Synthesis of the novel trimeric benzamides—potential inhibitors of protein–protein interactions†
Oleg V.
Kulikov
a and
Andrew D.
Hamilton
*ab
aDepartment of Chemistry, Yale University, New Haven, CT, 06511, USA
bDepartment of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, U.K. E-mail: Andrew.hamilton@chem.ox.ac.uk; andrew.hamilton@admin.ox.ac.uk; andrew.hamilton@yale.edu; Tel: +44 (0)1865 270242
First published on 2nd February 2012
Abstract
The synthesis of the family of new non-natural trimeric oligobenzamides (potential inhibitors of protein–protein interactions) bearing the following groups (R = isobutyl, isopropyl, CH2CONH2, (CH2)4NH2, benzyl) that mimic different amino acid residues (leucine, valine, asparagine, lysine, phenylalanine) was accomplished through an iterative (n + 1) elongation approach that includes reduction of the NO2-group (Pd/C, H2) and subsequent amide coupling in the presence of Mukaiyama's reagent.
Introduction
Over the years there has been a considerable interest in developing non-peptidic small-molecule alpha helix mimetics1 due to their potential ability to disrupt protein–protein interactions (PPIs). So far, a large variety of preorganized scaffolds2–6 (indanes, terphenyls, pyridyl-pyridones, pyridazines, polycyclic ethersetc.) where the substituents of the scaffold mimic the distance and angular orientation of amino-acid side-chains on an alpha-helix have been explored. Work from this lab3–6 and others2b–l has extended the design, and chemical synthesis of small-molecule inhibitors of therapeutically relevant protein targets important in cancer and infectious diseases, Alzheimer's, type II diabetes, HIV, etc.One important scaffold class has derived from oligomers of pyridyl or aryl benzamide with different side chain groups. Herein, we report the synthesis and self-assembly in the solid state of some novel benzamide backbone-based oligomers incorporating the various alkyl fragments that mimic amino acids such as leucine, valine, asparagine, lysine, phenylalanine. We further reasoned that introducing the branched alkyl side chains (isopropyl, isobutyl, CH2-3-pentyl) might strengthen the hydrophobic contacts between the targeted protein domain and the mimetic molecule. Water solubility of these derivatives can be improved by the incorporation of side chains bearing positive charges (e.g.ammonium or guanidinium groups mimicking Lys or Arg residues). These molecules have the potential to mimic three residues at positions i, i+4 and i+7 on one face of an alpha-helix. Current work represents the logical extension of our earlier efforts3,4 on the synthesis of alpha-helix mimetics derived from oligopyridylamide scaffolds.
Results and discussion
The synthesis and X-ray studies of trimeric benzamides.
The synthesis of the molecules in this series has been accomplished through a simple (n + 1) elongation that involves amide coupling with the corresponding aryl acid in the presence of 2-chloro-1-methylpyridinium iodide (Mukaiyama's reagent) followed by reduction (Pd/C, H2) of the nitro-component. Attempts to generate an acyl chloride of 3 by using oxalyl chloride and then reacting with amine 4 yielded a complex mixture of products from which diamide 5 was isolated. The lability of the Boc-protecting group under acidic conditions precluded formation of desired dimer 6, however the latter could be easily prepared when Mukaiyama's reagent was used as the coupling agent (Scheme 1).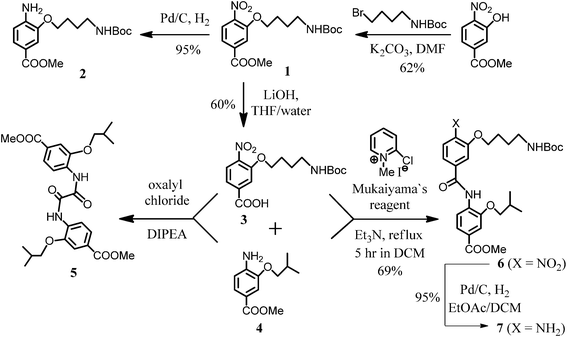 | ||
| Scheme 1 Synthesis of amide oligomers precursors. | ||
Coupling of amino-dimer 7 with various aryl acid monomers 3, 8a–g described earlier,7,8,9 afforded trimeric arylamides 9a–h. The Boc-protecting group was then successfully removed by addition of trifluoroacetic acid (TFA) to give the corresponding amino-derivatives 10a–h in nearly quantitative yields (Scheme 2).
