La(OTf)3-mediated self-organization of guanosine with an alkynyl-Au(I)PPh3 moiety to induce Au(I)–Au(I) interactions†
Xiangtai
Meng
a,
Toshiyuki
Moriuchi
*a,
Yuki
Sakamoto
a,
Masatoshi
Kawahata
b,
Kentaro
Yamaguchi
b and
Toshikazu
Hirao
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: moriuchi@chem.eng.osaka-u.ac.jp; hirao@chem.eng.osaka-u.ac.jp; Fax: 81 6-6879-7415; Tel: 81-6-6879-7413
bPharmaceutical Sciences at Kagawa Campus, Tokushima Bunri University 1314-1 Shido, Sanuki, Kagawa 769-2193, Japan. E-mail: yamaguchi@kph.bunri-u.ac.jp; Fax: 81-87-894-0181; Tel: 81-87-894-511
First published on 30th March 2012
Abstract
A bioorganometallic compound GPhAuPPh3 composed of the guanosine and alkynyl-Au(I)PPh3 moieties was prepared. The self-assembly properties in both the absence and presence of KPF6 or La(OTf)3 were studied by NMR, UV-vis, CD and fluorescence measurements. When monovalent KPF6 was added, the expected octamer was formed without any Au(I)–Au(I) interactions. This is due to the presence of the bulky phosphine moiety that makes the Au(I) moieties far away each other. In contrast, in the presence of trivalent La(OTf)3, a more closely stacked octamer was obtained than in the presence of KPF6, leading to Au(I)–Au(I) interactions.
Introduction
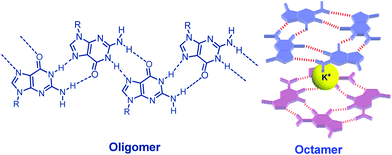
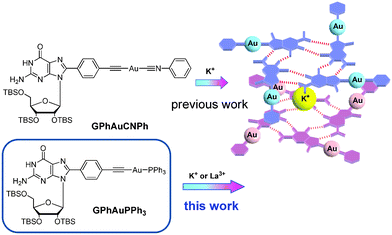
Since the pioneering work of Jaouen published at 1985,1 bioorganometallic chemistry has attracted wide attention. A series of bioorganometallic compounds were developed.2 Conjugation of organometallic compounds with biomolecules by a direct metal–carbon linkage has been proven to be a useful method to synthesize bioorganometallic compounds.3 Nucleobases are considered to be well-known biomolecules because of the assembly properties via hydrogen bonding. Among natural nucleobases, guanosine (G) derivatives certainly occupy a central role in supramolecular chemistry because they self-assemble into dimers, ribbons, G-octamers, G-hexadecamers and helices (Fig. 1).4 The most common mode of guanosine aggregates is the octamer, formed by two G-quartets and a cation (Fig. 1). The G-octamer architecture constitutes an attractive scaffold, in which π-functional molecules can be covalently attached and stacked in a well-defined arrangement. Therefore, various C8 substituted guanosine derivatives were developed.5Furthermore, some specific aggregates can be achieved by tuning or changing the solvent and cation as observed in porphyrin–G, pyrene–G, oligothiophene–G and oligo(p-phenylene-vinylene) (OPV)–G.6 Our strategy depends on the conjugation of guanosines and organometallic gold compounds at the C8 position.7 On the other hand, the architecture in Au(I) chemistry has received considerable interest, in particular with regard to the phenomenon of aurophilicity, resulting from Au(I)–Au(I) interactions.8 The synthesis and reactivity of alkynyl-Au(I)PPh3 compounds have drawn growing attention due to their intriguing photophysical properties.9 Very recently, the Au(I)–Au(I) interaction based on the formation of the G-octamer was demonstrated in a bioorganometallic compound GPhAuCNPh containing guanosine and an Au(I) isonitrile moiety in our previous paper (Fig. 2).7a These sterically undemanding nature of the isonitrile and alkynyl moieties are considered to be crucial for this kind of system. From this point of view, the design, synthesis and characterization of a new type bioorganometallic compound bearing guanosine and a bulky alkynyl-Au(I)PPh3 moiety was embarked upon in this paper.
