Influence of the antennas in starburst triphenylamine-based organic dye-sensitized solar cells: phenothiazine versus carbazole
Zhongquan
Wan
a,
Chunyang
Jia
*a,
Yandong
Duan
b,
Linlei
Zhou
a,
Jiaqiang
Zhang
c,
Yuan
Lin
*b and
Yu
Shi
a
aState Key Laboratory of Electronic Thin Films and Integrated Devices, School of Microelectronics and Solid-State Electronics, University of Electronic Science and Technology of China, Chengdu, 610054, P. R. China. E-mail: cyjia@ uestc.edu.cn; Fax: +86 28 83202569; Tel: +86 28 83202550
bCAS Key Laboratory of Photochemistry, Institute of Chemistry, BNLMS, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: linyuan@iccas.ac.cn; Fax: +86 10 8261 7315; Tel: +86 10 8261 5031
cBeijing Spacecrafts, China Academy of Space Technology, Beijing, 100190, P. R. China.
First published on 9th March 2012
Abstract
A new starburst triphenylamine-based organic dye (WD-1) with phenothiazine units as antenna groups was designed and synthesized and the corresponding dye (WD-4) containing carbazole antennas for the purpose of comparison was also synthesized. They were successfully applied in dye-sensitized solar cells (DSSCs). The photophysical and electrochemical properties of the dyes were investigated by UV-vis spectrometry and cyclic voltammetry. Under standard global AM 1.5 solar conditions, the WD-1 sensitized solar cell gave a short circuit photocurrent density (Jsc) of 9.2 mA cm−2, an open circuit voltage (Voc) of 625 mV, a fill factor (ff) of 0.79, corresponding to an overall conversion efficiency η of 4.54%. Under the same conditions, the WD-4 sensitized cell gave a Jsc of 7.3 mA cm−2, an Voc of 603 mV, and a ff of 0.74, corresponding to an overall conversion efficiency of 3.26%. An increase in η of about 39% was obtained from WD-4 to WD-1. Our findings demonstrate that the introduction of phenothiazine units as antennas in triphenylamine-based dyes could improve photovoltaic performance compared with carbazole units as antennas in DSSCs.
Introduction
Dye-sensitized solar cells (DSSCs) have attracted significant attention as low-cost alternatives to conventional inorganic photovoltaic devices.1 DSSCs based on the ruthenium sensitizers have shown very impressive solar-to-electric power conversion efficiencies, reaching 11% under standard AM 1.5G sunlight.2 However, the large-scale application of ruthenium dyes is limited because of costs and environmental issues. More and more efforts have been dedicated to the development of pure organic dyes which exhibit not only higher molar extinction coefficients, but also simple preparation and purification procedures at lower cost. Coumarin,3 merocyanine,4 indoline,5 polyene,6 hemicyanine,7 triphenylamine,8 fluorene,9 carbazole10 and tetrahydroquinoline11 based-organic dyes have been developed and showed good performance.The sensitizer is a crucial element in DSSCs, exerting significant influence on the power conversion efficiency as well as the stability of the cells. Most organic sensitizers are constituted by donor, linker and acceptor moieties and usually have a rod-like configuration. However, these rod-like molecules are elongated, which may facilitate the recombination of electrons with the triiodide and the formation of aggregates between molecules.12 Therefore, the dyes with a starburst conformation were designed and synthesized by introducing starburst donor units into the D-π-A molecule to form the D-D-π-A structure,13 which avoids the charge recombination processes of injected electrons with triiodide in the electrolyte. The improved performance of organic dyes with D-D-π-A structures over the simple D-π-A configuration can be achieved by molecular design.
Phenothiazine (PTZ) is a well-known heterocyclic compound with electron-rich sulfur and nitrogen heteroatoms, and the phenothiazine ring is nonplanar with a butterfly conformation in the ground state, which can impede the molecular aggregation and the formation of intermolecular excimers.14 There are few reports on the application of a dye containing a PTZ moiety in DSSCs so far.15
Based on these studies, a new starburst triphenylamine-based organic dye (WD-1) with phenothiazine units as antenna groups was designed and synthesized. The corresponding dye (WD-4) containing carbazole antennas for the purpose of comparison was also synthesized.13c They were successfully applied in DSSCs. The effect of different antennas on the performance of the starburst triphenylamine-based dye-sensitized solar cells was studied. The corresponding molecular structures of the two dyes are shown in Scheme 1.