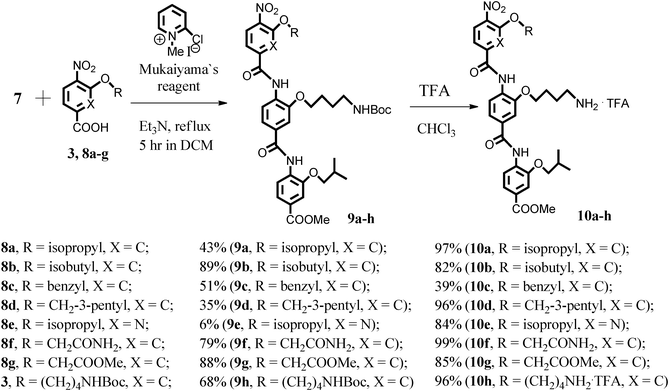 | ||
| Scheme 2 Synthesis and deprotection of aryl trimers. | ||
To test the scope of this approach, we synthesized a number of structural analogues.
The preparation of the isomeric trimer molecules, 13 and 16b, bearing the (CH2)4NH2-group employed a simple reordering of the synthetic steps described above (Scheme 3).
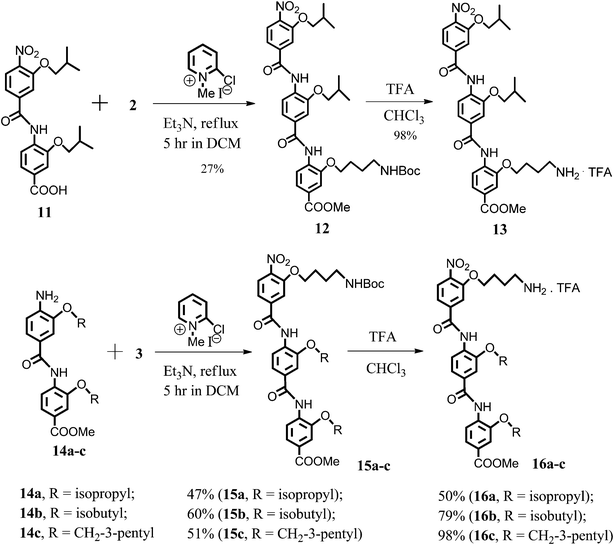 | ||
| Scheme 3 Synthesis of the structural analogues 13, 16a–c. | ||
The synthetic route leading to trimer 21—structural isomer of compound 10a—started from dimer 18 which was then hydrogenated on Pd/C to the respective amino-derivative 19. The further condensation of 19 and 3-isobutyloxy-4-nitrobenzoic acid (8b) led to the formation of the desired N-Boc-protected arylamide 20. The latter was Boc-deprotected in the moderate yield of 49% to furnish the final trimer 21 as its TFA salt (Scheme 4).
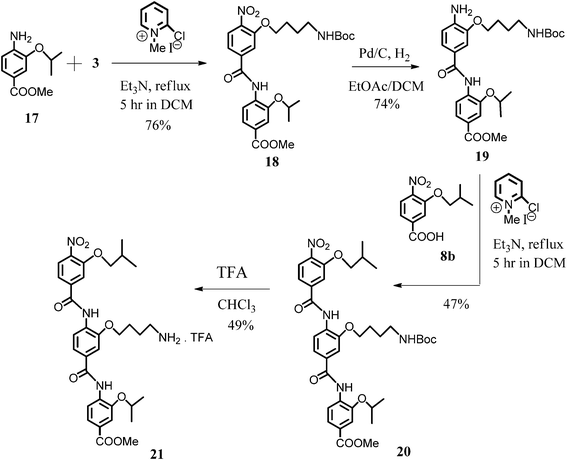 | ||
| Scheme 4 Synthesis of the trimer 21. | ||
Investigation of the solid state structures of dimer 7 and trimer 9f (Fig. 1, S1†) showed that the molecules are packed in a curved conformation with the side chains projected on one face. Notably, in the case of trimer 9f the N-terminal alkyl chain is disordered, thus only the molecular identity and connectivity could be confirmed. Partial crystal packing diagrams of 9f are depicted in the Figures S2–S6.† The curvature of the scaffolds (defined as the angle formed by the line drawn from the 1,4-aryl carbons linked to the amide carbonyl to the amide-N linked carbons) were similar, −158.8° for a NH2-dimer 7 (159.2° and 161.6° for a trimer 9f), to those described earlier9 for analogous dimeric amide foldamers. The intramolecular H-bond between the amide NH and the oxygen of the alkoxy group (dNH…O = 1.99–2.15 Å) appears to stabilize the curved backbone of both oligoamides.