 | ||
| Fig. 2 Structures of Au(I) compounds bearing the guanosine moiety. | ||
Results and discussion
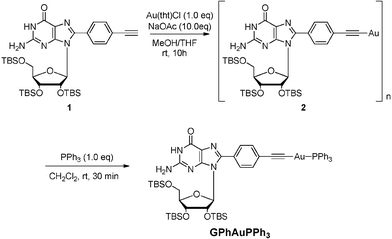
A synthetic route to GPhAuPPh3 is shown in Scheme 1. After addition of PPh3 to a dichloromethane suspension of 2, the desired Au(I) compound GPhAuPPh3 was obtained, which was purified by column chromotorography using ethyl acetate and dichloromethane as an eluent (v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The structure of GPhAuPPh3 was confirmed by 1H NMR, 13C NMR, 31P NMR, IR, HRMS, and two dimensional NMR including HSQC and HMBC. The formation of this type of Au–C
1). The structure of GPhAuPPh3 was confirmed by 1H NMR, 13C NMR, 31P NMR, IR, HRMS, and two dimensional NMR including HSQC and HMBC. The formation of this type of Au–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond is supported by the 1H NMR and IR. No assignable signal for the ethynyl proton was detected (Fig. 3). Additionally, the upfield-shift was observed for the phenylene (connected with the guanosine) protons in GPhAuPPh3 due to π-back donation from the Au(I) center (Fig. 3). A typical band due to ν(Au–C
C bond is supported by the 1H NMR and IR. No assignable signal for the ethynyl proton was detected (Fig. 3). Additionally, the upfield-shift was observed for the phenylene (connected with the guanosine) protons in GPhAuPPh3 due to π-back donation from the Au(I) center (Fig. 3). A typical band due to ν(Au–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) was observed at 2115 cm−1 in the IR spectrum of GPhAuPPh3.
C) was observed at 2115 cm−1 in the IR spectrum of GPhAuPPh3.
 | ||
| Scheme 1 Synthesis of GPhAuPPh3. | ||
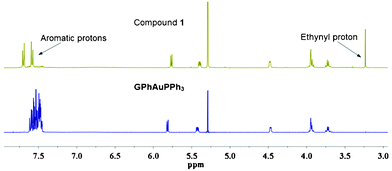 | ||
| Fig. 3 1H NMR spectra of GPhAuPPh3 and 1 in CD2Cl2 (1 × 10−2 M). | ||
We first studied the assembly properties of GPhAuPPh3 in the presence of 0.25 equivalents of KPF6. As shown in Fig. 4, after the addition of 0.25 equivalents of KPF6, the amino protons disappeared and a small upfield shift was observed for the amide proton at room temperature (Fig. 4b). Lowering the temperature to −45 °C, the amine protons split into two new sets of signals. One signal appeared at 10.12 ppm and another one at 4.61 ppm. This result suggests that the downfield signal (10.12 ppm) is involved in strong hydrogen bonding, thus considerably increasing the energy barrier for rotation about the C–N bond. Simultaneously, the amide signal became sharper and downfield shifted (Fig. 4c). In addition, analysis of the sample in chloroform by CSI-MS results in a spectrum showing a peak at m/z = 4759.5, which matches the mass [18 + 2Na]2+ (ESI†, Fig. S6). This is because alkali metal-mediated gas-phase binding of the G-quardruplex occurs in the order Na+ > K+.10 According to our previous results and the related literatures,4,7 these findings suggest the formation of an octamer.
