Morphology controlled synthesis of LiV205/Ag nanocomposite nanotubes with enhanced electrochemical performance†
Rahul S.
Diggikar
ab,
Vishal M.
Dhavale
c,
Dhanraj B.
Shinde
c,
Nihal S.
Kanbargi
d,
Milind V.
Kulkarni
b and
Bharat B.
Kale
*b
aP.G.Department of Chemistry, New Arts, Commerce and Science College, Parner, District- Ahmednagar, INDIA
bCentre For Materials For Electronics Technology (C-MET), Off Pashan Road, Pune, India. E-mail: bbkale@cmet.gov.in
cPhysical and Materials Chemistry Division, National Chemical Laboratory, Pune, India
dPolymer Chemistry Division, Indian Institute of Technology, Roorke, India
First published on 7th March 2012
Abstract
Uniformly embedded silver (Ag) nanoparticles in orthorhombic nanotubes (NTs) of lithium vanadium oxide (LVO) synthesized via a facile template-free hydrothermal treatment at low temperature exhibited an excellent morphology with good crystallinity and may act as an exceptional contender for electronic applications.
Polymorphic forms of lithium vanadium oxide (LVO) NTs, such as γ,1aα,β1bδ1c and ε1d have distinctive properties and are now widely used in electronic applications.2 The operating properties and applications of electronic devices depend not only on the oxidation state of vanadium, but also on its structure. So far, great efforts have been made in the field of synthesis of bulky or nanocrystalline LVO with different structural properties. However, due to the low conductivity, the efforts have not been highly successful. However, to the best of our knowledge, the synthesis of one dimensional (1D) nanostructures of silver–lithium vanadium oxide (LVO/Ag) nanocomposites of different morphologies with the same crystallographic structures has not yet been reported for electronic applications. Moreover, 1D nanostructures are excellent for the efficient transport of electrons.3 Recently, the 1D silver–vanadium oxide (SVO) nanowires showed excellent applications in electrochromic devices (ECDs) synthesized at higher temperatures with high conductivity.4,5
1D nanostructure appears to be an exciting field of research and could showcase great potential in addressing the problem of the space-confined transport phenomenon. The key to preparing 1D nanostructures lies in the way in which atoms or other building blocks are rationally assembled into a structure with nanometer sizes but are much larger in length under properly controlled conditions. This means that the formation of 1D nanostructures is thermodynamically preferable for many substances under certain conditions.3 Since, LVO is well known for its electronic applications; we have attempted the facile synthesis of the 1D nanostructured LVO/Ag nanocomposite.
In this communication, a controlled low-temperature hydrothermal method has been developed for the synthesis of a LVO/silver (Ag) 1D nanostructure by using LiNO3, AgNO3 and NH4VO3 at lower temperatures.
Scheme 1 elucidates the intercalation of silver in the layers of LVO.
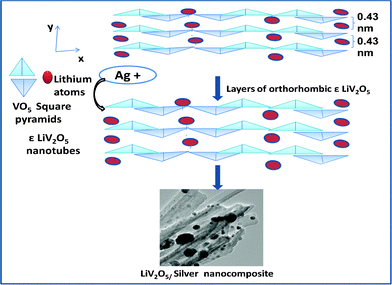 | ||
| Scheme 1 Staging of silver nanoparticles in NTs of LVO.1b | ||
The LVO NTs possess an orthorhombic structure (Fig. 1).The structure contains corrugated sheets that are orthogonal to the x-axis and parallel to the tubes direction, which is the y-axis direction. Because the length of the NTs determines the lateral dimensions of the sheet, the observed actuation must be produced by changes in the y-axis length.
