Morphology control of solution processable small molecule bulk heterojunction solar cellsviasolvent additives†
Jin Kuen
Park
a,
Chunki
Kim
a,
Bright
Walker
ac,
Thuc-Quyen
Nguyen
*a and
Jung Hwa
Seo
*b
aCenter for Polymers and Organic Solids, Department of Chemistry & Biochemistry, University of California, Santa Barbara, California 93106, United States. E-mail: quyen@chem.ucsb.edu
bDepartment of Materials Physics, College of Natural Science, Dong-A University, Busan, 604-714, Republic of Korea. E-mail: seojh@dau.ac.kr
cInterdisciplinary School of Green Energy, Ulsan National Institute of Science and Technology, Ulsan, 689-798, Republic of Korea.
First published on 6th February 2012
Abstract
A new donor–acceptor type small molecule with dithienothiophene and diketopyrrolopyrrole units has been synthesized for application in organic solar cells. In order to optimize the nanoscale morphology of the small molecule solar cells, additives such as 1,8-diiodooctane (DIO) and 1-chloronaphthalene (CN) are used. The use of CN significantly suppresses molecular aggregation and allows suitable phase separation, enhancing power conversion efficiency from 0.5% to 2.2%.
Organic solar cells based on solution processed bulk heterojunction (BHJ) systems are a promising technology for low-cost energy conversion devices.1–4 Progress with BHJ solar cells involving blends of conjugated copolymers and [6,6]-phenyl C71butyric acid methyl ester (PC71BM) has been rapid, in particular, the development of narrow band gap copolymers comprising alternating donor–acceptor (D–A) structural units have enabled highly efficient light absorption across the visible spectrum.5–8 Considerable research effort has been expended on the improvement of polymer/fullerene-based solar cells, leading to power conversion efficiencies (PCE) over 8%.
Solution processable conjugated small molecules have recently been recognized as a new class of donor material for BHJ solar cells, which have advantages including intrinsic monodispersity, good crystallinity and easy purification by column chromatography.9,10 However, small molecules often have a tendency to self-assemble into large, ordered domains, so that it is challenging to obtain the desired nanoscale morphology for efficient charge carrier generation and transport. Thus, the highest reported PCEs of solution-processed small molecule BHJ cells have so far reached only ∼6%.11–14 To achieve ideal phase separation in small molecule BHJ solar cells, several device process techniques have been utilized such as thermal annealing,10solvent vapor annealing,15 and choice of solvents.16,17 Recently, in polymer/fullerene based BHJ cells, the use of solvent additives has been demonstrated as an efficient way for controlling phase separation without thermal annealing.18,19 However, few applications of the additives process have been reported for the small molecule BHJ devices.20
In this contribution, we report the synthesis of a novel donor–acceptor (D–A) type small molecule consisting of a dithienothiophene (DTT) and two diketopyrrolopyrrole (DPP) chromophores (DTTDPP). Solution processed BHJ solar cells were fabricated using PC71BM as an electron acceptor and 1,8-diiodooctane (DIO) and 1-chloronaphthalene (CN) were used to control nanoscale morphology, where the DIO and CN were chosen as processing additives due to their higher boiling points than the parent solvent and selective solubility for the donor and the acceptor.18,19 We demonstrate that the use of CN in small molecule blends can be an effective strategy to achieve appropriate nanoscale morphology and leads to an increase in the PCE from 0.5% to 2.2%