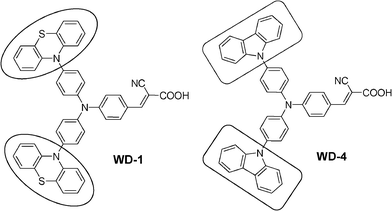 | ||
| Scheme 1 Molecular structures of WD-1 and WD-4. | ||
Experimental
Equipment
NMR spectra were recorded on a Bruker 400 MHz spectrometer. HRMS data were determined with an FTICR-APEX instrument. Absorption spectra were measured with a SHIMADZU (model UV1700) UV-vis spectrophotometer. The cyclic voltammograms of the dyes were obtained with a CH Instruments 660C electrochemical workstation using a normal three-electrode cell with a Pt working electrode, a Pt wire counter electrode, and Ag/AgCl as a reference electrode.Materials
All solvents and other chemicals were reagent grade and used without further purification. 3-hexyl-1-methylimidazolium iodide (HMII) was prepared according to the literature.16 Lithium iodide (LiI) was purchased from Acros. 2-Cyanoacetic acid, 4-tert-butylpyridine (TBP) and 3-methoxypropionitrile (MPN) were purchased from Aldrich. Phenothiazine, carbazole and triphenylamine were purchased from Astatech.Preparation of DSSCs
TiO2 colloid for DSSCs was prepared according to the literature.17 The FTO glass substrates were immersed in 40 mM TiCl4 aq. at 70 °C for 30 min and washed with water and ethanol. The 12 μm thick mesoporous nano-TiO2 films composed of 15–20 nm anatase TiO2 particles were coated on the FTO glass plates by a doctor blade. After drying the nanocrystalline TiO2 layer at 125 °C, a 4 μm thick second layer of 300–500 nm sized light scattering anatase particles (Shanghai Cai Yu Nano Technology Co., Ltd) was deposited by a doctor blade onto the first layer. The TiO2 electrodes were heated at 450 °C for 30 min. After the sintering, when the temperature cooled to about 90 °C, the electrodes were immersed in a dye bath containing 0.5 mM N3 in ethanol or 0.2 mM WD-1 and WD-4 in acetonitrile and left overnight. The films were then rinsed in ethanol to remove excess dye. Solar cells were assembled, using a 25 μm thick thermoplastic Surlyn frame, with a platinized counter electrode. An electrolyte solution was then introduced through the hole pre-drilled in the counter electrode, and the cell was sealed with thermoplastic Surlyn covers and a glass coverslip. The liquid electrolyte employed was a solution of 0.3 M HMII, 0.5 M LiI, 0.05 M I2 and 0.5 M TBP in MPN.Photovoltaic characterization
The irradiation source for the photocurrent density–voltage (J–V) measurement is an AM 1.5 solar simulator (91160A, Newport Co., USA). The incident light intensity was 100 mW cm−2 calibrated with a standard Si solar cell. The tested solar cells were masked to a working area of 0.2 cm2. Volt–current characteristics were performed on a Model 2611 Sourcemeter (Keithley Instruments, Inc., USA). A Keithley 2611 source meter and a Model spectrapro 300i monochromator (Acton research, USA) equipped with a 500 W xenon lamp (Aosiyuan Technology & Science Co., Ltd, China) were used for photocurrent action spectrum measurements. Electrochemical impedance spectroscopy (EIS) data were obtained under 100 mW cm−2 illuminations, using a perturbation of ± 10 mV over the open-circuit potential by using Solartron 1255B frequency analyzer and Solartron SI 1287 electrochemical interface system.Synthesis
Reagents: (a) POCl3, DMF, 1,2-dichloroethane, reflux, 12 h; (b) KI, KIO3, CH3COOH, 80 °C, 4 h; (c) phenothiazine or carbazole, 1,2-dichlorobenzene, Cu, K2CO3, 18-crown-6, reflux, 48 h; (d) 2-cyanoacetic acid, piperidine, acetonitrile, reflux, 10 h.The synthetic routes of the WD-1 and WD-4 were shown in Scheme 2. Compound 1 was prepared according to the reported procedures.18
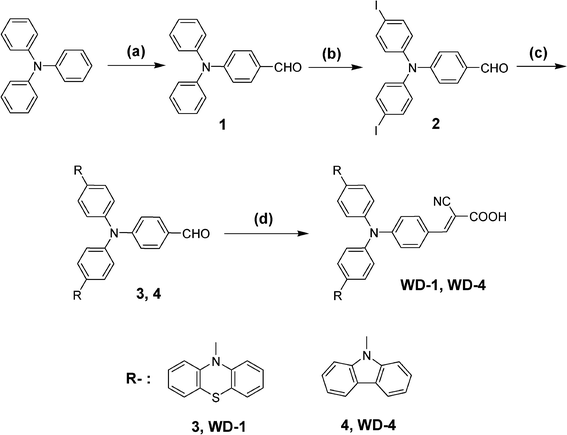 | ||
| Scheme 2 The synthetic procedure of the dyes. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) giving the product as a yellow powder (1.76 g, 70%). HRMS-EI (m/z): calcd for C19H13I2NO, 524.9087; found, 524.9102.
