Synthesis of bivalent neogalactolipids via modified Staudinger reaction†
Ekaterina A.
Ivanova
a,
Mikhail A.
Maslov
*a,
Nina G.
Morozova
a,
Galina A.
Serebrennikova
a and
Vladimir V.
Chupin
ab
aM. V. Lomonosov Moscow State University of Fine Chemical Technology, 86 Vernadskiy ave., Moscow, Russian Federation. E-mail: mamaslov@mail.ru; Fax: +7(495)9368901; Tel: +7(495)9368901
bShemyakin-Ovchinnikov Institute of bioorganic chemistry RAS, 16/10 Ul. Miklukho-Maklaya, Moscow, Russian Federation. E-mail: chupin@nmr.ru; Fax: +7(495)3355033; Tel: +7(495)3352733
First published on 20th March 2012
Abstract
The synthesis of bivalent galactose-containing neutral lipids for the development of a targeted gene delivery system to the hepatocytes has been described. For introducing galactosyl ligands into the lipid molecule, a convenient approach based on modified Staudinger reaction was used.
Molecular ‘recognition’ plays a critical role in cell activity, although many aspects of this phenomenon remain unclear and hypothetical. This applies to a large class of proteins called lectins, which can reversibly and selectively bind to a variety of carbohydrates. Lectin–carbohydrate interactions play an important role in different human biological processes, including the initiation of infection and disease, immune defence, recruitment of leukocytes to inflammatory sites, and cancer malignancy and metastasis.1,2 The carbohydrate recognition domain centres of lectins act as sensitive biosensors, detecting specific carbohydrate sequences in oligosaccharides, which are ligands in these carbohydrate–protein interactions.
Lectins located on the cell membrane can mediate endocytosis and are of great interest for the transport of exogenic therapeutic molecules. The ability to use carbohydrate ligands to target protein receptors (termed ‘glycotargeting’) was first demonstrated in 1971.3 This feature can be used for the targeted delivery of therapeutics via liposomes with incorporated synthetic glycolipids, thereby promoting the cell-specific transport of small drug molecules, proteins, and nucleic acids.
The binding affinity of synthetic saccharides with lectins is the most important criterion for the rational design of glycotargeting drug delivery systems, because these systems must ultimately compete for receptor binding with all other endogenous ligands present in vivo. Many of the developed cell-specific drug delivery systems based on carbohydrates have failed because of the low affinity of monovalent sugar ligands to their corresponding receptor proteins. The binding affinity of monovalent ligands is rather weak with a typical binding constant of approximately 10−4 M.4 Higher affinities can be attained by the introduction of a few sugar residues into the structure to produce multivalent systems with significantly improved affinities. The activity enhancement of multivalent carbohydrate ligands has been termed the ‘cluster glycoside effect’.5
Genetic disorders of liver cells lead to diseases such as haemophilia, deficiency of low-density lipoprotein receptors and α1-antitrypsin, Wilson's syndrome, hypercholesterolaemia, hyperuricaemia, and cancer.6,7 Thus, the liver has long been considered as an attractive target for gene therapy. Furthermore, several hepatocyte receptors have been used to direct gene transfer. The first reported mammalian lectin was the asialoglycoprotein receptor (ASGP-R), which is an integral membrane glycoprotein involved in the clearance of glycoproteins from the blood by endocytosis through the coated-pits pathway in hepatocytes.8 Although ASGP-R binds and internalizes most galactose and N-acetylgalactosamine9-terminated ligands, it exhibits significantly different affinities for these ligands depending on their structures. Monovalent ligands (free galactose, lactose, and monovalent galactosides) bind with millimolar dissociation constants, which are far below those needed to achieve glycotargeting under physiological conditions. In comparison, bivalent galactose-terminated oligosaccharides bind with affinities that are three-orders-of-magnitude higher.10 To develop a new hepatocyte-targeted delivery system, we synthesized bivalent galactose-containing neutral lipids 1a,b (Fig. 1) with improved affinity towards ASGP-R. In addition, we introduced galactosyl ligands into lipid molecules using a convenient approach based on a modified Staudinger reaction.