In Scheme 1, compound 3 and 4 were synthesized as reported in the literature with some modification.21–24 The compound 5 was afforded via a microwave-assisted Stille cross coupling reaction and brominated for further modification with 2-trimethylstannyl-5-hexylthiphene to obtain the final structure. Detailed procedures are described in the ESI.†
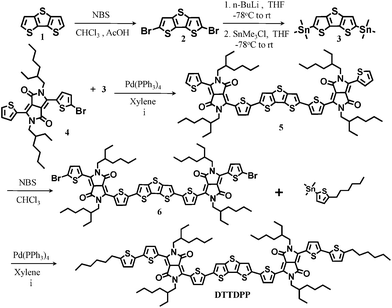 | ||
| Scheme 1 Synthetic procedure and molecular structures. | ||
Fig. 1a shows normalized UV-Vis absorption spectra of DTTDPP solution in chloroform and the film on a glass substrate. Peak maxima at 670 nm in both solution and film are nearly identical. However, the absorption onset of the film is red-shifted by 70 nm relative to the solution due to increased aggregation and electronic delocalization in the solid state. The optical gap (Eg) was estimated from the onset (827 nm) of the UV-Vis spectrum. Subtracting Eg from the HOMO obtained by ultraviolet photoelectron spectroscopy (UPS) yields an approximate LUMO value (UPS data is available in the ESI†). Thus, the energy levels of the materials in the solid state can be determined. The frontier orbital energies are summarized in Fig. 1b. According to the concept of band alignment between electron donors and electron acceptors in BHJ devices, one might expect that photoinduced electron transfer between DTTDPP and PC71BM would be efficient because of the large difference (0.7 eV) between the LUMO energies of DTTDPP and PC71BM.4,13
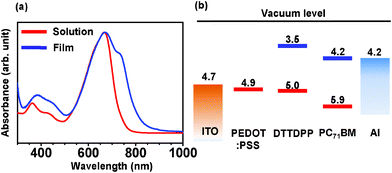 | ||
| Fig. 1 (a) Absorption spectra of dilute DTTDPP solution in chloroform (red line) and film on glass substrate (blue line). (b) Energy level diagram of DTTDPP:PC71BM solar cells. | ||
BHJ devices were fabricated by spin-casting DTTDPP:PC71BM mixtures from chloroform solution onto substrates consisting of glass/ITO/poly(ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). Fig. 2 shows current density–voltage (J–V) characteristics of the DTTDPP:PC71BM devices as a function of blend ratio. There is no significant change in the PCEs as the blend ratio is varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1
0.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; each ratio exhibits similar a short-circuit current (Jsc), open circuit voltage (Voc), and fill factor (FF).
2; each ratio exhibits similar a short-circuit current (Jsc), open circuit voltage (Voc), and fill factor (FF).
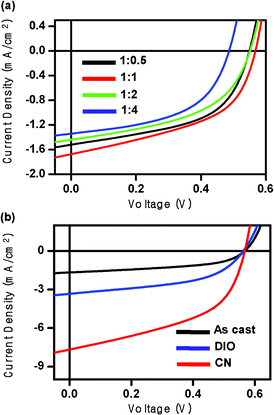 | ||
Fig. 2
J–V characteristics of DTTDPP:PC71BM solar cells (a) as a function of blend ratio and (b) DTTDPP:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solar cells without and with 1% additives under AM 1.5G irradiation at 100 mW cm−2. 1) solar cells without and with 1% additives under AM 1.5G irradiation at 100 mW cm−2. | ||
To examine the influence of solvent additives on device performance, we used 1 vol% DIO and CN in the active layer. We note that DTTDPP:PC71BM films processed with DIO and CN showed no residual I and Cl in X-ray photoelectron spectra (ESI†), suggesting that the additive volatilizes during film processing. The use of additives significantly enhances device performance, especially in the Jsc (Fig. 2b). Table 1 provides a summary of device parameters. Although 1% DIO doubled the Jsc from 1.67 to 3.33 mA cm−2, the FF decreased. The use of CN, however, results in markedly enhanced Jsc and a constant FF, leading to a big improvement in PCE from 0.65% to 2.19%. This dramatic increase in Jsc suggests that solvent additives improve the efficiency of charge carrier photo-generation and/or transport, presumably through changes in the film morphology.