5) giving the product as a yellow powder (1.76 g, 70%). HRMS-EI (m/z): calcd for C19H13I2NO, 524.9087; found, 524.9102.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH (10
CH3OH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) as eluent to afford the dye WD-1 as a dark red solid (125 mg, yield 66%). 1H NMR (400 MHz, DMSO): δ (ppm) = 8.23 (s, 1H), 8.05 (d, 2H), 7.45 (m, 8H), 7.25 (d, 2H), 7.12 (m, 4H), 7.03 (m, 4H), 6.91 (m, 4H), 6.43 (m, 4H). HRMS-EI (m/z): calcd for C46H30N4O2S2, 734.1810; found, 734.1790.
v) as eluent to afford the dye WD-1 as a dark red solid (125 mg, yield 66%). 1H NMR (400 MHz, DMSO): δ (ppm) = 8.23 (s, 1H), 8.05 (d, 2H), 7.45 (m, 8H), 7.25 (d, 2H), 7.12 (m, 4H), 7.03 (m, 4H), 6.91 (m, 4H), 6.43 (m, 4H). HRMS-EI (m/z): calcd for C46H30N4O2S2, 734.1810; found, 734.1790.
Results and discussion
Absorption properties in solutions and on TiO2 films
The UV-vis spectra of the dyes WD-1 and WD-4 in acetonitrile were measured (Fig. 1) and the characterisation data are collated in Table 1. The two dyes both exhibit one intense absorption band in the visible region. The absorption spectrum of WD-1 has one absorption band centerd at 422 nm with a molar extinction coefficient of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 367 M−1 cm−1. On the other hand, WD-4 exhibits an absorption band centerd at 424 nm with a molar extinction coefficient of 13
367 M−1 cm−1. On the other hand, WD-4 exhibits an absorption band centerd at 424 nm with a molar extinction coefficient of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 M−1 cm−1. The maxima absorption peak of WD-1 has a little blue shift compared to that of WD-4. It has been speculated that it may be caused by the decrease of co-planarity between the electron donor and the electron acceptor in the ground-state due to the introduction of phenothiazine unit. Notably, the molar extinction coefficient (29
834 M−1 cm−1. The maxima absorption peak of WD-1 has a little blue shift compared to that of WD-4. It has been speculated that it may be caused by the decrease of co-planarity between the electron donor and the electron acceptor in the ground-state due to the introduction of phenothiazine unit. Notably, the molar extinction coefficient (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 367 M−1 cm−1) of WD-1 is higher than that of WD-4 (13
367 M−1 cm−1) of WD-1 is higher than that of WD-4 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 M−1 cm−1), indicating a good ability for light harvesting in the DSSCs.
834 M−1 cm−1), indicating a good ability for light harvesting in the DSSCs.
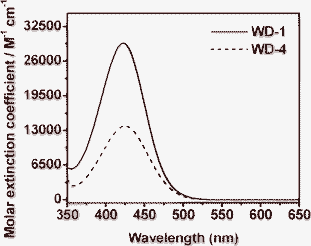 | ||
| Fig. 1 Absorption spectra of WD-1 and WD-4 in acetonitrile. | ||
| Dye | λ max a/nm (εb/M−1 cm−1) | λ max c/nm | E ox d/V (vs. NHE) | E g e/eV | E ox* f/V(vs. NHE) |
|---|---|---|---|---|---|
| a Absorption spectra were measured in acetonitrile (2.5 × 10−5 M). b The molar extinction coefficient at λmax of the absorption spectra. c Absorption spectra of the dyes adsorbed on TiO2 electrodes. d E ox was measured in DMF with 0.1 M n-Bu4NPF6 as electrolyte (scanning rate: 100 mV s−1, working electrode and counter electrode: Pt wires, and reference electrode: Ag/AgCl), potentials measured vs. Ag/AgCl were converted to normal hydrogen electrode (NHE) by the addition of +0.2 V. e E g was estimated from the absorption spectra of TiO2 electrodes sensitized by the dyes. f E ox* was calculated from Eox−Eg. | |||||
| WD-1 | 422 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 367) 367) |
416 | 0.64 | 2.63 | −1.99 |
| WD-4 | 424 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834) 834) |
424 | 0.45 | 2.55 | −2.10 |
Fig. 2 shows the absorption spectra of WD-1 and WD-4 on 3 μm thick TiO2 films after 12 h adsorption. The maximal absorption peaks for WD-1 and WD-4 on the TiO2 films are at λ = 416 nm and 424 nm, respectively. The absorption spectra of the two dyes adsorbed onto TiO2 electrodes are similar to those of the corresponding solution spectra. In addition, the absorption spectra of the dyes were broadened after adsorption on the TiO2 surface, which should favor the light harvesting of the solar cells and thus increase the photocurrent response region, leading to the increase of Jsc. More importantly, the maximum absorption wavelengths of the dyes on TiO2 films did not show a big difference in comparison with those in acetonitrile, this may be ascribed to the starburst antenna groups linked to the triphenylamine, which prevented the strong tendency of the dyes to aggregate on the TiO2 film in a large degree. This method for the prevention of aggregation might be better than using co-absorption of the dyes with deoxycholic acid, which was also able to prevent aggregation and hence improve the photovoltaic performance by means of improving both photocurrent and photovoltage, but the unavoidably significant decreased dye adsorption and limited the photovoltaic performance. It is confirmed that this is an effective way to lower the tendency to aggregate by introducing a starburst antenna group into triphenylamine dye.