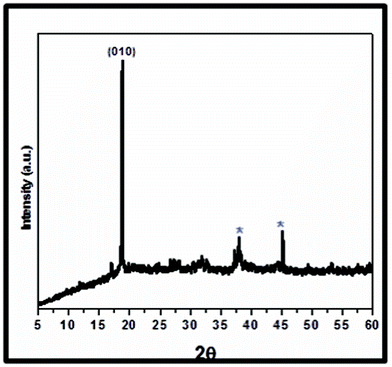 | ||
| Fig. 1 XRD pattern of LVO/Ag nanocomposite NTs. | ||
In a typical reaction, LVO/Ag NTs were prepared by the hydrothermal polycondensation of ammonium metavanadate (Aldrich) and reduction of AgNO3 in an autoclave where 0.3 g of ammonium metavanadate (A) was dissolved in 30 ml of deionized (DI) water containing 2 ml of 1 M HNO3. This mixture was stirred at room temperature for 7 h, then 40 mg of lithium nitrate (C) and 1.1 g of AgNO3 (B) were added. After stirring for 1 h, the mixture was transferred to a 50 ml Teflon-lined autoclave and treated at 80 °C for 24 h. The resulting precipitate was dispersed in 50 ml of DI water with vigorous stirring. The product was suction-filtered and rinsed with DI water and acetone. The NTs were dried at 40 °C for 12 h. The LVO/Ag nanocomposite peeled off the filter paper as a sheet. The same procedure was carried out at different concentrations of the lithium salt. The chemical reaction can be formulated as:4
| AgNO3 + 2NH4VO3 + LiNO3 → LiV2O5/Ag + 2NH3 + H2O + 2NO3− | (1) |
The entropy change may be considered as a cumulative effect of structural, electric and order-disorder processes. Assuming that these effects are additive, it may be possible to make a quantitative estimate of the individual contributions so as to determine the predominant factor in driving the reaction. Entropy of reaction (1) is ∼32.42 J mol−1k−1,6a–c implying the tendency of the reaction to progress towards the right-hand side. Basically, the silver was found to be mobile in LVO at appropriate concentrations of lithium, as reported by West et al.7
The phase purity of the product was examined by X-ray diffraction (XRD) (Fig. 1). The peak at 2θ = 19.0° showed the existence of ε-LiV2O5 and is in good agreement with JCPDS data (card no.01-0089-8320, ICSD-88643) with the lattice constants a = 11.3552, b = 4.6548, c = 3.5732 nm of space group Pmn. The corresponding strong (010) plane indicates the existence of an orthorhombic structure of the LVO.1b Two weak peaks at 2θ = 38.1 (111) and 44.3° (200) shown by (*) are characteristics of silver.4,4a It is concluded that due to the layered structure of LVO, silver is intercalated and forms nanocomposites in the form of NTs under the present synthetic conditions with this particular composition. The domain structure influences the intensities and line widths of all reflections. At a given temperature, the conductivity of LVO/Ag having a (010) plane is more than 1 order of magnitude.8a This indicates that electron conduction preferentially depends on the concentration of lithium (C).1d,8 Hence, we chose 1D NTs of LVO/Ag for further studies which has lower concentration of lithium.
As shown in Fig. 2, the LVO/Ag sample shows NTs of LVO with silver. HRTEM images reveal that silver nanoparticles of sizes in the range 2–10 nm were embedded in the LVO NTs having diameters of 40–50 nm and lengths of 5–10 μm. The single tube has been indexed, and shows existence of LVO NTs and silver with (010), (200) planes respectively (Fig. 3). However, the morphologies obtained are very sensitive to experimental conditions used, as explained in S1†, the reasons for this is unclear but the formation of NTs is supported by Zhang et al.8b
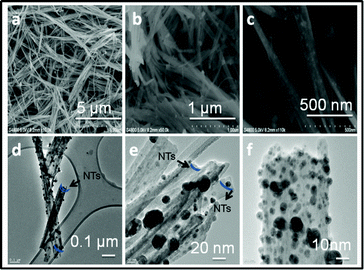 | ||
| Fig. 2 (a) FESEM image of LVO/Ag NTs; (b) silver nanoparticles coated on LVO NTs; (c) silver nanoparticles are intercalated in LVO NTs with their corresponding TEM images. (d)–(f) TEM images of LVO/Ag NTs at different magnifications. | ||
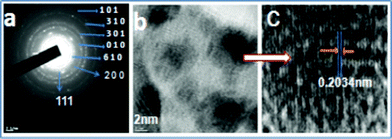 | ||
| Fig. 3 (a) SAED pattern, (b) HRTEM, and (c) clear fringes of LVO/Ag NTs. | ||
It is apparent that Ag+ and V2O5 co-exist with the corresponding ions of Li+ in all typical reactions reported herewith. The Li+ ion concentration plays an important role in the synthesis of the desired crystal structure and morphology of the product. When the reaction is performed at different concentrations of lithium salt, a decrease in lithium concentration leads to a product with a much slimmer outlook. Which means, that with an increase in the lithium concentration, an uneven morphology of LVO/Ag can be obtained.7
The mechanism of the reaction with silver and LVO differs from the classical mechanisms, which are based either on reversible insertion or deinsertion of silver into LVO. This difference is due to the facts that most of such materials crystallize in a rock-salt structure (ESI, S1d,e†) that does not contain any available empty sites for Ag+ ions, and also that none of the 3d metals considered forms alloys with Lithium7a (ESI, S1a†).