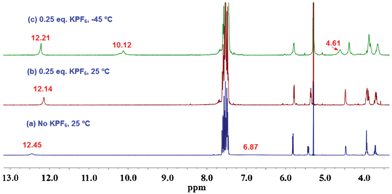 | ||
| Fig. 4 1H NMR spectra of GPhAuPPh3 in CD2Cl2 at various temperatures (1 × 10−2 M). | ||
Furthermore, the assembling properties of GPhAuPPh3 were also studied by CD, UV-vis and emission spectra in the absence and presence of KPF6. After addition of 0.25 equivalents of KPF6, the CD spectrum changed with three positive bands at 233, 268 and 325 nm (Fig. 5a). The spectral changes observed upon addition of KPF6 indicate a change in the conformation and/or secondary structure of GPhAuPPh3. However, there are no big differences in the UV and emission spectra after addition of KPF6 (Fig. 5b and 5c). These findings indicate that no Au(I)–Au(I) interaction is present in the assembled octamer. In our previous paper, the bioorganometallic compound GPhAuCNPh bearing the Au(I) isonitrile and guanosine moieties induced Au(I)–Au(I) interactions in the presence of KPF6 due to the sterically undemanding of the isonitrile (Fig. 2). In contrast, the bulky PPh3 moiety of GPhAuPPh3 made the Au(I) moieties far away from each other.
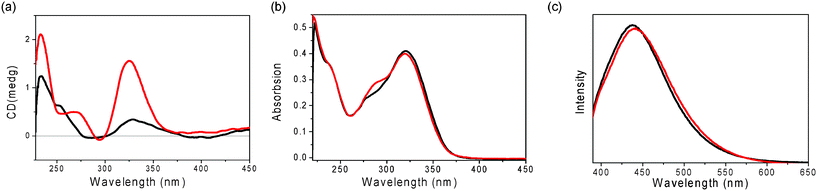 | ||
| Fig. 5 (a) CD, (b) UV-vis and (c) emission spectra (λex = 380 nm) of GPhAuPPh3 in the absence (black line) and presence (0.25 eq.) of KPF6 (red line) in CH2Cl2 (1 × 10−5 M). | ||
How can two Au(I) moieties be brought close together? One way is to shorten the distance between the two quartets in the octamer. Wu and Shinoda11 reported that the octamer formed by trivalent cations is more closely stacked than those formed by mono- and divalent cations. Therefore, addition of 0.25 equivalents of La(OTf)3 to a chloroform solution of GPhAuPPh3 was examined to reveal the assembling properties. Similar to the behavior with the monovalent cation at −45 °C, the amine protons split into new two signals (9.77 and 4.51 ppm) and the amide signal became sharper and more downfield-shifted (Fig. 6). The downfield amino signal (9.77 ppm) and the sharp amide signal (12.74 ppm) indicate that these protons are involved in strong hydrogen bonding. With reference to previous studies on the assembly properties of guanosine derivatives, an octamer appeared to be formed. In fact, the CSI-MS spectrum of the sample in chloroform shows a peak at m/z = 4966.4 corresponding to [(18 + La)OTf + NaOTf]2+ (ESI†, Fig. S7).
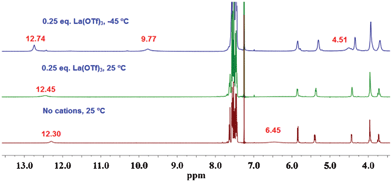 | ||
| Fig. 6 1H NMR spectra of GPhAuPPh3 in CDCl3 at various temperatures (1 × 10−2 M). | ||
Further studies on the assembling properties were carried out using UV-vis, CD and emission spectra in the absence and presence of La(OTf)3. CD spectroscopy provides insight into the chirality of this assembly in solution. As shown in Fig. 7a, the CD spectrum of GPhAuPPh3 in chloroform in the absence of La(OTf)3 at 298 K showed a strong positive band and two weak negative bands due to the formation of the oligomeric species. In sharp contrast, in the presence of 0.25 equivalents of La(OTf)3, a significant change was observed in the CD spectrum: three positive bands at 257, 288, 330 nm appeared. This difference indicates a change in the conformation and/or secondary structure of GPhAuPPh3 after the addition of La(OTf)3.