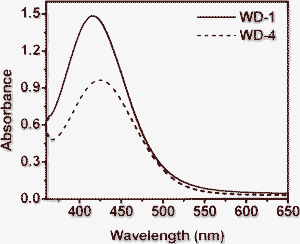 | ||
| Fig. 2 Absorption spectra of TiO2 electrodes sensitized by WD-1 and WD-4. | ||
Based on the Tauc relation, the energy gap (Eg) can be obtained by plotting (ahv)2vs. hv and extrapolating the linear portion of (ahv)2 to zero as shown in Fig. 3.19 The blue shift of WD-1 can be evidenced by the higher energy band-gap as compared WD-4. The Eg of WD-1 and WD-4 are estimated to be 2.63 eV and 2.55 eV, respectively.
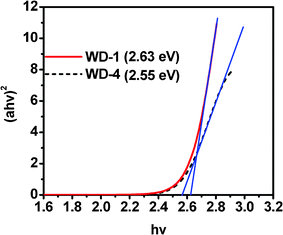 | ||
| Fig. 3 Plot of (ahv)2vs. hv. | ||
Electrochemical properties
Cyclic voltammetry measurements were performed in dimethylformamide (DMF) solution using 0.1 M tetrabutyl ammonium tetrafluoroborate as supporting electrolyte (Fig. 4), and the corresponding data are summarized in Table 1.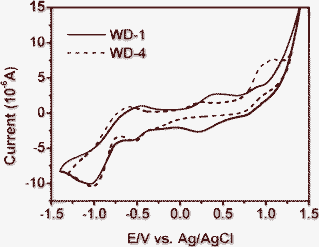 | ||
| Fig. 4 Cyclic voltammograms of WD-1 and WD-4 dissolved in DMF. | ||
The oxidation potential vs. NHE (Eox) corresponded to the highest occupied molecular orbital (HOMO), while the excited state oxidation potential vs. NHE (Eox*), which corresponded to the lowest unoccupied molecular orbital (LUMO). In this study, the first oxidation potentials (Eox) of WD-1 and WD-4 are 0.64 V and 0.45 V vs. NHE, respectively. The Eox of the two dyes are more positive than the iodine/iodide redox potential (0.4 V vs. NHE), and indicate that the oxidized dyes formed from respective electron injection into conduction band of TiO2 will favorably accept electrons from I− ions in thermodynamic property. The excited state oxidation potentials (Eox*) of the dyes can be obtained by the first oxidation potentials (Eox) and the energy gaps (Eg) of the dyes, namely, Eox−Eg. The Eox* of the WD-1 and WD-4 are −1.99 V and −2.10 V vs. NHE, respectively. The Eox* of the dyes are far more negative than the band edge energy of the nanocrystalline TiO2 electrode (−0.5 V vs. NHE),20 indicating that the electron injection process from the excited dye molecule to the TiO2 conduction band is energetically permitted.