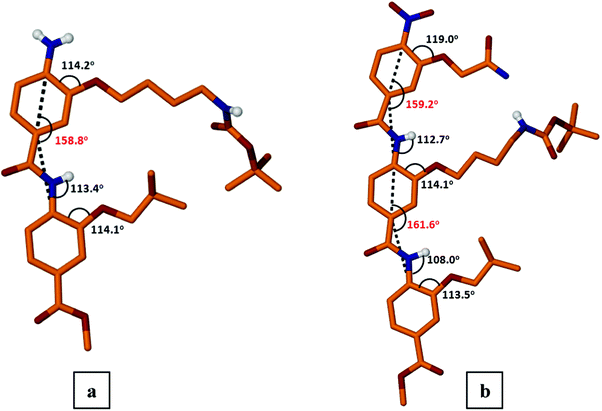 | ||
| Fig. 1 Molecular structures of dimer 7 (panel a) and trimer 9f (panel b). Carbon hydrogen atoms not shown for clarity. | ||
In the crystal (Fig. 2), molecules of dimer 7 interact through intermolecular H-bonds that involve CO and NH2-groups (NH2…O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 2.54 Å).
C, 2.54 Å).
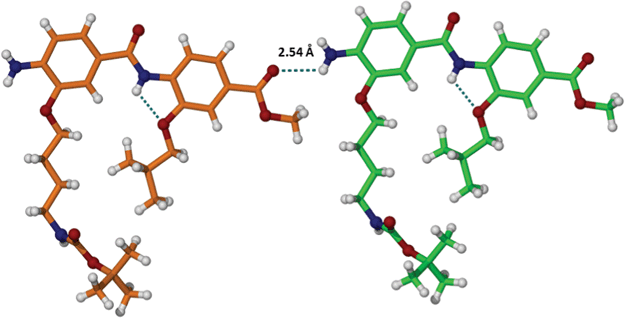 | ||
| Fig. 2 Intermolecular H-bonding between COOMe and NH2 groups of the neighbouring dimer 7 molecules. | ||
In addition (Fig. 3) intermolecular H-bonds for NH2-dimer 7 are formed through the interaction of NH2…O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Boc), 2.12 Å (panel a) and CONH…O
C(Boc), 2.12 Å (panel a) and CONH…O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH, 2.05 Å (panel b). More details on dimer 7 crystal packing are given in supplementary section (Fig. S7, S8†).
C–NH, 2.05 Å (panel b). More details on dimer 7 crystal packing are given in supplementary section (Fig. S7, S8†).
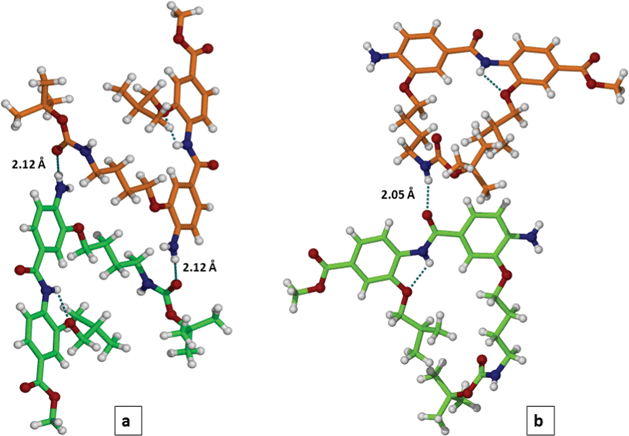 | ||
| Fig. 3 Formation of an intermolecular H-bonding network between dimer 7 molecules in crystal: amine/amide (panel a) and amide/amide (panel b) contacts. | ||
X-ray studies on NH2-dimers 14a, 14c showed that the molecules have the same curved backbone as 7 with the side chains projected on the different faces (Fig. 4).
