Blue fluorescence-assisted SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) xCryO3 for efficient persistent photocatalysis†
xCryO3 for efficient persistent photocatalysis†
Huihui
Li
*ab,
Shu
Yin
a,
Yuhua
Wang
b and
Tsugio
Sato
a
aInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1, Katahira, Aoba-ku, Sendai 980-8577, Japan. E-mail: lihuihui@mail.tagen.tohoku.ac.jp; Fax: 81 22 217 5598; Tel: 81 22 217 5599
bLanzhou University, China
First published on 16th February 2012
Abstract
The visible light induced photocatalyst SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 was synthesized by microwave-assisted solvothermal reaction, followed by coupling with CaAl2O4:(Eu,Nd) via mild planetary ball milling. The photocatalytic activities of the SrTi1
xCryO3 was synthesized by microwave-assisted solvothermal reaction, followed by coupling with CaAl2O4:(Eu,Nd) via mild planetary ball milling. The photocatalytic activities of the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3-based composites in the oxidative destruction of NO under solar light were investigated. The results show that CaAl2O4:(Eu,Nd)/SrTi1-xO3Cry composites exhibit prolonged photocatalytic activity for the persistent destruction of NO even after turning off light for 180 min.
xCryO3-based composites in the oxidative destruction of NO under solar light were investigated. The results show that CaAl2O4:(Eu,Nd)/SrTi1-xO3Cry composites exhibit prolonged photocatalytic activity for the persistent destruction of NO even after turning off light for 180 min.
Extensive efforts have been made to study titanium dioxide based photocatalysts due to their wide applications in environmental remediation and solar energy conversion. Recently, perovskite type strontium titanate (SrTiO3) has also attracted considerable attention due to its unique photocatalytic activity, high stability, nontoxicity, etc. However, since neither strontium titanate or titanium dioxide is a wide gap semiconductor with a band gap energy of ca. 3.2 eV, it requires UV light to generate the photocatalytic activities, which is only 5% of the natural solar light. It is of great significance to develop photocatalysts that can be used in both UV irradiation (290–400 nm) and visible light (400–700 nm) to enhance the photocatalysis efficiency.1–13 One approach is to dope TiO2 with the N anion followed by CaAl2O4:(Eu,Nd) coupling. CaAl2O4:(Eu,Nd) has characteristics of high luminescent brightness of around 400–500 nm, long afterglow time, good chemical stability and low toxicity.14,15 Doping TiO2 with N anions27 results in a visible light response (above 600 nm) and photocatalytic activity under visible light irradiation, whereas coupling TiO2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy with CaAl2O4:(Eu,Nd) prolongs the photocatalytic activity even after the UV light irradiation is stopped.16,17,25,26 One valid question is whether this novel fluorescence assisted system of TiO2 with a visible light response modified by anion doping is unique. To clarify the above-mentioned question, a small amount chromium cation-doped SrTiO318 was synthesized to couple with CaAl2O4:(Eu,Nd) in the present work. The photocatalytic activity of the CaAl2O4:(Eu,Nd)/Cr-doped SrTiO3 composite photocatalyst under a solar simulator irradiation is evaluated by monitoring the degradation of NO in a continuous gas flow system. The origin of the visible-light response and the dependence of the photocatalytic activity on the mass ratio of the CaAl2O4:(Eu,Nd)/Cr-doped SrTiO3 composite are discussed.
xNy with CaAl2O4:(Eu,Nd) prolongs the photocatalytic activity even after the UV light irradiation is stopped.16,17,25,26 One valid question is whether this novel fluorescence assisted system of TiO2 with a visible light response modified by anion doping is unique. To clarify the above-mentioned question, a small amount chromium cation-doped SrTiO318 was synthesized to couple with CaAl2O4:(Eu,Nd) in the present work. The photocatalytic activity of the CaAl2O4:(Eu,Nd)/Cr-doped SrTiO3 composite photocatalyst under a solar simulator irradiation is evaluated by monitoring the degradation of NO in a continuous gas flow system. The origin of the visible-light response and the dependence of the photocatalytic activity on the mass ratio of the CaAl2O4:(Eu,Nd)/Cr-doped SrTiO3 composite are discussed.
Blue fluorescence-assisted SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite photocatalysts were fabricated by a simple precipitation method. The mass ratios of SrTi1
xCryO3 composite photocatalysts were fabricated by a simple precipitation method. The mass ratios of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 to (SrTi1
xCryO3 to (SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 + CaAl2O4:(Eu, Nd)) were 0, 20, 30, 40, 50, 60, and 100 mass%.
xCryO3 + CaAl2O4:(Eu, Nd)) were 0, 20, 30, 40, 50, 60, and 100 mass%.