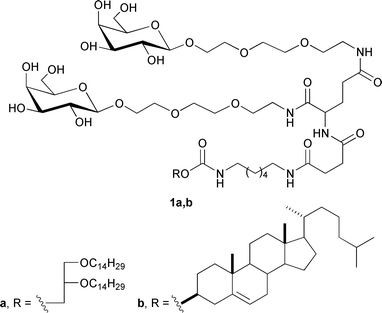 | ||
| Fig. 1 Structures of synthesized neoglycolipids. | ||
The ligand affinity towards ASGP-R is determined not only by the number of exposed terminal non-reducing D-galactose units but also by their distances within the cluster. In addition to this inter-galactose distance, the correct orientation of one residue with respect to the other also appears important.11–13 To obtain branched galactose-containing lipids 1a,b, L-glutamic acid was chosen as the core molecule to provide efficient distance and ligand orientation due to its polyfunctionality. To distance the targeting units within L-glutamic acid, a commercially available triethylene glycol spacer was chosen because it provides an independent orientation of galactose residues in the space; moreover, it is commercially available and can be easily derivatized. Cholesterol and 1,2-di-O-tetradecyl-rac-glycerol were used as the hydrophobic domains required for incorporation into a lipid bilayer. Hydrophobic domains were attached to L-glutamic acid through a complex spacer based on 1,6-diaminohexane and succinic acid.
Compound 2a was synthesized from 1,2-di-O-tetradecyl-rac-glycerol as previously described.14 Cholesterol derivative 2b was obtained in 82% yield by the treatment of 1,6-diaminohexane with cholesterol chloroformate in the presence of Et3N (Scheme 1). Amino derivatives 2a,b were treated with succinic anhydride in the presence of Et3N to afford carboxyl containing hydrophobic components 3a,b in 68% and 66% yields, respectively.
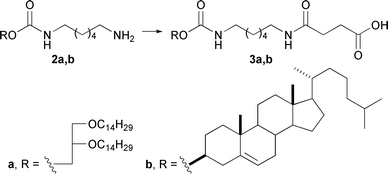 | ||
| Scheme 1 Synthesis of hydrophobic components based on cholesterol and 1,2-di-O-tetradecyl-rac-glycerol. Reagents and conditions: succinic anhydride, Et3N, DCM, 40 °C. | ||
The glycosylation of alcohol 4 with 2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl bromide in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 under modified Helferich reaction conditions15 in the presence of mercury cyanide and dried crushed molecular sieves (4 Å) produced the desired β-galactoside 5 in 75% yield (Scheme 2)†.
1.5 under modified Helferich reaction conditions15 in the presence of mercury cyanide and dried crushed molecular sieves (4 Å) produced the desired β-galactoside 5 in 75% yield (Scheme 2)†.
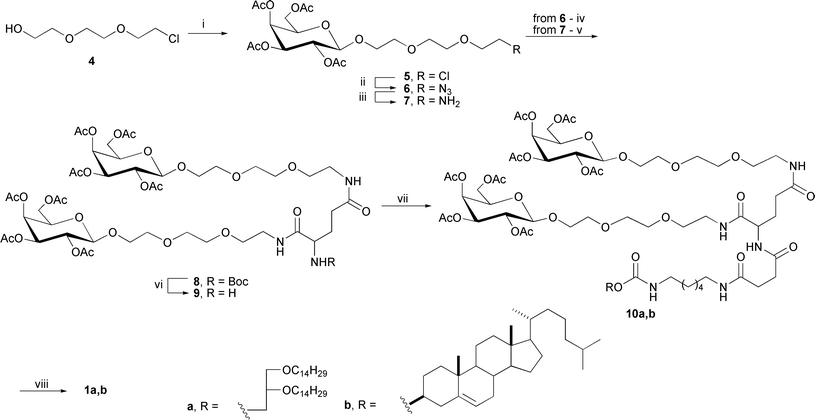 | ||
| Scheme 2 Synthesis of galactose-containing clusters. Reagents and conditions: (i) 2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl bromide, Hg(CN)2, crushed molecular sieves 4 Å, DCM, 40 °C; (ii) NaN3, DMF, 80 °C; (iii) 10% Pd/C, ammonium formate, MeOH; (iv) N-tert-butyloxycarbonyl-L-glutamic acid, PBu3, DIC, HOBt, THF, 0 °C → RT; (v) N-tert-butyloxycarbonyl-L-glutamic acid, HBTU, DIPEA, DMF, 4 °C; (vi) TFA, DCM, RT; (vii) 3a,b, HBTU, DIPEA, DMF, 0 °C; (viii) 0,04N MeONa/MeOH, 0 °C. | ||
The treatment of compound 5 with sodium azide in anhydrous DMF afforded azide 6 in 85% yield. Amine 7 was obtained via a Pd/C-catalyzed reduction of compound 6 in the presence of ammonium formate. Unfortunately, amine 7 was isolated by column chromatography only in 25% yield. The yield of amine 7 increased up to 90% (without chromatographic isolation) when TsOH was added to the reaction mixture. Further coupling of galactose-containing amine 7 and Boc-protected L-glutamic acid in the presence of HBTU (O-benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate) and DIPEA led to the formation of mono- and di-substituted derivatives of L-glutamic acid. The desired cluster 8 was isolated in 30% yield, whereas mono-substituted L-glutamic acid was obtained in 50% yield. The low yield of cluster 8 may potentially be due to the spatial hindrance that results from attaching the second molecule of galactoside 7 to the α-carboxyl group of amino acid as well as insufficient reactivity of the activated glutamic acid.