For better electrochemical performances, small-sized, highly dispersed nanoparticles with good morphology and crystallinity are more important than the bulk materials.4a,9,10,11 We exhibited the excellent 1D morphology with good crystallinity having a conductivity of around 0.9 S cm−1, which is greater than that previously reported.4 Due to the low concentration and immobility of alkali ions (Li+ ions),11a the diffusion activity of Li is low at the macro level and higher in nano level, hence the enhancement in conductivity.11b Li et al.4a demonstrated decoration of silver on NTs of vanadium oxide, however, we presented silver, which was not only decorated but also intercalated in the LVO NTs. During the synthesis process, the products may be influenced by two factors: (a) the dispersion effect of Ag in LVO layer and (b) the concentration of lithium nitrate, which directly controls the morphology.
The specific surface area of the vanadium oxides is 11.26 m2 g−1,13 whereas for LVO/Ag it is 8.2771 m2 g−1 which indicates the growth of LVO/Ag NTs (ESI, S1 and S2†). In the IR spectrum, the relative intensity of the band at 542 cm−1 strongly decreased due to the introduction of Ag+ into the LVO NT’s framework, which interrupted the linkages of Li–O–V and produced more edge-sharing V–O bonds and terminal V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (ESI, S3†).The intercalation of silver in LVO framework enlarged the interlayer spacing as well as coordinated water. These contributed to Li ion diffusion in LVO NTs.1,4,12–14b
O bonds (ESI, S3†).The intercalation of silver in LVO framework enlarged the interlayer spacing as well as coordinated water. These contributed to Li ion diffusion in LVO NTs.1,4,12–14b
The electrochemical performance of LVO/Ag nanocomposite NTs is presented in Fig. 4. The results show that the volume capacitance is observed to be 124 F g−1 which is three times higher than the reported 42 F g−1.15 In cyclic voltammetery (CV) study; two oxidation peaks can be seen at −0.35 and −0.75 V (Fig. 4a). As reported in the literature, V4+ ions were partially oxidized and leads a mixture of V4+and V5+. The remaining V4+ also further oxidized to V5+ at −0.35 V.4 The reverse reactions take place as indicated by the two reduction peaks at −0.42 and −0.75 V. The same was supported by Young et al.,15awhere the Li+ ion diffusion is illustrated at a maximum in the silver-doped material.1,4 However, Coustier et al.15b reported that the conductivity and electrochemical performance of vanadate was enhanced by 2 to 4 orders of magnitude on successful intercalation of silver in particular molar ratios. This is also supported by the conductivity measurements of the samples (ESI, S1†). The LVO/Ag NTs shows excellent stability toward repeated charge/discharge cycling (Fig. 4b) due to the intercalation of silver in LVO NTs (S4†) and the lowest energy position there is a flat potential energy surface for Ag+ to move in the LVO channel along x-axis. Each position shows that the unit cell parameters along the x and y-axis may change greatly. Therefore, the nature and amplitude of the structural changes induced by Ag+ incorporation leads to enhance electrochemical performance.1b,16 However, the vanadate is a Mott insulator (at a certain temperature) with a low band gap, and the intercalated silver can change the applied properties of LVO. To explain the applied properties, it was proposed that a multilayer structure was formed in the LVO during synthesis in which the outer layer was largely responsible for the electronic conductivity. The enhancement of conductivity in the material is a bulk enhancement and is not only confined to the outer interfaces of silver and LVO.1 Konta et al.15c presented that the band gaps of vanadate are usually smaller than real band gaps due to substituting Ag for Li in a LVO. It is reasonable for the Ag+ ions to have such an influence, which depends on the redox potential of Ag+/Ag0. This result provides important information of enhancement in electrochemical properties. Further investigation is in process.
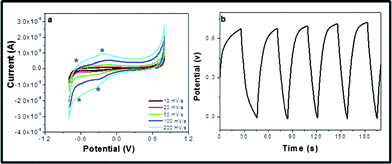 | ||
| Fig. 4 (a) CV, and (b) charge–discharge curves of the LVO/Ag NTs. | ||
In conclusion, a scalable, low temperature, reproducible and template free process for the synthesis of NTs of a LVO/Ag nanocomposite was developed. It is found that the synthesis conditions, such as the concentration and temperature, do not have an influence on the crystal structure but do strongly affect the morphology and its applications. Our investigations suggest that LVO/Ag nanocomposite is good contenders for supercapacitors. This method opens up new avenues for synthesis and applications of Au-LVO, Pt-LVO nanocomposites NTs.
This work was financially supported by the University Grants Commission, New Delhi and A. J. M. V. P. Samaj, Ahmednagar (reputed educational institutes) to R. S. D. under the FIP scheme (file no. 34-34/08) and the work was carried out in the C-MET, Pune India.
References
- (a) Y. W. Wang, H. Y. Xu, H. Wang, Y. C. Zhang, Z. Q. Song, H. Yan and C. R. Wan, Solid State Ionics, 2004, 167, 419–424 CrossRef CAS; (b) J. Galy, C. Satto, P. Sciau and P. Millet, J. Solid State Chem., 1999, 146, 129–136 CrossRef CAS; (c) P. Millet, C. Satto, P. Sciau and J. Galy, J. Solid State Chem., 1998, 136, 56–62 CrossRef CAS; (d) H. Katzke, Z. Kristallogr, 2001, 216, 278–283.