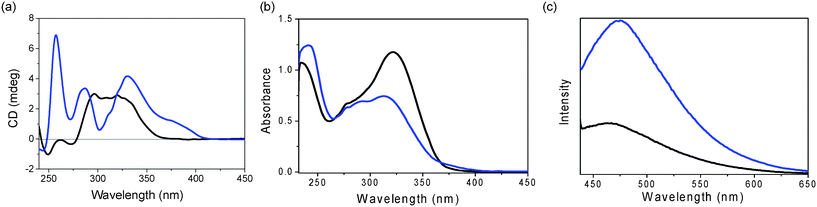 | ||
| Fig. 7 (a) CD, (b) UV-Vis and (c) emission spectra (λex = 420 nm) of GPhAuPPh3 in the absence (black line) and presence (0.25 eq.) of La(OTf)3 (blue line) in CHCl3 (1 × 10−4 M). | ||
Upon addition of La(OTf)3 to a solution of GPhAuPPh3 in chloroform, a drop in intensity of the absorption band at 313 nm was observed, together with the concomitant growth of a new low-energy shoulder in the region of approximately 370–420 nm in the UV-vis spectrum, as shown in Fig. 7b, indicating the conversion of GPhAuPPh3 to a new chemical species, namely the octamer.
Luminescence is another attractive property of Au(I) compounds. The luminescence response of GPhAuPPh3 towards La(OTf)3 was investigated (Fig. 7c). In the absence of La(OTf)3, the bioorganometallic compound GPhAuPPh3 exhibited an emission band around at 440–650 nm in chloroform. Upon addition of La(OTf)3 to a chloroform solution of GPhAuPPh3, a new low energy band at ca. 475 nm appeared. The new emission band is derived from an excitation band at around 420 nm, which coincides with the new absorption shoulder at around 370–420 nm in the UV-vis spectrum resulting from a Au(I)–Au(I) interaction. The low energy emission band is considered to be attributed to the Au(I)–Au(I) interaction, because the octamer formed by trivalent cations was reported to be more closely stacked than those formed by mono- and divalent cations (Fig. 8).7a
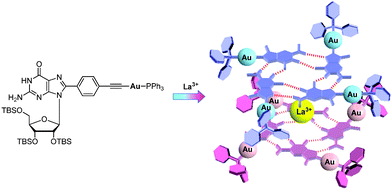 | ||
| Fig. 8 A possible diagram showing the formation of the Au(I)–Au(I) interaction upon addition of La(OTf)3. | ||
Experimental section
All reagents and solvents were purchased from commercial sources and were further purified by the standard methods, if necessary. Solvents employed were dried by refluxing in the presence of appropriate drying reagents and distilled under nitrogen. Analytical thin-layer chromatography (TLC) was performed on Merck TLC plate (silica gel 60 F254, 0.25 mm). Column chromatography was conducted on silica gel (Wakogel C-200). NMR spectra were recorded on a JNM-ECS 400 (400 MHz for 1H NMR, 100 MHz for 13C NMR and 162 MHz for 31P NMR). Compound 1 was prepared according to published procedure.7a All compounds were dried under vacuum for overnight to remove any trace amounts solvent and water.Synthesis of alkynyl-Au(I)PPh3 compound GPhAuPPh3
