of the immobilization system is suitable for long-term operations. In particular, the average pH change over three repeated fed-batch runs was 2.1, which is comparable to the value observed for the first immobilization run.
Repeated fed-batch operations performed by the removal of the exhausted medium and replacement with fresh culture medium had a positive effect on hydrogen production, both in terms of the rate and yield. In the repeated runs, we observed an average increase of the biogas production rate by 27.1% with respect to the first immobilization run, reaching a mean value of 50.6 mL L−1 h−1. Similarly, the hydrogen yield increased, showing values close to the theoretical 4 moles H2 per mole of glucose consumed. These values are comparable with those reported in the literature.10,25–27 The positive effect on hydrogen production observed for the repeated fed-batch runs can also be related to the increase in the acetate–lactate ratio, that was found to be on average 3.6.
Overall, these results suggest that repeated fed-batch runs enabled acclimation of the immobilized cells, which reached a steady state with improved culture performance.
The stable adhesion of T. neapolitana cells onto the polymeric support after each repeated fed-batch run in a bioreactor was confirmed by Scanning Electron Microscopy (SEM). Fig. 2 shows micrographs of the hydrogel surface after I, II and III repeated cell growth cycles. In the case of cycle I (Fig. 2a), few isolated cells were present on the surface, massive matrix colonization could not be observed and no apparent biofilm was developed. Successive fermentation runs on HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 significantly increased the number of cells bound to the hydrogel surface. Indeed observation of the HMD 5
3 significantly increased the number of cells bound to the hydrogel surface. Indeed observation of the HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 surface after the III repeated growth cycle showed a very different picture (Fig. 2c): numerous cells were aggregated onto most of the matrix’s surface and they were evenly distributed and connected to each other forming a multi-layer in a biofilm-like arrangement. Moreover, bacterial cells were covered by a continuous layer, so they were not clearly morphologically visible. It can be hypothesized that T. neapolitana produced exopolysaccharides useful for cell attachment to the hydrogel surface, in accordance with several reports which described exopolysaccharide production by Thermotogales under optimal growth conditions.6
3 surface after the III repeated growth cycle showed a very different picture (Fig. 2c): numerous cells were aggregated onto most of the matrix’s surface and they were evenly distributed and connected to each other forming a multi-layer in a biofilm-like arrangement. Moreover, bacterial cells were covered by a continuous layer, so they were not clearly morphologically visible. It can be hypothesized that T. neapolitana produced exopolysaccharides useful for cell attachment to the hydrogel surface, in accordance with several reports which described exopolysaccharide production by Thermotogales under optimal growth conditions.6
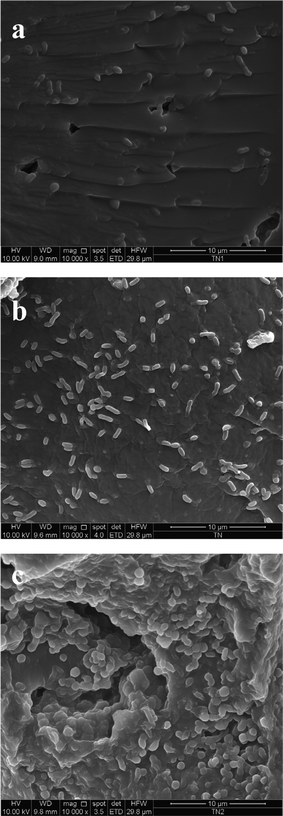 | ||
Fig. 2 SEM microphotographs of the HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 surface with immobilized T. neapolitana cells after I (a), II (b) and III (c) repeated fed-batch growth cycles. 3 surface with immobilized T. neapolitana cells after I (a), II (b) and III (c) repeated fed-batch growth cycles. | ||
In conclusion, an amine-bearing acrylic hydrogel (HMD 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was prepared and used as a support in a bioreactor for the stable immobilization of the hydrogen-producing bacterium T. neapolitana. A series of repeated fed-batch experiments using glucose as the substrate demonstrated that cells immobilized on this hydrogel showed constant hydrogen production and glucose consumption, comparable to that of buffered suspended cultures and higher than that of unbuffered suspended cultures. The positive effect is mainly due to the intrinsic pH-buffering properties of the hydrogel. Notably, repeated fed-batch runs improved cell attachment capacity, and increased hydrogen production. Therefore, polyacrylic cationic hydrogels with amine groups are suitable for immobilization applications in thermophilic, long-term H2 production, which is an essential prerequisite to developing the biotechnological potential of biohydrogen. Towards this view and to extend the applicability of this immobilization material, studies are underway for the testing of a longer-term series of fed-batch experiments and continuous stirred fermentation.