Fig. 1(A) displays a comparison of the photocatalytic NO destruction behaviors of the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 and CaAl2O4:(Eu, Nd)/50 mass% SrTi1
xCryO3 and CaAl2O4:(Eu, Nd)/50 mass% SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites under artificial solar light irradiation and after turning off the light. Both samples possessed excellent photocatalytic NO destruction activity under artificial solar light irradiation. As expected, the degree of NO destruction immediately decreased after turning off the irradiation light, but CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 composites under artificial solar light irradiation and after turning off the light. Both samples possessed excellent photocatalytic NO destruction activity under artificial solar light irradiation. As expected, the degree of NO destruction immediately decreased after turning off the irradiation light, but CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 retained the NO destruction ability even after turning off the light for more than 200 min.
xCryO3 retained the NO destruction ability even after turning off the light for more than 200 min.
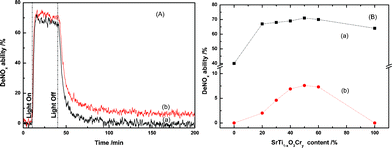 | ||
Fig. 1 (A) The photocatalytic NO destruction activity of the (a) SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 and (b) CaAl2O4:(Eu, Nd)/50 mass% SrTi1 xCryO3 and (b) CaAl2O4:(Eu, Nd)/50 mass% SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites during artificial solar light irradiation with a light intensity of 69.7 W m−2 for 30 min, followed by turning off the light; (B) effect of the SrTi1 xCryO3 composites during artificial solar light irradiation with a light intensity of 69.7 W m−2 for 30 min, followed by turning off the light; (B) effect of the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content in the CaAl2O4:(Eu,Nd)/SrTi1 xCryO3 content in the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite on the persistent degradation of NO (a) under light irradiation and (b) at 1 h after turning off the light. xCryO3 composite on the persistent degradation of NO (a) under light irradiation and (b) at 1 h after turning off the light. | ||
Fig. 1(B) shows the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content-dependent NO destruction of CaAl2O4:(Eu, Nd)/SrTi1
xCryO3 content-dependent NO destruction of CaAl2O4:(Eu, Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 under light irradiation and at 60 min after turning off the light. The NO destruction ability under light irradiation greatly increased by the addition of a small amount of SrTi1
xCryO3 under light irradiation and at 60 min after turning off the light. The NO destruction ability under light irradiation greatly increased by the addition of a small amount of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3, such as 20 mass%, but did not change significantly after further increase in the SrTi1
xCryO3, such as 20 mass%, but did not change significantly after further increase in the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content. In contrast, the prolonged NO destruction ability after turning off the irradiation light increased at first with an increase in SrTi1
xCryO3 content. In contrast, the prolonged NO destruction ability after turning off the irradiation light increased at first with an increase in SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content up to 50 mass% and then decreased. The increase in the NO destruction ability in the initial stage may be due to the increase in the amount of photocatalyst, and the decrease by the addition of excess SrTi1
xCryO3 content up to 50 mass% and then decreased. The increase in the NO destruction ability in the initial stage may be due to the increase in the amount of photocatalyst, and the decrease by the addition of excess SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 may be due to the decrease in the long afterglow phosphor content, indicating that the optimum SrTi1
xCryO3 may be due to the decrease in the long afterglow phosphor content, indicating that the optimum SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content is around 50 mass%. For comparison purposes, the performance of two recognized visible light-sensitive photocatalysts for NO evolution, TiO2
xCryO3 content is around 50 mass%. For comparison purposes, the performance of two recognized visible light-sensitive photocatalysts for NO evolution, TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy (prepared according to ref. 16, 17) and SrTi1
xNy (prepared according to ref. 16, 17) and SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3, under the same experimental conditions, are also shown in supporting information (Fig. S1, ESI†). It was found that SrTi1
xCryO3, under the same experimental conditions, are also shown in supporting information (Fig. S1, ESI†). It was found that SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 is higher performing than TiO2
xCryO3 is higher performing than TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy in the darkness after turning off the light, although the superiority is slight. More specifically, the optimum TiO2
xNy in the darkness after turning off the light, although the superiority is slight. More specifically, the optimum TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy content is ca. 40 mass%17 in this novel fluorescence-assisted composite photocatalyst.
xNy content is ca. 40 mass%17 in this novel fluorescence-assisted composite photocatalyst.