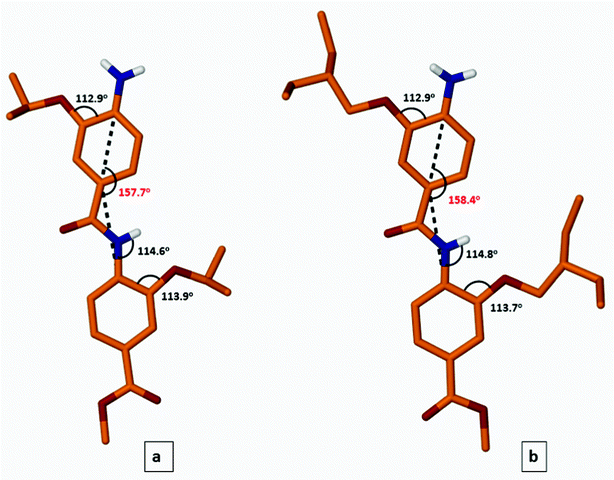 | ||
| Fig. 4 Molecular structures of dimer 14a (panel a) and dimer 14c (panel b). Carbon hydrogen atoms not shown for clarity. | ||
Partial crystal packing diagram of 14a showcasing the formation of intermolecular H-bonds is depicted in Fig. 5 (See also Fig. S9, S10 for more details on packing†).
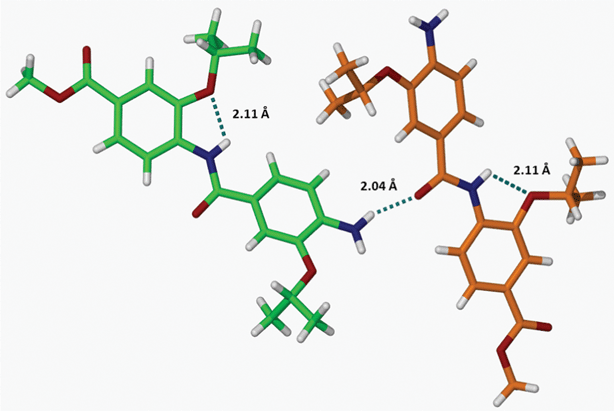 | ||
| Fig. 5 Formation of an intermolecular H-bonding network between dimer 14a molecules through the interaction of NH2 and CONH groups. | ||
Similarly, the molecules of 14c are involved in a hydrogen bonding network as a result of the interaction between aromatic amino and the methoxycarbonyl groups (NH2…O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OMe, 2.03 Å, Fig. 6, S11–S15†):
C–OMe, 2.03 Å, Fig. 6, S11–S15†):
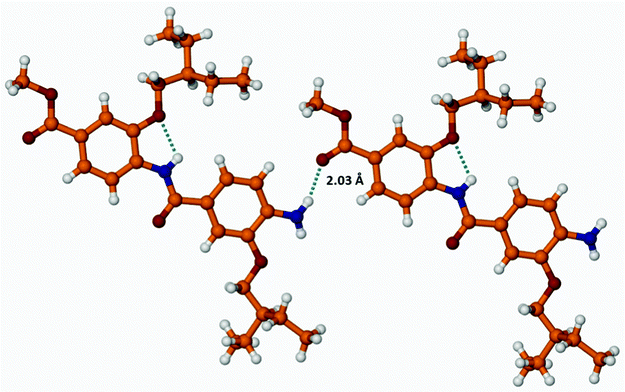 | ||
Fig. 6 Formation of an intermolecular H-bonds between dimer 14c molecules through the interaction of NH2–and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OMe groups. C–OMe groups. | ||
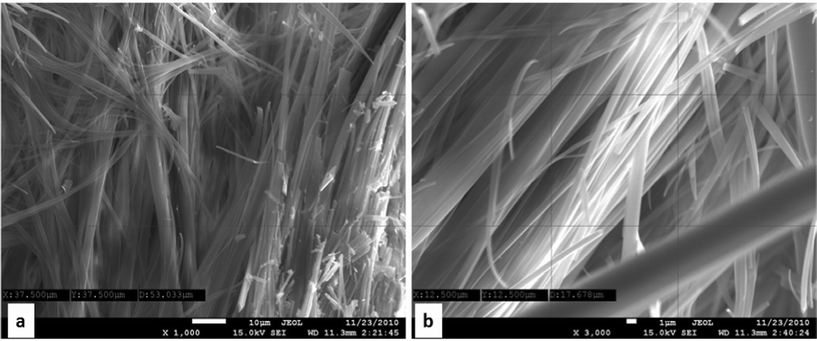 | ||
| Fig. 7 SEI images of dimer 7: the formation of microfibrillar network (panel a, scale bar 10 μm, magnification 1k; panel b, scale bar 1 μm, magnification 3k) . | ||
An extremely long aggregate (> 600 μm) was detected in the micrograph shown in the Fig. 8a. The behavior of the corresponding NO2-dimer 6 resembles the aggregates observed in the case of dimer 7, however, the fibers derived from 6 tend to be considerably shorter (under 100 μm, Fig. 8b, S16, S17†).