Theoretical calculations
To get further insight into the molecular structure and electron distribution of the two dyes, their geometries were optimized by density functional theory (DFT) calculations at the B3LYP/6-31G(d,p) level with Gaussian 03.21 From the optimized geometries of the dyes (Fig. 5) and the selected dihedral angles (Table 2), we can know that all dihedral angles between phenothiazine/carbazole and benzene are all non-coplanar, which can help to inhibit the close π–π aggregation effectively between the starburst structures. This result is consistent with the absorption spectra of TiO2 electrodes sensitized by the dyes.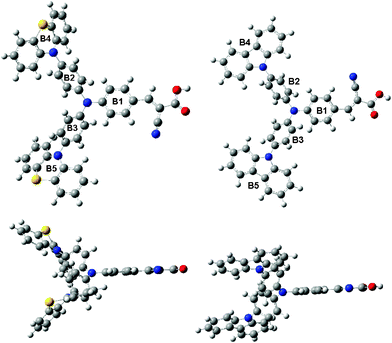 | ||
| Fig. 5 Optimized geometries of WD-1 (left) and WD-4 (right). | ||
| Dihedral angles | B1–B2 | B1–B3 | B2–B4 | B3–B5 |
|---|---|---|---|---|
| WD-1 | 30.76° | 30.82° | 81.91° | 82.21° |
| WD-4 | 29.51° | 30.10° | 54.35° | 54.08° |
Fig. 6 shows the electron distribution of the HOMOs and LUMOs of WD-1 and WD-4. At the ground states, HOMO-1 and HOMO are mainly distributed on carbazole and carbazole/triphenylamine in WD-4, respectively, but electrons are only localized on a phenothiazine ring in WD-1. The difference in HOMOs between WD-1 and WD-4 might be attributed to the plane of phenothiazine almost being vertical to the plane of benzene in triphenylamine. Therefore, base on the theory of LCAO-MO, the orbitals of phenothiazine are localized on their own zone to form quasi-degenerate HOMO-1 and HOMO orbitals. At the excited state (LUMO), intramolecular charge-transfer occurs, thereby resulting in the electron movement to the electron-acceptor part (cyanoacrylic acid). The frontier molecular orbitals of the dyes reveals that HOMOs–LUMO excitation moves the electron-density distribution from the electron-donor moiety to the electron-acceptor moiety through π bridge. Thus, an efficient charge separation is obtained based on the change of electron distribution, which is induced by photoexcitation.
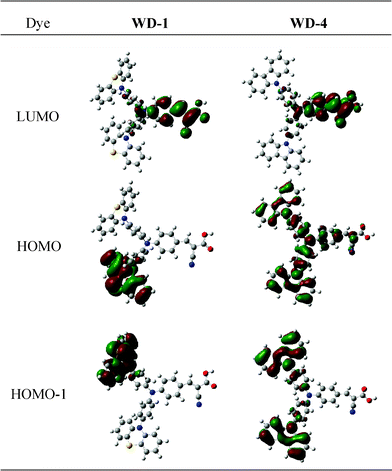 | ||
| Fig. 6 Frontier molecular orbitals of WD-1 and WD-4. | ||
Photovoltaic performances of DSSCs
Fig. 7 shows the incident monochromatic photon-to-current conversion efficiency (IPCE) obtained with a sandwich-type two electrode cell using 0.3 M HMII, 0.5 M LiI, 0.05 M I2 and 0.5 M TBP in MPN as redox electrolyte. In the range of 400–500 nm, the solar cells based on WD-1 and WD-4 show high IPCE above 44% and with the highest value of 70% at 470 nm for WD-1 and 57% at 470 nm for WD-4, respectively. In comparison with WD-4, WD-1 gives the higher IPCE value, which implies the dye would show a relatively large photocurrent in DSSCs. The higher IPCE value of WD-1 might be attributed to the higher molar extinction coefficient. On the other hand, the IPCE values for WD-1 and WD-4 are obviously decreased above 500 nm in the long wavelength region, which can be attributed to the decrease in light harvesting for the dyes. These results are consistent with the absorption spectra in solution and on the TiO2 films of the dyes.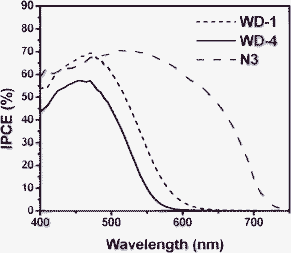 | ||
| Fig. 7 The IPCE spectra of solar cells based on WD-1, WD-4 and N3. | ||
Fig. 8 shows the current–voltage characteristics of dye-sensitized solar cells employing WD-1, WD-4 and N3 as sensitizers under standard global AM 1.5G solar light. The WD-1-sensitized cell gave a short-circuit photocurrent density (Jsc) of 9.2 mA cm−2, an open-circuit voltage (Voc) of 625 mV, and a fill factor (ff) of 0.79, corresponding to an overall conversion efficiency of 4.54% (see Table 3). The WD-4-sensitized solar cell gave a Jsc of 7.3 mA cm−2, an Voc of 603 mV, and a ff of 0.74, corresponding to an overall conversion efficiency of 3.26%. These values were comparable to those of the cell based on N3 (Jsc = 15.6 mA cm−2, Voc = 656 mV, ff = 0.72, and η = 7.37%) measured in the same conditions.