In addition, the characterization system used in the present research was similar to that of the Japanese Industrial Standard which was established at the beginning of 2004.19 In this JIS standard, it is recommended that the photocatalytic activity of photocatalyst should be characterized by measuring the decrease in concentration of NO at the outlet of a continuous reactor. One ppm NO gas with a flow rate of 3.0 dm3 min−1 is introduced to a reactor then irradiated by a lamp with light wavelength of 300–400 nm.
The mechanism of photocatalytic deNOx had been researched carefully by M.Anpo.20 During the deNOx photocatalytic reaction, the nitrogen monoxide reacts with these reactive oxygen radicals, molecular oxygen, and very small amount of water in air to produce HNO2 or HNO3. It was confirmed that about 20% of the nitrogen monoxide was decomposed to nitrogen and oxygen directly.20
To investigate the persistent photocatalytic deNOx mechanism of the blue-fluorescence-assisted SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 photocatalyst, the prepared samples were thoroughly characterized. Fig. 2(A) shows a comparison of the XRD patterns of the SrTi1
xCryO3 photocatalyst, the prepared samples were thoroughly characterized. Fig. 2(A) shows a comparison of the XRD patterns of the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 synthesized by the microwave-assisted solvothermal reaction, the CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 synthesized by the microwave-assisted solvothermal reaction, the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite consisted of 50 wt% SrTi1
xCryO3 composite consisted of 50 wt% SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 prepared by planetary ball milling, and purchased CaAl2O4:(Eu,Nd) powders. The SrTi1
xCryO3 prepared by planetary ball milling, and purchased CaAl2O4:(Eu,Nd) powders. The SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 sample exhibited a single phase of perovskite type SrTiO3 and no extra peak was observed, indicating that doping of Cr3+ into SrTiO3 did not introduce any possible impurities.18 The CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 sample exhibited a single phase of perovskite type SrTiO3 and no extra peak was observed, indicating that doping of Cr3+ into SrTiO3 did not introduce any possible impurities.18 The CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite exhibited sharp peaks corresponding to CaAl2O4 and SrTiO3, indicating the coupling of CaAl2O4:(Eu,Nd) and SrTi1
xCryO3 composite exhibited sharp peaks corresponding to CaAl2O4 and SrTiO3, indicating the coupling of CaAl2O4:(Eu,Nd) and SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3.
xCryO3.
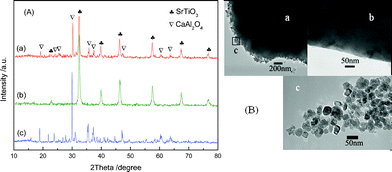 | ||
Fig. 2 (A) XRD patterns of (a) CaAl2O4:(Eu, Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 consisted of 50 wt% SrTi1 xCryO3 consisted of 50 wt% SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3, (b) pure SrTi1 xCryO3, (b) pure SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 and (c) pure CaAl2O4:(Eu, Nd), and (B) TEM images of (a) CaAl2O4:(Eu, Nd)/SrTi1 xCryO3 and (c) pure CaAl2O4:(Eu, Nd), and (B) TEM images of (a) CaAl2O4:(Eu, Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite, (b) pure CaAl2O4:(Eu, Nd) and (c) pure SrTi1 xCryO3 composite, (b) pure CaAl2O4:(Eu, Nd) and (c) pure SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3. xCryO3. | ||
Fig. 2(B) shows the transmission electron microscopy (TEM) images of the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite. As can be seen from these images, a layer of SrTi1
xCryO3 composite. As can be seen from these images, a layer of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 nanoparticles with a diameter of 25–40 nm was deposited on the surface of the CaAl2O4:(Eu,Nd) particles.
xCryO3 nanoparticles with a diameter of 25–40 nm was deposited on the surface of the CaAl2O4:(Eu,Nd) particles.