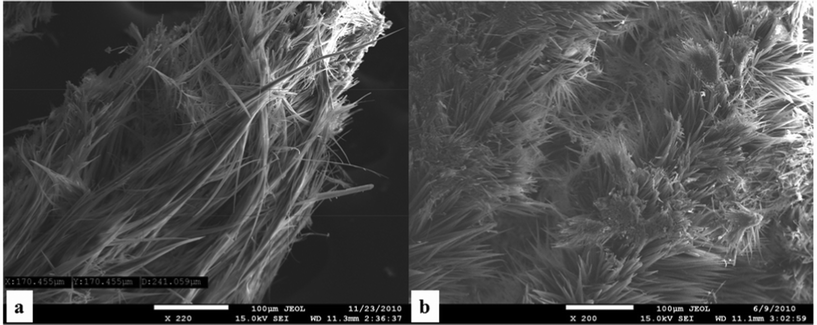 | ||
| Fig. 8 SEI images of NH2-dimer 7 (panel a) vs.NO2-dimer 6 (panel b); scale bar 100 μm. | ||
Intermolecular hydrogen bonding likely plays a crucial role in the organization of molecules into supramolecular aggregates, however the contribution of hydrophobic side chain/side chain interactions9 should not be underestimated. Thus, the specific fibrillar arrangements observed by using SEI analysis likely reflect both intermolecular influences. The visualization by electron microscopy of the synthetic peptides and artificial foldamers self-assembling into supramolecular fibrous structures has been reported.10
Conclusions
In summary, a small library of trimeric benzamides—potential alpha-helix mimetics—was prepared through Mukayama's reagent mediated amide coupling to expand the range of key amino acids residues applicable to the targeting of protein–protein interactions. Inspection of the solid state structures of three amino-dimers and the benzamide trimer molecule confirmed that they adopt a curved conformation with intramolecular H-bonding between the amide NH and the alkoxy oxygen of the neighboring aromatic fragment (dNH…O ∼2 Å) and showed the standard values of angles of inclination (∼160°). In the crystal, dimer molecules are organized into a hydrogen bonded network. Electron micrographs of the dimers showed a fibrous aggregated morphology (average width of fibers ranges from 1 to 3 μm, length 10–50 μm). This behavior likely results from the extensive intermolecular interactions observed in the crystal.Acknowledgements
We wish to thank Dr Chris Incarvito for the assistance with X-ray crystallographic analysis and Dr Jim Eckert for the acquisition of electron microscopy images. This work was supported by the National Institute of Health (CA67771) and the National Science Foundation (CHE-0750357).References
- (a) Foldamers: Structure, Properties and Applications, (ed.: S. Hecht, I. Huc), WILEY-VCH, Weinheim, 2007 Search PubMed; (b) J. Iriondo-Alberdi, K. Laxmi-Reddy, B. Bouguerne, C. Staedel and I. Huc, Chem. Bio. Chem, 2010, 11, 1679 CrossRef CAS; (c) B. Baptiste, C. Douat-Casassus, K. Laxmi-Reddy, F. Godde and I. Huc, J. Org. Chem., 2010, 75, 7175 CrossRef CAS; (d) X. Zhao and Zh-T. Li, Chem. Commun., 2010, 46, 1601 RSC; (e) G. N. Tew, R. W. Scott, M. L. Klein and W. F. DeGrado, Acc. Chem. Res., 2010, 43, 30 CrossRef CAS; (f) Q. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grélard, H. Jiang and I. Huc, Science, 2011, 331, 1172 CrossRef CAS; (g) N. Delsuc, S. Massip, J.-M. Léger, B. Kauffmann and I. Huc, J. Am. Chem. Soc., 2011, 133, 3165 CrossRef CAS.