To increase the yield of cluster 8 and to shorten the number of synthetic steps, we proposed another chemical approach for the synthesis of 8 based on a modified Staudinger reaction. The classical Staudinger reaction is used to convert azides into amines in the presence of a phosphine reagent and proceeds through an aza-ylide intermediate. Various modifications of the Staudinger reaction are widely applied in many fields of bioorganic chemistry, including the modification of cell surfaces,16 synthesis of peptides,17 specific labelling of nucleic acids,18 and proteomic studies,19,20 as a basic approach for various bioconjugations.21,22 This reaction is widely used in the synthesis of N-glycoproteins23 because it significantly simplifies the synthesis of glycosylamines and gives high yields.
The key reagents in the Staudinger reaction are derivatives of phosphines, including trialkylphosphines, triarylphosphines, and phosphinotioesters. The coupling of azide 6 with N-Boc-L-glutamic acid in the presence of triphenylphosphine, DIC (N,N′-diisopropylcarbodiimide) and HOBt (N-hydroxybenzotriazole) did not afford the desired product 8, which is most likely due to the low reactivity of triphenylphosphine and the steric hindrance of the aza-ylide intermediate. When triphenylphosphine was replaced with tributylphosphine while maintaining the other conditions and reagent ratios, the yield of the desired cluster 8 increased to 48%. Varying the reagent ratios led to an increase in the yield of cluster 8 up to 65%. Notably, the formation of mono-substituted derivatives of L-glutamic acid was not observed. After removing the Boc-protecting group with TFA, the bivalent galactose-containing cluster 9 was obtained in 72% yield.
The attachment of bivalent cluster 9 to the hydrophobic components 3a,b was carried out in the presence of HBTU and DIPEA in anhydrous DMF, providing compounds 10a,b in 72% and 80% yields, respectively (Scheme 2). The deacetylation of compounds 10a,b under the Zemplén reaction with 0.04 N sodium methoxide in methanol yielded the desired bivalent galactose-containing neutral lipids 1a,b in 90% yields. The structures of the synthesized neogalactolipids 1a,b were confirmed by 1H and 13C-NMR spectroscopy as well as 1H1H-COSY 2D-NMR spectroscopy and mass spectrometry.
To estimate the accessibility and affinity of galactosyl ligands towards ASGP-R, a ricin agglutination test was performed. Ricinus communis lectin (RCA120) promotes the aggregation of liposomes that exhibit galactose moieties on their surface.24 Cationic liposomes composed of polycationic lipid 2D3,25 neutral lipid DOPE, and neogalactolipid 1a (from 0% to 5%) were prepared as previously described.25 Liposomes containing 1a were agglutinated in the presence of RCA120 (1 mg mL−1), whereas conventional liposomes without lipid 1a were unaffected (Fig. 2). The addition of free galactose (100 mg mL−1) resulted in decreasing absorbance levels, demonstrating the binding specificity of the galactose-containing liposomes with RCA120. It should be noted that the agglutination level depends on the amount of neoglycolipid 1a incorporated into liposomes.