In addition, Fig. 3(A) shows the diffuse reflectance spectrum of undoped SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 and the emission spectrum of CaAl2O4:(Eu,Nd). CaAl2O4:(Eu,Nd) emitted blue luminescent light by UV light irradiation (325 nm) which peaked at 440 nm, while SrTi1
xCryO3 and the emission spectrum of CaAl2O4:(Eu,Nd). CaAl2O4:(Eu,Nd) emitted blue luminescent light by UV light irradiation (325 nm) which peaked at 440 nm, while SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 showed absorption of visible light up to 700 nm, indicating a nice overlap between the diffuse reflectance spectrum of SrTi1
xCryO3 showed absorption of visible light up to 700 nm, indicating a nice overlap between the diffuse reflectance spectrum of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 and the emission spectrum of CaAl2O4:(Eu, Nd). Therefore, it implied the possibility of a CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 and the emission spectrum of CaAl2O4:(Eu, Nd). Therefore, it implied the possibility of a CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite as the luminescence assisted photocatalyst which can use the long afterglow from the phosphor as the light source for the photocatalytic reaction. Our previous research proved that SrTi1
xCryO3 composite as the luminescence assisted photocatalyst which can use the long afterglow from the phosphor as the light source for the photocatalytic reaction. Our previous research proved that SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 could induce photocatalytic activity by a long wavelength and weak LED light irradiation of 627 nm and 2.0 mW cm−2, respectively18. This result also strongly implied the potential application of the composite as a luminescence assisted photocatalyst.
xCryO3 could induce photocatalytic activity by a long wavelength and weak LED light irradiation of 627 nm and 2.0 mW cm−2, respectively18. This result also strongly implied the potential application of the composite as a luminescence assisted photocatalyst.
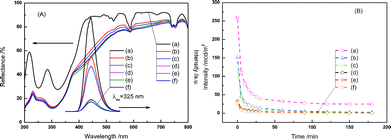 | ||
Fig. 3 (A) Overlap of the diffuse reflectance spectra and emission spectra (λex = 325 nm), and (B) emission decay curves of the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites consisted of (a) 0, (b) 20, (c) 30, (d) 40, (e) 50, and (f) 60 mass% of SrTi1 xCryO3 composites consisted of (a) 0, (b) 20, (c) 30, (d) 40, (e) 50, and (f) 60 mass% of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 after irradiation by solar light for 30 min. xCryO3 after irradiation by solar light for 30 min. | ||
To further justify the property of the blue fluorescence-assisted SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites, photoluminescence (PL) emission spectra have been used to study the transfer behavior of the photogenerated electrons and holes and understand the separation and recombination of photogenerated charge carriers. Phosphorescence from the CaAl2O4:(Eu,Nd) crystals has been considered due to the 5d–4f transition of the Eu2+ ions in the crystals. The long afterglow from CaAl2O4:(Eu,Nd) is proposed based on hole trapping by the Nd3+ ions added as an auxiliary activator. The holes generated by the excitation of Eu2+ were trapped by co-doped Nd3+ ions and/or native defects. Holes trapped at Nd and/or the defects are released slowly, then recombine with electrons from the Eu2+ ions. This process is thought to be the origin of the long persistent phosphorescence from CaAl2O4:(Eu,Nd).21,22 In order to investigate the photoelectric properties of CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 composites, photoluminescence (PL) emission spectra have been used to study the transfer behavior of the photogenerated electrons and holes and understand the separation and recombination of photogenerated charge carriers. Phosphorescence from the CaAl2O4:(Eu,Nd) crystals has been considered due to the 5d–4f transition of the Eu2+ ions in the crystals. The long afterglow from CaAl2O4:(Eu,Nd) is proposed based on hole trapping by the Nd3+ ions added as an auxiliary activator. The holes generated by the excitation of Eu2+ were trapped by co-doped Nd3+ ions and/or native defects. Holes trapped at Nd and/or the defects are released slowly, then recombine with electrons from the Eu2+ ions. This process is thought to be the origin of the long persistent phosphorescence from CaAl2O4:(Eu,Nd).21,22 In order to investigate the photoelectric properties of CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3, PL spectra were acquired for different composite samples after excitation by UV light irradiation, as shown in Fig. 3(A). The PL intensity greatly decreased with increasing the content of SrTi1
xCryO3, PL spectra were acquired for different composite samples after excitation by UV light irradiation, as shown in Fig. 3(A). The PL intensity greatly decreased with increasing the content of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 loading on the surface of the CaAl2O4:(Eu,Nd). From the Fig. 2(A), the peak wavelengths of the phosphorescence spectra did not to vary with the SrTi1
xCryO3 loading on the surface of the CaAl2O4:(Eu,Nd). From the Fig. 2(A), the peak wavelengths of the phosphorescence spectra did not to vary with the SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content. It implies that the crystal field of CaAl2O4:(Eu,Nd), which affects the 5d electron states of Eu2+ ions, was not changed dramatically by the compositional variations of the CaAl2O4:(Eu,Nd)/SrTi1
xCryO3 content. It implies that the crystal field of CaAl2O4:(Eu,Nd), which affects the 5d electron states of Eu2+ ions, was not changed dramatically by the compositional variations of the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3.
xCryO3.