| Blend ratio | Additive | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
none | 0.55 | 1.51 | 51 | 0.42 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
none | 0.57 | 1.67 | 50 | 0.48 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
none | 0.56 | 1.45 | 50 | 0.41 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
none | 0.49 | 1.34 | 51 | 0.33 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DIO | 0.57 | 3.33 | 34 | 0.65 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CN | 0.57 | 7.67 | 50 | 2.19 |
To gain insight into the surface morphology of the BHJ layers, atomic force microscopy (AFM) was performed. Fig. 3 represents surface topographic images (top) and height profiles (bottom) of DTTDPP:PC71BM as a function of blend ratio. Uneven and round-shaped features with varying average diameters from 170 nm to 265 nm were observed as the amount of PC71BM increases from 0.5 wt% to 1 wt%. Such large aggregated domains could decrease exciton dissociation efficiency since the exciton diffusion length for most organic semiconductors is estimated to be in the range of 5–20 nm.25
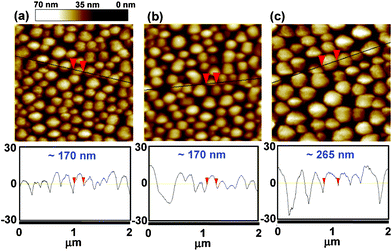 | ||
Fig. 3 Surface topographic AFM images (size : 2 μm × 2 μm) of DTTDPP:PC71BM devices with blend ratio of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, (b) 1 0.5, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, and (c) 1 0.7, and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The surface morphology changes of DTTDPP:PC71BM after adding 1% DIO and CN are shown in Fig. 4. Top images display the surface topographies, while phase images follow in the bottom. There is some degree of aggregation in the DTTDPP:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) films prepared using chloroform solution with 1% DIO (Fig. 4a and c). In contrast, the addition of 1% CN appears to suppress aggregation, leading to smooth surface and desired phase separation as shown in Fig. 4b and d. This observation helps explain the high Jsc obtained from the device processed from CN additive. These results demonstrate that more ideal percolating networks are achieved using CN additive. Although the possible effect of the additives on crystallinity of these features remains poorly understood at this stage, a more detailed investigation of mechanisms by which additives affect the DTTDPP:PC71BM BHJ system is currently under way.
1) films prepared using chloroform solution with 1% DIO (Fig. 4a and c). In contrast, the addition of 1% CN appears to suppress aggregation, leading to smooth surface and desired phase separation as shown in Fig. 4b and d. This observation helps explain the high Jsc obtained from the device processed from CN additive. These results demonstrate that more ideal percolating networks are achieved using CN additive. Although the possible effect of the additives on crystallinity of these features remains poorly understood at this stage, a more detailed investigation of mechanisms by which additives affect the DTTDPP:PC71BM BHJ system is currently under way.
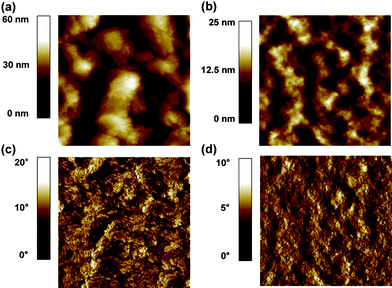 | ||
Fig. 4 Surface topographic AFM images (size : 2 μm × 2 μm) of DTTDPP:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) devices with (a) 1% DIO and (b) 1% CN. (c) and (d) Phase images of devices corresponding to (a) and (b). 1) devices with (a) 1% DIO and (b) 1% CN. (c) and (d) Phase images of devices corresponding to (a) and (b). | ||
Conclusions
We introduce a new D–A type of solution-processable, narrow band gap conjugated small molecule, DTTDPP. Control devices of DTTDPP:PC71BM (no additives) exhibit the large aggregated domains, resulting in poor device performance. Our finding is that the use of additives such as DIO and CN in chloroform dramatically improves the Jsc, concomitant with changes in the nanoscale morphology. Such methodologies for optimization should be applicable to other conjugated small molecules and further studies of solvent additive effects on small molecule BHJ solar cells.Acknowledgements
CK is supported by the NSF-SOLAR program. BW is supported by the Office of Naval Research. TQN thanks the Camille Dreyfus Teacher Scholar and the Alfred Sloan Fellowship. JHS thanks a grant (F0004012-2011-34) from Information Display R&D Center, one of the Knowledge Economy Frontier R&D Program funded by the Ministry of Knowledge Economy of Korean government and the framework of international cooperation program managed by National Research Foundation of Korea (2011-0030885) for financial support. All authors specially thank Prof. G. C. Bazan and Prof. A. J. Heeger (UCSB) for the material design and their facilities.References
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15 CrossRef CAS.