- (a) C. G. Cummings and A. D. Hamilton, Curr. Opin. Chem. Biol., 2010, 14, 341 CrossRef CAS; (b) P. Restorp and J.Jr. Rebek, Bioorg. Med. Chem. Lett., 2008, 18, 5909 CrossRef CAS; (c) J. P. Plante, T. Burnley, B. Malkova, M. E. Webb, S. L. Warriner, T. A. Edwards and A. J. Wilson, Chem. Commun., 2009, 5091 RSC; (d) L. Moisan, S. Odermatt, N. Gombosuren, A. Carella and J.Jr. Rebek, Eur. J. Org. Chem., 2008, 1673 CrossRef CAS; (e) S. Marimganti, M. N. Cheemala and J.-M. Ahn, Org. Lett., 2009, 11, 4418 CrossRef CAS; (f) P. Tosovska and P. S. Arora, Org. Lett., 2010, 12, 1588 CrossRef CAS; (g) V. Haridas, Eur. J. Org. Chem., 2009, 5112 CrossRef CAS; (h) A. J. Wilson, Chem. Soc. Rev., 2009, 38, 3289 RSC; (i) A. Shaginian, L. R. Whitby, S. Hong, I. Hwang, B. Farooqi, M. Searcey, J. Chen, P. K. Vogt and D. L. Boger, J. Am. Chem. Soc., 2009, 131, 5564 CrossRef CAS; (j) J. H. Lee, Q. Zhang, S. Jo, S. C. Chai, M. Oh, W. Im, H. Lu and H.-S. Lim, J. Am. Chem. Soc., 2011, 133, 676 CAS; (k) M. De Giorgi, A.-S. Voisin-Chiret, J. S-O. Santos, F. Corbo, C. Franchini and S. Rault, Tetrahedron, 2011, 67, 6145 CrossRef CAS; (l) T.-K. Lee and J.-M. Ahn, ACS Comb. Sci., 2011, 13, 107 CrossRef CAS.
- I. Saraogi, C. D. Incarvito and A. D. Hamilton, Angew. Chem., Int. Ed., 2008, 47, 9691 CrossRef CAS.
- I. Saraogi, J. A. Hebda, J. Becerril, L. A. Estroff, A. D. Miranker and A. D. Hamilton, Angew. Chem. Int. Ed., 2010, 49, 736 CAS.
- S. Fletcher and A. D. Hamilton, Curr. Opin. Chem. Biol., 2005, 9, 632 CrossRef CAS.
- (a) I. Saraogi and A. D. Hamilton, Biochem. Soc. Trans., 2008, 36, 1414 CrossRef CAS; (b) I. Saraogi and A. D. Hamilton, Chem. Soc. Rev., 2009, 38, 1726 RSC.
- V. Pomel, J. C. Rovera, A. Godard, F. Marsais and G. Queguiner, J. Heterocycl. Chem., 1996, 33, 1995 CrossRef CAS.
- J. Plante, F. Campbell, B. Malkova, C. Kilner, S. L. Warriner and A. J. Wilson, Org. Biomol. Chem., 2008, 6, 138 CAS.
- O. V. Kulikov, C. D. Incarvito and A. D. Hamilton, Tetrahedron Lett., 2011, 52, 3705 CrossRef CAS.
- (a) S. Rele, Y. Song, R. P. Apkarian, Zh. Qu, V. P. Conticello and E. L. Chaikof, J. Am. Chem. Soc., 2007, 129, 14780 CrossRef CAS; (b) L. Huang, R. A. McMillan, R. P. Apkarian, B. Pourdeyhimi, V. P. Conticello and E. L. Chaikof, Macromolecules, 2000, 33, 2989 CrossRef CAS; (c) G. Angelici, G. Falini, H.-J. Hofmann, D. Huster, M. Monari and C. Tomasini, Chem.–Eur. J., 2009, 15, 8037 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 851713–851714. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01153k |
| This journal is © The Royal Society of Chemistry 2012 |