3) was prepared and used as a support in a bioreactor for the stable immobilization of the hydrogen-producing bacterium T. neapolitana. A series of repeated fed-batch experiments using glucose as the substrate demonstrated that cells immobilized on this hydrogel showed constant hydrogen production and glucose consumption, comparable to that of buffered suspended cultures and higher than that of unbuffered suspended cultures. The positive effect is mainly due to the intrinsic pH-buffering properties of the hydrogel. Notably, repeated fed-batch runs improved cell attachment capacity, and increased hydrogen production. Therefore, polyacrylic cationic hydrogels with amine groups are suitable for immobilization applications in thermophilic, long-term H2 production, which is an essential prerequisite to developing the biotechnological potential of biohydrogen. Towards this view and to extend the applicability of this immobilization material, studies are underway for the testing of a longer-term series of fed-batch experiments and continuous stirred fermentation.
Acknowledgements
The authors thank the Italian Minister for University and Scientific Research (MIUR) for financially supporting this research under contract F.I.S.R. n. 1756 “Metodologie innovative per la produzione di idrogeno da processi biologici”. The work was also supported by the Italian Minister of Agriculture (MIPAAF) under the project “BIORES-BIOdiesel programmi specifici per il REcupero di Sottoprodotti.”References
-
(a) Renewables 2011 Global Status Report, REN2011 - Renewable energy policy network for the 21st century, http://energy4farms.eu/wp-content/uploads/2011/08/Global-REN-2011.pdf;
(b) R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 RSC
; (c) N. Ramroop Singh, in The Biofuels Handbook, ed. J. G. Speight, Royal Society of Chemistry, Cambridge, 2011, ch. 5, pp. 160–198 Search PubMed
.
- D. Das and T. N. Veziroğlu, Int. J. Hydrogen Energy, 2001, 26, 13–28 CrossRef CAS
.
- P. C. Hallenbeck and J. R. Benemann, Int. J. Hydrogen Energy, 2002, 27, 1185–1193 CrossRef CAS
.
-
J. H. Reith, R. H. Wijffels and H. Barten, Dutch Biological Hydrogen Foundation, 2003, ISBN:90-9017165-7
.
-
(a) D. B. Levin, L. Pitt and M. Love, Int. J. Hydrogen Energy, 2004, 29, 173–185 CrossRef CAS
; (b) L. Lu, N. Ren, X. Zhao, H. Wang, D. Wu and D. Xing, Energy Environ. Sci., 2011, 4, 1329–1336 RSC
.
- R. Huber and M. Hannig, Prokaryotes, 2006, 7, 899–922 CrossRef
.
- G. d'Ippolito, L. Dipasquale, F. M. Vella, I. Romano, A. Gambacorta, A. Cutignano and A. Fontana, Int. J. Hydrogen Energy, 2010, 35, 2290–2295 CrossRef CAS
.
- S. E. Craven, Appl. Environ. Microbiol., 1988, 54, 2042–2048 CAS
.
- B. F. Liu, N. Q. Ren, D. F. Xing, J. Ding, G. X. Zheng, W. Q. Guo, J. F. Xu and G. J. Xie, Bioresour. Technol., 2009, 100, 2719–2723 CrossRef CAS
.