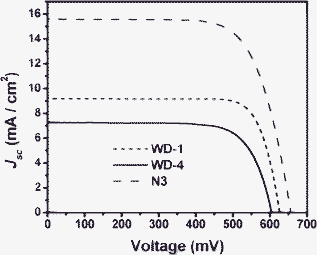 | ||
| Fig. 8 Current density–voltage curves of solar cells based on WD-1, WD-4 and N3. | ||
| Dyes | J sc/mA cm−2 | V oc/mV | ff | η/% | τ e/ms |
|---|---|---|---|---|---|
| WD-1 | 9.2 | 625 | 0.79 | 4.54 | 70.15 |
| WD-4 | 7.3 | 603 | 0.74 | 3.26 | 63.95 |
| N3 | 15.6 | 656 | 0.72 | 7.37 | 87.01 |
According to Fig. 8 and Table 3, it is clear that the photovoltaic performances of the DSSCs can be evidently affected by the phenothiazine/carbazole antennas in the dye molecules. In comparison with WD-4, the Jsc and Voc values of WD-1 are improved by introducing phenothiazine antennas. The higher Jsc value of WD-1 might be due to the IPCE of WD-1 being higher than that of WD-4. The higher Voc of WD-1 than that of WD-4 might be due to the comparatively lower Eox (HOMO) of the former, which offers enough of a larger driving force for the reduction of the oxidized dye. This, in turn, will lead to a slower back electron transfer from TiO2 to the dye and result in a lager Voc value.22 The higher Jsc and Voc for the DSSCs of WD-1 lead to the higher efficiency finally, and an increase in η of about 39% was obtained from WD-4 to WD-1.
Electrochemical impedance spectroscopic (EIS) analysis was performed to elucidate the photovoltaic findings further. EIS is a useful tool for characterizing important interfacial charge transfer processes in DSSCs.23 In this study, impedance spectra of the solar cells based on the dyes were used to investigate mechanisms at the internal interfaces of the DSSCs. This examination was conducted by subjecting the cells to constant AM 1.5G 100 mW cm−2 illumination and to bias at the open-circuit voltage (Voc) of the cells.
Fig. 9a shows the Nyquist plots for solar cells based on the dyes. For the frequency range investigated (0.1 Hz to 100 KHz), a larger semicircle occurs in the lower-frequency range and a smaller semicircle occurs in the higher-frequency range. With the bias illumination and voltage applied, the larger semicircle at lower frequencies corresponded to the charge transfer processes at the TiO2/dye/electrolyte interface, while the smaller semicircle at higher frequencies corresponded to the charge transfer processes at the Pt/electrolyte interface. As shown in Fig. 9a, the order of the larger semicircle radius decreases from WD-4 to WD-1, thus indicating the more efficient electron generation and transport in the solar cell based on WD-1.
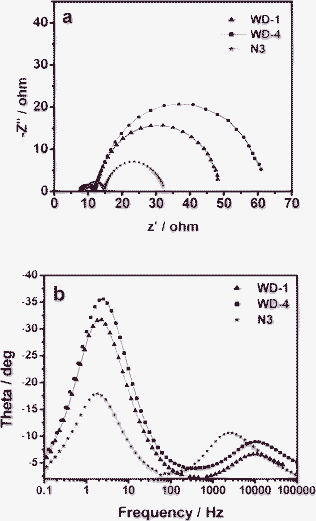 | ||
| Fig. 9 Impedance spectra of DSSCs based on dyes. (a) Nyquist plots; (b) Bode phase plots. | ||
In the Bode phase plots (Fig. 9b), the middle-frequency peak (in the 0.1–100 Hz range) is indicative of the charge-transfer process of injected electrons in TiO2. This finding is related to the electron life time and is ultimately correlated with Voc. The electron life time is obtained from the middle-frequency peak in the Bode phase plot using eqn (1), in which f is the frequency of the peak.
| τe = 1/ω = 1/2πf | (1) |
The injected electron lifetime of the WD dyes increases from WD-4 (63.95 ms) to WD-1 (70.15 ms) (Table 3). This result is correlated with the fact that WD-1, which have the phenothiazine units as antennas, possess higher Voc value (625 mV) than WD-4 (603 mV). The device based on WD-1 showed the longer electron life time. Thus, the presence of phenothiazine units in the WD dyes as antennas increases not only charge generation and injection but also the electron life time, which improves Voc and Jsc, and ultimately the overall power conversion efficiency. The EIS results are in good agreement with results of the short-circuit currents, open circuit voltages and the overall power conversion efficiencies of the DSSCs based on WD-1 and WD-4.