Fig. 3(B) shows the phosphorescence decay profiles of CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites containing various amounts of SrTi1
xCryO3 composites containing various amounts of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3. Although the fluorescence intensity decreased with an increase in SrTi1
xCryO3. Although the fluorescence intensity decreased with an increase in SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content, the fluorescence life time did not change so much. Therefore, the decrease in the fluorescence intensity seems to be due to the absorption of the fluorescence light by SrTi1
xCryO3 content, the fluorescence life time did not change so much. Therefore, the decrease in the fluorescence intensity seems to be due to the absorption of the fluorescence light by SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 particles.
xCryO3 particles.
Table 1 summarized the apparent fluorescence quantum efficiencies (QEf) of the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite consisting of different amounts of SrTi1
xCryO3 composite consisting of different amounts of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 under fluorescence irradiation at 440 nm after turning off the artificial solar light. The photocatalytic deNOx abilities, absorbed afterglow intensity, and the fluorescence intensity of the composite samples 60 min after turning off the light are also listed. The apparent fluorescence quantum efficiency (QE) was calculated using the following equation22,23 (eqn(1)), which takes into account the deNOx abilities and average absorption degrees of the fluorescence light (λ = 440 nm).
xCryO3 under fluorescence irradiation at 440 nm after turning off the artificial solar light. The photocatalytic deNOx abilities, absorbed afterglow intensity, and the fluorescence intensity of the composite samples 60 min after turning off the light are also listed. The apparent fluorescence quantum efficiency (QE) was calculated using the following equation22,23 (eqn(1)), which takes into account the deNOx abilities and average absorption degrees of the fluorescence light (λ = 440 nm).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composites under fluorescence at 440 nm, 60 min after turning off the light
xCryO3 composites under fluorescence at 440 nm, 60 min after turning off the light
SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 content/% xCryO3 content/% |
Fluorescence intensity/mcd m−2 | Absorbed afterglow/mcd m−2 | DeNOx ability/% | Quantum efficiency/% |
|---|---|---|---|---|
| 20 | 8.1 | 24.4 | 2.0 | 0.17 |
| 30 | 4.9 | 27.6 | 4.6 | 0.37 |
| 40 | 2.5 | 30.0 | 6.9 | 0.50 |
| 50 | 2.5 | 30.0 | 7.6 | 0.56 |
| 60 | 1.9 | 30.6 | 7.3 | 0.52 |
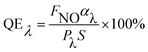
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy using the same flow type reactor was less than 0.04%, but it is clear that the CaAl2O4:(Eu,Nd)/SrTi1
xNy using the same flow type reactor was less than 0.04%, but it is clear that the CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 composite possessed quite high QE under luminescence irradiation after turning off the irradiation light, i.e., the composites consisted of more than 40 mass% of SrTi1
xCryO3 composite possessed quite high QE under luminescence irradiation after turning off the irradiation light, i.e., the composites consisted of more than 40 mass% of SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 showed more than 0.1% of QE. This result indicated that the luminescent light of CaAl2O4:(Eu,Nd) might be utilized effectively, although it is very weak.
xCryO3 showed more than 0.1% of QE. This result indicated that the luminescent light of CaAl2O4:(Eu,Nd) might be utilized effectively, although it is very weak.
Herein we demonstrated a facile and general procedure for the fabrication of perovskite type SrTiO3 with a high visible light response and stable deNOx ability under fluorescence assistance. Furthermore, studies of their photocatalytic performance have clearly revealed that the TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xNy nanoparticles do not exhibit superior or unique photocatalytic properties in this novel fluorescence-assisted system in comparison with CaAl2O4:(Eu,Nd)/SrTi1
xNy nanoparticles do not exhibit superior or unique photocatalytic properties in this novel fluorescence-assisted system in comparison with CaAl2O4:(Eu,Nd)/SrTi1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCryO3 for the persistent degradation of continuous flowing NO gas. After the long afterglow phosphor CaAl2O4:(Eu,Nd) is supported, the composite photocatalyst may be further modified to tune the photocatalytic performance. This new fluorescence-assisted photocatalyst provides new opportunities for the relationship between fluorescence and photocatalytic performance, and aids in the rational design of fluorescence-assisted composite photocatalysts.
xCryO3 for the persistent degradation of continuous flowing NO gas. After the long afterglow phosphor CaAl2O4:(Eu,Nd) is supported, the composite photocatalyst may be further modified to tune the photocatalytic performance. This new fluorescence-assisted photocatalyst provides new opportunities for the relationship between fluorescence and photocatalytic performance, and aids in the rational design of fluorescence-assisted composite photocatalysts.