To a tetrahydrofuran–methanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, 45 mL) of 1 (136 mg, 0.187 mmol) were added Au(tht)Cl (59.5 mg, 0.187 mmol) and NaOAc (153 mg, 1.87 mmol) at room temperature. The mixture was stirred for 10 h. The yellow precipitate 2 (131 mg, yield 76%) was filtered, washed with water and methanol and dried under vacuum. To a dichloromethane suspension (3 mL) of 2 (131 mg, 0.142 mmol) was added PPh3 (37 mg, 0.142 mmol) under nitrogen. The mixture was stirred for 30 min at room temperature and became clear. The solvent was removed under reduced pressure. An analytically pure sample GPhAuPPh3 (80 mg, yield: 48%) was obtained by column chromatography on silica gel using ethyl acetate and dichloromethane (4
9 v/v, 45 mL) of 1 (136 mg, 0.187 mmol) were added Au(tht)Cl (59.5 mg, 0.187 mmol) and NaOAc (153 mg, 1.87 mmol) at room temperature. The mixture was stirred for 10 h. The yellow precipitate 2 (131 mg, yield 76%) was filtered, washed with water and methanol and dried under vacuum. To a dichloromethane suspension (3 mL) of 2 (131 mg, 0.142 mmol) was added PPh3 (37 mg, 0.142 mmol) under nitrogen. The mixture was stirred for 30 min at room temperature and became clear. The solvent was removed under reduced pressure. An analytically pure sample GPhAuPPh3 (80 mg, yield: 48%) was obtained by column chromatography on silica gel using ethyl acetate and dichloromethane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as eluent. 1H NMR (400 M, CD2Cl2): δ 12.43 (s, 1H, NH), 7.45–7.61 (m, 19 H, Ar), 6.80 (br, 2H, NH2), 5.82 (d, J = 8.0 Hz, 1H), 5.42 (dd, J = 5.9, 4.5 Hz, 1H), 4.47 (dd, J = 4.3, 2.1 Hz, 1H), 3.91–3.97 (m, 2H), 3.69–3.75 (m, 1H), 0.88 (s, 9H), 0.84(s, 9H), 0.73 (s, 9H), 0.11 (s, 3H), 0.09 (s, 3H), 0.01 (s, 3H), 0.006 (s, 3H), −0.06 (s, 3H), −0.28 (s, 3H). 13C NMR (100 M, CD2Cl2): 159.2, 153.1, 152.5, 149.2, 134.4, 134.3, 132.3, 131.6, 130.2, 129.6, 129.3, 129.2, 129.0, 117.9, 103.8, 103.5, 88.9, 85.2, 72.5, 71.7, 62.8, 25.8, 25.8, 25.6, 18.2, 18.0, 17.9, −4.6, −4.7, −4.8, −5.3, −5.5 ppm. 31P NMR (162 M, CD2Cl2): 42.76 ppm. HRMS (FAB) m/z calcd for C54H74AuN5O5PSi3 (([M + H]+)), 1184.4401; found, 1184.4406.
1 v/v) as eluent. 1H NMR (400 M, CD2Cl2): δ 12.43 (s, 1H, NH), 7.45–7.61 (m, 19 H, Ar), 6.80 (br, 2H, NH2), 5.82 (d, J = 8.0 Hz, 1H), 5.42 (dd, J = 5.9, 4.5 Hz, 1H), 4.47 (dd, J = 4.3, 2.1 Hz, 1H), 3.91–3.97 (m, 2H), 3.69–3.75 (m, 1H), 0.88 (s, 9H), 0.84(s, 9H), 0.73 (s, 9H), 0.11 (s, 3H), 0.09 (s, 3H), 0.01 (s, 3H), 0.006 (s, 3H), −0.06 (s, 3H), −0.28 (s, 3H). 13C NMR (100 M, CD2Cl2): 159.2, 153.1, 152.5, 149.2, 134.4, 134.3, 132.3, 131.6, 130.2, 129.6, 129.3, 129.2, 129.0, 117.9, 103.8, 103.5, 88.9, 85.2, 72.5, 71.7, 62.8, 25.8, 25.8, 25.6, 18.2, 18.0, 17.9, −4.6, −4.7, −4.8, −5.3, −5.5 ppm. 31P NMR (162 M, CD2Cl2): 42.76 ppm. HRMS (FAB) m/z calcd for C54H74AuN5O5PSi3 (([M + H]+)), 1184.4401; found, 1184.4406.