- S. A. Munro, S. H. Zinder and L. P. Walker, Biotechnol. Prog., 2009, 25, 1035–1042 CrossRef CAS
.
- M. A. Basile, L. Dipasquale, A. Gambacorta, M. F. Vella, A. Calarco, P. Cerruti, M. Malinconico and G. Gomez d'Ayala, Bioresour. Technol., 2010, 101, 4386–4394 CrossRef CAS
.
-
(a) A. A. Tsygankov, Y. Hirata, M. Miyake, Y. Asada and J. Miyake, J. Ferment. Bioeng., 1994, 77, 575–578 CrossRef CAS
; (b) A. Léonard, J. C. Rooke, C. F. Meunier, H. Sarmento, J.-P. Descy and B.-L. Su, Energy Environ. Sci., 2010, 3, 370–377 RSC
; (c) A. Léonard, P. Dandoy, E. Danloy, G. Leroux, C. F. Meunier, J. C. Rooke and B.-L. Su, Chem. Soc. Rev., 2011, 40, 860–885 RSC
.
- K. K. Rao and D. O. Hall, J. Mar. Biotechnol., 1996, 4, 10–15 CAS
.
- J. S. Dickson and M. Koohmaraie, Appl. Environ. Microbiol., 1989, 55, 832–836 CAS
.
- F. Berlutti, F. Rosso, P. Bosso, F. Giansanti, M. Ajello, A. De Rosa, E. Farina, G. Antonini and P. Valenti, J. Biomed. Mater. Res., 2003, 67A, 18–25 CrossRef CAS
.
- W. Jaeger, J. Bohrisch and A. Laschewsky, Prog. Polym. Sci., 2010, 35, 511–577 CrossRef CAS
.
- C. Wandrey, J. Hernández-Barajas and D. Hunkeler, Adv. Polym. Sci., 1999, 145, 123–182 CrossRef CAS
.
- Y. F. Chu, C. H. Hsu, P. K. Soma and Y. M. Lo, Bioresour. Technol., 2009, 100, 3167–3174 CrossRef CAS
.
- A. M. Funhoff, C. F. van Nostrum, G. A. Koning, N. M. E. Schuurmans-Nieuwenbroek, D. J. A. Crommelin and W. E. Hennink, Biomacromolecules, 2004, 5, 32–39 CrossRef CAS
.
- E. Ranucci, P. Ferruti, E. Lattanzio, A. Manfredi, M. Rossi, P. R. Mussini, F. Chiellini and C. Bartoli, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6977–6991 CrossRef CAS
.
- T. Zeegers-Huyskens and G. Bator, Vib. Spectrosc., 1996, 13, 41–49 CrossRef CAS
.
- X. Peng and X. Peng, J. Appl. Polym. Sci., 2006, 101, 1381–1385 CrossRef CAS
.
- K. Nath and D. Das, Appl. Microbiol. Biotechnol., 2004, 65, 520–529 CrossRef CAS
.
- T. de Vrije, A. E. Mars, M. A. W. Budde, M. H. Lai, C. Dijkema and P. de Waard, Appl. Microbiol. Biotechnol., 2007, 74, 1358–1367 CrossRef CAS
.
- S. A. Van Ooteghem, A. Jones, D. van der Lelie, B. Dong and D. Mahajan, Biotechnol. Lett., 2004, 26, 1223–1232 CrossRef CAS
.
- N. T. Eriksen, T. M. Nielsen and N. Iversen, Biotechnol. Lett., 2008, 30, 103–109 CrossRef CAS
.
- T. A. D. Nguyen, S. J. Han, J. P. Kim, M. S. Kim and S. J. Sim, Bioresour. Technol., 2010, 101, S38–S41 CrossRef CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, solid-state 13C-NMR and FTIR spectra. See DOI: 10.1039/c2ra01025a/ |
| This journal is © The Royal Society of Chemistry 2012 |