This research was supported in part by the Management Expenses Grants for National Universities Corporations from the Ministry of Education, Culture, Sports, Science for Technology of Japan (MEXT), and by the Grant-in-Aid for Science Research (No.20360293 & No. 22651022).
References
- H. Irie, Y. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 5483 CrossRef CAS.
- T. Umebayashi, T. Yamaki, S. Tanaka and K. Asai, Chem. Lett., 2003, 32, 330 CrossRef CAS.
- A. Kudo, R. Niishiro, A. Iwase and H. Kato, Chem. Phys., 2007, 339, 104 CrossRef CAS.
- A. Ismail and D. Bahnemann, Green Chem., 2011, 13, 428 RSC.
- R. Asahi, T. Morikawa, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS.
- T. Ohno, T. Mitsui and M. Matsumura, Chem. Lett., 2003, 32, 364 CrossRef CAS.
- J. Wang, S. Yin, Q. Zhang, F. Saito and T. Sato, J. Mater. Chem., 2003, 13, 2348 RSC.
- P. Pathak, M. Meziani, L. Castillo and Y. Sun, Green Chem., 2005, 7, 667 RSC.
- I. Justicia, P. Ordejon, G. Canto, J. L. Mozos, J. Fraxedas, G. A. Battiston, R. Gerbasi and A. Figueras, Adv. Mater., 2002, 14, 1399 CrossRef CAS.
- J. Wang, S. Yin, M. Komatsu, Q. Zhang, F. Saito and T. Sato, Appl. Catal., B, 2004, 52, 11 CrossRef CAS.
- J. Wang, S. Yin, M. Komatsu and T. Sato, J. Eur. Ceram. Soc., 2005, 25, 3207 CrossRef CAS.
- S. Yin, Y. Aita, M. Komatsu, J. Wang, Q. Tang and T. Sato, J. Mater. Chem., 2005, 15, 674 RSC.
- W. Feng, G. Wu, L. Li and N. Guan, Green Chem., 2011, 13, 3265 RSC.
- C. Chang, J. Xu, L. Jiang, D. Mao and W. Ying, Mater. Chem. Phys., 2006, 98, 509 CrossRef CAS.
- C. Zhao and D. Chen, Mater. Lett., 2007, 61, 3673 CrossRef CAS.
- H. Li, S. Yin and T. Sato, Nanoscale Res. Lett., 2011, 6, 5 Search PubMed.
- H. Li, S. Yin and T. Sato, Appl. Catal., B, 2011, 106, 586 CrossRef CAS.
- U. Sulaeman, S. Yin and T. Sato, Appl. Phys. Lett., 2010, 97, 103102 CrossRef.
- Japanese Industrial Standard (JIS R 1701-1:2004(J)), Test method for air purification performance of photocatalytic materials—Part 1: Removal of nitric oxide, Japanese Standards Association, Established on 2004-01-20.
- M. Anpo, in “Recent Development on Visible Light Response Type Photocatalyst” (ISBN4-86043-009-03), NTS, Tokyo, 2002, p9 Search PubMed.
- Y. Lin, Z. Tang, Z. Zhang and C. Nan, J. Eur. Ceram. Soc., 2003, 23, 175 CrossRef CAS.
- X. Teng, W. Zhuang, Y. Hu, C. Zhao, H. He and X. Huang, J. Alloys Compd., 2008, 458, 446 CrossRef CAS.
- S. Yin, B. Liu, P. Zhang, T. Morikawa, K. Yamanaka and T. Sato, J. Phys. Chem. C, 2008, 112, 12425 CAS.
- S. Yin, K. Ihara, Y. Aita, M. Komatsu and T. Sato, J. Photochem. Photobiol., A, 2006, 179, 105 CrossRef CAS.
- H. Li, S. Yin, Y. Wang and T. Sato, J. Catal., 2012, 286, 273 CrossRef CAS.
- H. Li, S. Yin and T. Sato, IOP Conf. Ser.: Mater. Sci. Eng., 2011, 18, 172003 CrossRef.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95(1), 69 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental preparation and photocatalytic degradation characterization. See DOI: 10.1039/c2ra20278f/ |
| This journal is © The Royal Society of Chemistry 2012 |