- H. Manjunatha, G. S. Suresh and T. V. Venkatesha, J. Solid State Electrochem., 2011, 15, 431–445 CrossRef CAS.
- X. Wang and Y. Li, J. Am. Chem. Soc., 2002, 124, 12 CrossRef.
- (a) C. Xiong, A. E. Aliev, B. Gnade and K. J. Balkus, ACS Nano, 2008, 2, 293–301 CrossRef CAS; (b) J. Li, L. F. Zheng, K. F. Zhang, X. Q. Feng, Z. X. Su and J. T. Ma, Mater. Res. Bull., 2008, 43, 2810–2817 CrossRef CAS.
- L. Mai, X. Xu, C. Han, Y. Luo, L. Xu, Y. A. Wu and Y. Zhao, Nano Lett., 2011, 11, 4992–4996 CrossRef CAS.
- (a) J. S. Bae and Su-II Pyun, J. Alloys Compd., 1995, 217, 52–58 CrossRef CAS; (b) W. M. Latimer and B. S. Greensfelder, J. Am. Chem. Soc., 1928, 60, 2202 CrossRef; (c) H. L. Johnston and E. C. Kerr, J. Am. Chem. Soc., 1950, 2(7), 4733 CrossRef.
- (a) K. West and A. M. Crespi, J. Power Sources, 1995, 54, 334–337 CrossRef CAS; (b) H. Liu, PhD thesis, University of Wollongong, 2001 Search PubMed.
- (a) F. Huguenin, R. M. Torresi and D. A. Buttry, J. Electrochem. Soc., 2002, 149, A546 CrossRef CAS; (b) B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 3 CrossRef; (c) X. Zhang, J. S. Lee, G. S. Lee, D. K. Cha, M. J. Kim, D. J. Yang and S. K. Manoher, Macromolecules, 2006, 39, 470–472 CrossRef CAS.
- R. Baddour-Hadjean, V. Golabkan, J. P. Pereira-Ramos, A. Mantoux and D. Lincot, J. Raman Spectrosc., 2002, 33, 631 CrossRef CAS.
- D. Ahn, Y. M. Koo, M. G. Kim, N. Shin, J. Park, J. Eom, J. Cho and T. J. Shin, J. Phys. Chem. C, 2010, 114, 3675 CAS.
- (a) Y. Q. Qiao, X. L. Wang, J. P. Zhou, J. Zhang, C. D. Gu and J. P. Tu, J. Power Sources, 2012, 198, 287–293 CrossRef CAS; (b) R. J. Barczynski and L. Murawski, Material Science-Poland, 2006, 1, 221–227 Search PubMed; (c) J. Liu, Y. Zhou, J. Wang, Y. Pan and D. Xue, Chem. Commun., 2011, 47, 10380–10382 RSC.
- L. Z. you, C. D. meng and Z. K. chao, Trans. Nonferrous Met. Soc. China, 2007, 17, 443 CrossRef.
- B. M. Reddy, K. N. Rao, G. K. Reddy and P. Bharal, J. Mol. Catal. A: Chem., 2006, 253, 44 CrossRef CAS.
- (a) N. V. Kosova, E. T. Devyatkina and V. V. Kaichev, Inorg. Mater., 2007, 43, 185 CrossRef CAS; (b) M. Wang and N. J. Alexandra, J. Solid State Chem., 2005, 178, 1230 CrossRef CAS.
- (a) G. Gu, M. Schmid, P. Chiu, A. Minett, J. Fraysse, G. Kim, S. Roth, M. Kozlov, E. Munoz and R. H. Baughman, Nat. Mater., 2003, 2, 316–319 CrossRef CAS; (b) H. R. Young and K. Kiyoshi, J. Electrochem. Soc., 2004, 151, A1406–1411 CrossRef; (c) F. Coustier, S. Passerini, J. Hill and W. H. Smyrl, MRS Online Proc. LIbr., 1997, 496, 353 Search PubMed; (d) R. Konta, H. Kato, H. Kobayashi and A. Kudo, Phys. Chem. Chem. Phys., 2003, 5, 3061–3065 RSC.
- Q. Bao, S. Bao, C. M. Li, X. Qi, C. Pan, J. Zang, W. Wang and D. Y. Tang, Chem. Mater., 2007, 19, 5965–5972 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: S1, S2, S3, S4. See DOI: 10.1039/c2ra01289h |
| This journal is © The Royal Society of Chemistry 2012 |