Conclusion
In summary, a new starburst triphenylamine-based organic dye, WD-1, that contained phenothiazine units as antennas has been designed and synthesized. We also synthesized the corresponding dye, WD-4, containing carbazole units as antennas for the purpose of comparison. The effect of different antennas to the performance of the starburst triphenylamine-based dye-sensitized solar cells was studied. Under standard global AM 1.5 solar conditions, the WD-1 sensitized solar cell gave a short circuit photocurrent density (Jsc) of 9.2 mA cm−2, an open circuit voltage (Voc) of 625 mV, a fill factor (ff) of 0.79, corresponding to an overall conversion efficiency η of 4.54%. Under the same conditions, the WD-4 sensitized cell gave a Jsc of 7.3 mA cm−2, an Voc of 603 mV, and a ff of 0.74, corresponding to an overall conversion efficiency of 3.26%. An increase in η of about 39% was obtained from WD-4 to WD-1. EIS analysis was performed to elucidate the photovoltaic findings. The EIS results are in good agreement with results of the short-circuit currents, open circuit voltages and the overall power conversion efficiencies of the DSSCs based on WD-1 and WD-4. Our findings demonstrate that the introduction of phenothiazine units as antennas in triphenylamine-based dyes could improve photovoltaic performances compared with the carbazole units as antennas in DSSCs. Because the spectral response of WD-1 is not enough, the work to extend the spectral response of the dye aiming for even better performance of DSSCs is in progress. It was expected that, by adjusting the molecular structure of this kind of starburst triphenylamine-based dyes, higher efficiency could be achieved.Acknowledgements
We thank the National Natural Science Foundation of China (Grant Nos. 20602005, 20873015), the Fundamental Research Funds for the Central Universities (Grant No. ZYGX2010J035), the Innovation Funds of State key Laboratory of Electronic Thin Films and Integrated Device (Grant No. CXJJ201104) and the Beijing National Laboratory for Molecular Sciences (BNLMS) for financial support.References
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CAS; (b) M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 Search PubMed.
- (a) M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CAS; (b) F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 10720 CAS; (c) C. Chen, M. Wang, J. Li, N. Pootrakulchote, C. Alibabaei, J. Decoppet, J. Tsai, C. Grätzel, C. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103 CAS.
- (a) K. Hara, K. Sayama, Y. Ohga, A. Shinpo, S. Suga and H. Arakawa, Chem. Commun., 2001, 569 CAS; (b) Z. S. Wang, Y. Cui, Y. Dan-Oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2008, 112, 17011 CAS; (c) K. D. Seo, H. M. Song, M. J. Lee, M. Pastore, C. Anselmi, F. D. Angelis, M. K. Nazeeruddin, M. Gräetzel and H. K. Kim, Dyes Pigm., 2011, 90, 304 CAS.
- (a) K. Sayama, K. Hara, N. Mori, M. Satsuki, S. Suga, S. Tsukagoshi, Y. Abe, H. Sugihara and H. Arakawa, Chem. Commun., 2000, 1173 CAS; (b) W. J. Wu, J. L. Hua, Y. H. Jin, W. H. Zhan and H. Tian, Photochem. Photobiol. Sci., 2008, 7, 63 CAS.
- (a) T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, J. Am. Chem. Soc., 2004, 126, 12218 CAS; (b) T. Dentani, Y. Kubota, K. Funabiki, J. Y. Jin, T. Yoshida, H. Minoura, H. Miura and M. Matsui, New J. Chem., 2009, 33, 93 CAS; (c) M. Matsui, M. Kotani, Y. Kubota, K. Funabiki, J. Y. Jin, T. Yoshida, S. Higashijima and H. Miura, Dyes Pigm., 2011, 91, 145 CAS.
- K. Hara, T. Sato, R. Katoh, A. Furube, T. Yoshihara, M. Murai, M. Kurashige, S. Ito, A. Shinpo, S. Suga and H. Arakawa, Adv. Funct. Mater., 2005, 15, 246 CAS.
- (a) Z. S. Wang, F. Y. Li and C. H. Huang, Chem. Commun., 2000, 2063 CAS; (b) Q. H. Yao, F. S. Meng, F. Y. Li, H. Tian and C. H. Huang, J. Mater. Chem., 2003, 13, 1048 CAS.
- (a) D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt and L. C. Sun, Chem. Commun., 2006, 2245 CAS; (b) H. N. Tian, J. X. Pan, R. K. Chen, M. Liu, Q. Y. Zhang, A. Hagfeldt and L. C. Sun, Adv. Funct. Mater., 2008, 18, 3461 CAS; (c) P. Qin, H. J. Zhu, T. Edvinsson, G. Boschloo, A. Hagfeldt and L. C. Sun, J. Am. Chem. Soc., 2008, 130, 8570 CAS; (d) D. H. Lee, M. J. Lee, H. M. Song, B. J. Song, K. D. Seo, M. Pastore, C. Anselmi, S. Fantacci, F. D. Angelis, M. K. Nazeeruddin, M. Gräetzel and H. K. Kim, Dyes Pigm., 2011, 91, 192 CAS.