Physical measurements
UV-vis spectra were obtained with a Hitachi U-3500 spectrophotometer under nitrogen at 298 K using 1 mm pathlength quartz cuvettes. Emission spectra were collected with a Shimadzu RF-5300PC spectrofluorophotometer under nitrogen at 298 K using 5 mm pathlength quartz cuvettes. CD (circular dichromism) spectra of GPhAuPPh3 were recorded using a JASCO J-720 spectropolarimeter under nitrogen at 298 K using 5 mm pathlength quartz cuvettes.Conclusions
In conclusion, the bioorganometallic compound GPhAuPPh3 bearing the guanosine and alkynyl-Au(I)PPh3 moiety was designed and synthesized. GPhAuPPh3 was demonstrated to form octamers in the presence of KPF6 or La(OTf)3. The Au(I)–Au(I) interaction was observed in the La(OTf)3-mediated octamer due to close stacking. Studies on the application of the bioorganometallic G-octamer to functional materials and catalysts are now in progress.Acknowledgements
The author X. M. expresses special thanks for the Global COE (center of excellence) Program “Global Education and Research Center for Bio-Environmental Chemistry” of Osaka University. This work was supported by Grant-in-Aids for Science Research on Innovative Areas (Nos. 22108516 and 23111711) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. Thanks are also due to the Analytical Center, Graduate School of Engineering, Osaka University.References
- (a) G. Jaouen and A. Vessières, Pure Appl. Chem., 1985, 57, 1865 CrossRef CAS; (b) S. Top, G. Jaouen, A. Vessières, J. P. Abjean, D. Davoust, C. A. Rodger, B. G. Sayer and M. J. McGlinchey, Organometallics, 1985, 4, 2143 CrossRef CAS.
- (a) R. H. Fish and G. Jaouen, Organometallics, 2003, 22, 2166 CrossRef CAS; (b) G. Jaouen, ed., Bioorganometallics; Biomolecules, labeling, Medicine, Wiley-VCH, Weinheim, 2006 and references therein Search PubMed; (c) R. Severin, R. Bergs and W. Beck, Angew. Chem., Int. Ed., 1998, 37, 1634 CrossRef; (d) R. H. Fish, Aust. J. Chem., 2010, 63, 1505 CrossRef CAS.
- T. Moriuchi and T. Hirao, Acc. Chem. Res., 2010, 43, 1040 CrossRef CAS.
- (a) J. T. Davis, Angew. Chem., Int. Ed., 2004, 43, 668 CrossRef CAS; (b) J. T. Davis and G. P. Spada, Chem. Soc. Rev., 2007, 36, 296 RSC; (c) S. Lena, S. Masiero, S. Pieraccini and G. P. Spada, Mini-Rev. Org. Chem., 2008, 5, 262 CrossRef CAS; (d) C. Aimé, R. Nishiyabu, R. Gondo, K. Kaneko and N. Kimizuka, Chem. Commun., 2008, 6534 RSC.
- (a) J. L. Sessler, M. Sathiosatham, K. Doerr, V. Lynch and K. A. Abboud, Angew. Chem., Int. Ed., 2000, 39, 1300 CrossRef CAS; (b) M. S. Kaucher and J. T. Davis, Tetrahedron Lett., 2006, 47, 6381 CrossRef CAS; (c) M. García-Arriaga, G. Hobley and J. M. Rivera, J. Am. Chem. Soc., 2008, 130, 10492 CrossRef; (d) J. E. Betancourt, M. Martín-Hidalgo, V. Gubala and J. M. Rivera, J. Am. Chem. Soc., 2009, 131, 3186 CrossRef CAS; (e) M. del C. Rivera-Sánchez, I. Andújar-de-Sanctis, M. García-Arriaga, V. Gubala, G. Hobley and J. M. Rivera, J. Am. Chem. Soc., 2009, 131, 10403 CrossRef; (f) Z. Lin, C. M. Lawrence, D. Xiao, V. V. Kireev, S. S. Skourtis, J. L. Sessler, D. N. Beratan and I. V. Rubtsov, J. Am. Chem. Soc., 2009, 131, 18060 CrossRef CAS; (g) Y. Saito, A. Suzuki, K. Imai, N. Nemoto and I. Saito, Tetrahedron Lett., 2010, 51, 2606 CrossRef CAS; (h) A. Dumas and N. W. Luedtke, J. Am. Chem. Soc., 2010, 132, 18004 CrossRef CAS; (i) M. Martín-Hidalgo and J. M. Rivera, Chem. Commun., 2011, 47, 12485 RSC.