- G. Li, V. Shrotriya, Y. Yao, J. Huang and Y. Yang, J. Mater. Chem., 2007, 17, 3126 RSC.
- K. M. Coakley and M. D. McGehee, Chem. Mater., 2004, 16, 4533 CrossRef CAS.
- M. C. Scharber, D. Wuhlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. L. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 CrossRef CAS.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- C. M. Amb, S. Chen, K. R. Graham, J. Subbiah, C. E. Small, F. So and J. R. Reynolds, J. Am. Chem. Soc., 2011, 133, 10062 CrossRef CAS.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS.
- M. T. Lloyd, A. C. Mayer, S. Subramanian, D. A. Mourey, D. J. Herman, A. Bapat, V. J. E. Anthony and G. G. Malliaras, J. Am. Chem. Soc., 2007, 129, 9144 CrossRef CAS.
- B. Walker, A. B. Tamayo, X.-D. Dang, P. Zalar, J. H. Seo, A. Garcia, M. Tantiwiwat and T.-Q. Nguyen, Adv. Funct. Mater., 2009, 19, 3063 CrossRef CAS.
- B. Walker, C. Kim and T.-Q. Nguyen, Chem. Mater., 2011, 23, 470 CrossRef CAS.
- F. Silvestri, M. D. Irwin, L. Beverina, A. Facchetti, G. A. Pagani and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 17640 CrossRef CAS.
- P. F. Xia, X. J. Feng, J. Lu, S.-W. Tsang, R. Movileanu, Y. Tao and M. S. Wong, Adv. Mater., 2008, 20, 4810 CrossRef CAS.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2011, 11, 44 CrossRef.
- G. Wei, R. R. Lunt, K. Sun, S. Wang, M. E. Thompson and S. R. Forrest, Nano Lett., 2010, 10, 3555 CrossRef CAS.
- K. N. Winzenberg, P. Kemppinen, G. Fanchini, M. Bown, G. E. Collis, C. M. Forsyth, K. Hegedus, Th. B. Singh and S. E. Watkins, Chem. Mater., 2009, 21, 5701 CrossRef CAS.
- B. Walker, A. Tamayo, D. T. Duong, X.-D. Dang, C. K. Kim, J. Granstrom and T.-Q. Nguyen, Adv. Energy Mater., 2011, 212, 221 CrossRef.
- C. V. Hoven, X.-D. Dang, R. C. Coffin, J. Peet, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2010, 22, E63 CrossRef CAS.
- J. Jo, D. Gendron, A. Najari, J. S. Moon, S. Cho, M. Leclerc and A. J. Heeger, Appl. Phys. Lett., 2010, 97, 203303 CrossRef.
- H. Fan, H. Shang, Y. Li and X. Zhan, Appl. Phys. Lett., 2010, 97, 133302 CrossRef.
- A. B. Tamayo, M. Tantiwiwat, B. Walker and T.-Q. Nguyen, J. Phys. Chem. C, 2008, 112, 15543 CAS.
- J. Mei, K. R. Graham, R. Stalder, S. P. Tiwari, H. Cheun, J. Shim, M. Yoshio, C. Nuckolls, B. Kippelen, R. K. Castellano and J. R. Reynolds, Chem. Mater., 2011, 23, 2285 CrossRef CAS.
- X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS.
- J. Frey, S. Proemmel, M. A. Armitage and A. B. Holmes, Org. Synth., 2006, 83, 209 CAS.
- K. R. Graham, J. Mei, R. Stalder, J. W. Shim, H. Cheun, F. Steffy, F. So, B. Kippelen and J. R. Reynolds, ACS Appl. Mater. Interfaces, 2011, 3, 1210 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, external quantum efficiency (EQE) profiles, UPS spectrum, and DFT calculation. See DOI: 10.1039/c2ra01182d |
| This journal is © The Royal Society of Chemistry 2012 |