- (a) S. Kim, J. K. Lee, S. O. Kang, J. Ko, J. H. Yum, S. Fantacci, F. D. Angelis, D. D. Censo, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 16701 CAS; (b) D. Kim, J. K. Lee, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 1913 CAS; (c) H. Choi, J. K. Lee, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 3115 CAS; (d) W. Q. Li, Y. Z. Wu, X. Li, Y.S. Xie and W. H. Zhu, Energy Environ. Sci., 2011, 4, 1830 CAS.
- (a) C. Zafer, B. Gultekin, C. Ozsoy, C. Tozlu, B. Aydin and S. Icli, Sol. Energy Mater. Sol. Cells, 2010, 94, 655 CAS; (b) C. Teng, X. C. Yang, C. Z. Yuan, C. Y. Li, R. K. Chen, H. N. Tian, S. F. Li, A. Hagfeldt and L. C. Sun, Org. Lett., 2009, 11, 5542 CAS; (c) K. Hara, Z. S. Wang, Y. Cui, A. Furube and N. Koumura, Energy Environ. Sci., 2009, 2, 1109 CAS; (d) Z. S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CAS.
- R. Q. Chen, X. C. Yang, H. N. Tian and L. C. Sun, J. Photochem. Photobiol., A, 2007, 189, 295 CAS.
- J. Tang, W. J. Wu, J. L. Hua, J. Li and H. Tian, Energy Environ. Sci., 2009, 2, 982 CAS.
- (a) J. Tang, J. L. Hua, W. J. Wu, J. Li, Z. G. Jin, Y. T. Long and H. Tian, Energy Environ. Sci., 2010, 3, 1736 CAS; (b) Z. J. Ning, Q. Zhang, W. J. Wu, H. C. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3791 CAS; (c) C. Teng, X. C. Yang, S. F. Li, M. Cheng, A. Hagfeldt, L. Z. Wu and L. C. Sun, Chem.–Eur. J., 2010, 16, 13127 CAS; (d) L. Alibabaei, J. H. Kim, M. Wang, N. Pootrakulchote, J. Teuscher, D. Di Censo, R. Humphry-Baker, J.-E. Moser, Y.-J. Yu, K.-Y. Kay, S. M. Zakeeruddin and M. Grätzel, Energy Environ. Sci., 2010, 3, 1757 CAS.
- X. X. Kong, A. P. Kulkarni and S. A. Jenekhe, Macromolecules, 2003, 36, 8992 CAS.
- (a) Z. Q. Wan, C. Y. Jia, J. Q. Zhang, Y. D. Duan, Y. Lin and Y. Shi, J. Power Sources, 2012, 199, 426 CAS; (b) H. N. Tian, X. C. Yang, J. Y. Cong, R. K. Chen, C. Teng, J. Liu, Y. Hao, L. Wang and L. C. Sun, Dyes Pigm., 2010, 84, 62 CAS; (c) S. S. Park, Y. S. Won, Y. C. Choi and J. H. Kim, Energy Fuels, 2009, 23, 3732 CAS; (d) Z. B. Xie, A. Midya, K. P. Loh, S. Adams, D. J. Blackwood, J. Wang, X. J. Zhang and Z. K. Chen, Prog. Photovoltaics, 2010, 18, 573 CAS; (e) W. J. Wu, J. B. Yang, J. L. Hua, J. Tang, L. Zhang, Y. T. Long and H. Tian, J. Mater. Chem., 2010, 20, 1772 CAS.
- P. Bonhôte, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168 Search PubMed.
- J. Liu, H. T. Yang, W. W. Tan, X. W. Zhou and Y. Lin, Electrochim. Acta, 2010, 56, 396 CAS.
- W. Xu, B. Peng, J. Chen, M. Liang and F. S. Cai, J. Phys. Chem. C, 2008, 112, 874 CAS.
- C. H. Yang, H. L. Chen, Y. Y. Chuang, C. G. Wu, C. P. Chen, S. H. Liao and T. L. Wang, J. Power Sources, 2009, 188, 627 CAS.
- A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49 CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Jr. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, revision B.05; Gaussian, Inc.: Wallingford, CT, 2004.
- K. R. J. Thomas, Y. Hsu, J. T. Lin, K. Lee, K. Ho, C. Lai, Y. Cheng and P. Chou, Chem. Mater., 2008, 20, 1830 CAS.
- B. J. Song, H. M. Song, I. T. Choi, S. K. Kim, K. D. Seo, M. S. Kang, M. J. Lee, D. W. Cho, M. J. Ju and H. K. Kim, Chem.–Eur. J., 2011, 17, 11115 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