- (a) S. Masiero, G. Gottarelli and S. Pieraccini, Chem. Commun., 2000, 1995 RSC; (b) S. Martic, X. Liu, S. Wang and G. Wu, Chem.–Eur. J., 2008, 14, 1196 CrossRef CAS; (c) G. P. Spada, S. Lena, S. Masiero, S. Pieraccini, M. Surin and S. Samori, Adv. Mater., 2008, 20, 2433 CrossRef CAS; (d) S. Pieraccini, S. Bonacchi, S. Lena, S. Masiero, M. Montalti, N. Zaccheroni and G. P. Spada, Org. Biomol. Chem., 2010, 8, 774 RSC; (e) D. Gonzalez-Rodriguez, P. G. A. Janssen, R. Martıin-Rapun, I. De Cat, S. De Feyter, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2010, 132, 4710 CrossRef CAS.
- (a) X. Meng, T. Moriuchi, M. Kawahata, K. Yamaguchi and T. Hirao, Chem. Commun., 2011, 47, 4682 RSC; (b) X. Meng, T. Moriuchi, N. Tohnai, M. Miyata, M. Kawahata, K. Yamaguchi and T. Hirao, Org. Biomol. Chem., 2011, 9, 5633 RSC.
- (a) H. Schmidbaur, Chem. Soc. Rev., 1995, 24, 391 RSC; (b) P. Pyykkö, Chem. Rev., 1997, 97, 597 CrossRef; (c) M. J. Katz, K. Sakai and D. B. Leznoff, Chem. Soc. Rev., 2008, 37, 1884 RSC; (d) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931 RSC; (e) X. He and V. W.-W. Yam, Coord. Chem. Rev., 2011, 255, 2111 CrossRef CAS.
- (a) W. Y. Wong, Y. Guo and C. L. Ho, J. Inorg. Organomet. Polym. Mater., 2009, 19, 46 CrossRef CAS; (b) I. O. Koshevoy, Y. C. Lin, A. J. Karttunen, P. T. Chou, P. Vainiotalo, S. P. Tunik, M. Haukka and T. A. Pakkanen, Inorg. Chem., 2009, 48, 2094 CrossRef CAS; (c) X. He, N. Zhu and V. W.-W. Yam, Organometallics, 2009, 28, 3621 CrossRef CAS; (d) S. M. Aly, C. L. Ho, W. Y. Wong, D. Fortin and P. D. Harvey, Macromolecules, 2009, 42, 6902 CrossRef CAS; (e) M. Shiotsuka, N. Nishiko, Y. Tsuji, N. Kitamura, S. Onaka and K. Sako, Transition Met. Chem., 2010, 35, 129 CrossRef CAS; (f) M. H. Nauyen and J. H. K. Yip, Organometallics, 2010, 29, 2422 CrossRef.
- T. Aggerholm, S. C. Nanita, K. J. Koch and R. G. Cooks, J. Mass Spectrom., 2003, 38, 87 CrossRef CAS.
- (a) I. C. M. Kwan, Y. M. She and G. Wu, Chem. Commun., 2007, 4286 RSC; (b) S. Shinoda, T. Noguchi, M. Ikeda, Y. Habata and H. Tsukube, J. Inclusion Phenom. Macrocyclic Chem., 2011, 71, 523 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR and CSI-MS spectra of GPhAuPPh3. See DOI: 10.1039/c2ra01196d |
| This journal is © The Royal Society of Chemistry 2012 |