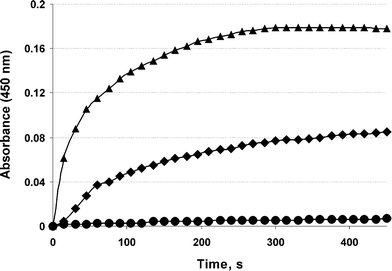 | ||
| Fig. 2 Agglutination of cationic liposomes by Ricinus communis lectin (RCA120). Circle: liposomes 2D3-DOPE-1a (0%), square: liposomes 2D3-DOPE-1a (2.5%), triangle: liposomes 2D3-DOPE-1a (5%). | ||
In conclusion, we have synthesized bivalent neoglycolipids designed for the development of liposomal drug delivery systems targeted to hepatocytes. In addition, an optimized Staudinger reaction was successfully applied to reduce the number of synthetic steps and to increase the yields of the resulting neoglycolipids. One of the newly synthesized glycolipids was incorporated into cationic liposomes and the specific binding of these galactose-containing liposomes was demonstrated using the ricin agglutination test.
References
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637 CrossRef CAS.
- M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754 CrossRef.
- J. C. Rogers and S. Kornfeld, Biochem. Biophys. Res. Commun., 1971, 45, 622–629 CrossRef CAS.
- D. T. Connolly, R. R. Townsend, K. Kawaguchi, W. R. Bell and Y. C. Lee, J. Biol. Chem., 1982, 257, 939 CAS.
- Y. C. Lee, in Neoglycoconjugates: Preparation and Applications, ed. Y. C. Lee and R. T. Lee, Academic Press, London, 1994, p. 3 Search PubMed.
- J. Prieto, C. Qian, R. Hernandez-Alcoceba, G. Gonzalez-Aseguinolaza, G. Mazzolini, B. Sangro and G. Kramer, Expert Opin. Biol. Ther., 2004, 4, 1073 CrossRef CAS.
- K. Kunath, A. von Harpe, D. Fischer and T. Kissel, J. Controlled Release, 2003, 88, 159 CrossRef CAS.
- R. J. Stockert, Physiol. Rev., 1995, 75, 591 CAS.
- J. U. Baenziger and Y. Maynard, J. Biol. Chem., 1980, 255, 4607 CAS.
- T. Aastrupb, M. Barboiu and E. Mahon, Chem. Commun., 2010, 46, 5491 RSC.
- P. V. Balaji, P. K. Qasba and V. S. R. Rao, Biochemistry, 1993, 32, 12599 CrossRef CAS.
- T. K. Dam and C. F. Brewer, Adv. Carbohydr. Chem. Biochem., 2010, 63, 139 CrossRef CAS.
- R. J. Pieters, Org. Biomol. Chem., 2009, 7, 2013 CAS.
- E. V. Shmendel, M. A. Maslov, N. G. Morozova and G. A. Serebrennikova, Russ. Chem. Bull., 2010, 59, 2281 CrossRef CAS.
- B. Helferich and K. Weis, Chem. Ber., 1956, 89, 314 CrossRef CAS.
- E. Saxon, S. J. Luchansky, H. C. Hang, C. Yu, S. C. Lee and C. R. Bertozzi, J. Am. Chem. Soc., 2002, 124, 14893 CrossRef CAS.
- K. L. Kiick, E. Saxon, C. R. Bertozzi and D. A. Tirrell, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 19 CrossRef CAS.
- C. C.-Y. Wang, T. S. Seo, Z. Li, H. Ruparel and J. Ju, Bioconjugate Chem., 2003, 14, 697 CrossRef CAS.
- H. C. Hang, C. Yu, D. L. Kato and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14846 CrossRef CAS.
- D. J. Vocadlo, H. C. Hang, E. J. Kim, J. A. Hanover and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9116 CrossRef CAS.
- M. B. Soellner, K. A. Dikson, B. L. Nilsson and R. T. Rainers, J. Am. Chem. Soc., 2003, 125, 11790 CrossRef CAS.
- M. Kohn, R. Wacker, C. Peters, H. Schroder, L. Soulere, R. Breinbauer, C.M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2003, 42, 5830 CrossRef.
- Y. He, J. H. Ronald, J. Chang and L. L. Kiessling, Org. Lett., 2004, 6, 4479 CrossRef CAS.
- S. R. S. Ting, G. Chen and M. H. Stenzel, Polym. Chem., 2010, 1, 1392 RSC.
- O. O. Markov, N. L. Mironova, M. A. Maslov, I. A. Petukhov, N. G. Morozova, V. V. Vlassov and M. A. Zenkova, J. Controlled Release, 2011 DOI:10.1016/j.jconrel.2011.11.034.
Footnote |
| † Electronic Supplementary Information (ESI) available: NMR and mass-spectra of synthesized compounds. See DOI: 10.1039/c2ra01356h/ |
| This journal is © The Royal Society of Chemistry 2012 |
