Ammonium–crown ether based host–guest systems: N–H⋯O hydrogen bond directed guest inclusion featuring N–H donor functionalities in angular geometry†
Monima
Sarma
,
Tanmay
Chatterjee
and
Samar K.
Das
*
School of Chemistry, University of Hyderabad, Central University P.O., Hyderabad, 500 046, Andhra Pradesh, India. E-mail: skdsc@uohyd.ernet.in; Fax: +91 40 2301 2460; Tel: +91 40 2301 1007
First published on 31st January 2012
Abstract
This article demonstrates crown ether based host–guest adduct formation in a series of crystalline solids 1–7, in which ammonium-type cationic guests have been integrated with various crown ethers. Addition of more than two equivalents of perchloric acid to an acetonitrile solution of 3-aminopyridine (3AmPy), 4,4′-diaminodiphenylmethane (DADPM) or 4,4′-diaminodiphenylether (DADPE) generates the doubly protonated anilinium (or ammonium) cationic species (written as 3AmPyH2, DADPMH2 and DADPEH2 respectively) which act as guests to the various crown ether hosts present in solution. The relevant compounds crystallize as their perchlorate salts and they have been structurally characterized through X-ray diffraction. The major driving force towards host–guest complexation in the compounds 1–7 is found to be the N–H⋯O hydrogen-bond interactions between the aforementioned guests and the crown ether hosts.
Introduction
Since their discovery, crown ethers have been extensively used as hosts for the recognition of a diverse class of guest species.1,2 The nature of the guest integration with the crown polyethers is largely governed by their cavity dimension and the stoichiometry of the resulting host–guest complexes is usually administered by the structure of the host and the size of the guest, although violation of the straightforward structural prediction is sometimes registered. The most characteristic feature of crown ethers is their lipophobic/hydrophilic cavity surrounded by a lipophilic/hydrophobic ring unit. In the area of crystal engineering, these hosts have been extensively used to build simple to complex architectures taking into account the fact that, (a) the crown ethers act as the hydrogen bond acceptors through the electronegative heteroatoms and (b) they also participate in weak hydrogen bond donation through their saturated carbon atoms.3–5 The metal ions which possess a spherical geometry, are usually bound through the X:→ M dative (coordinate covalent) linkages (X = heteroatom) whereas, D–H⋯X interactions (D = H–bond donor) between the cations (e.g. H3O+, NH4+, RNH3+etc.) and the crown ethers (X = acceptor heteroatom O and N being the most common) are mainly responsible for the guest encapsulation via non-covalent molecular recognition process.The tetrahedral guest cation, ammonium ion (NH4+), or organic ammonium ion (RNH3+, ArNH3+etc.) is generally perched into the oxy-crown ether cavity through the N–H⋯O hydrogen bond. The binding of this guest system has mostly been studied with the 18-crown-6 (18C6) derivatives, whereas associated entries with other crown ether systems e.g. 12-crown-4 (12C4), 15-crown-5 (15C5) or 21-crown-7 (21C7) or their derivatives are relatively less. We have recently reported a series of NH4+/ArNH3+-crown ether supramolecular adducts in association with the isopoly- and heteropoly-anions and demonstrated, how the symmetry and/or cavity size of the crown ethers and the symmetry of the polyanions govern the crystal packing feature.6 In continuation with our endeavour towards achieving diverse topologies based on molecular recognition processes by the macrocyclic hosts, we have been interested to gain more insight into the molecular packing of crown ether inclusion complexes incorporating guest units that bear hydrogen bonding sites in an angular geometrical fashion. In the present work, we have chosen doubly protonated 3-amino pyridine (hereafter 3AmPyH2), diamino diphenyl ether (hereafter DADPEH2) and diamino diphenyl methane (hereafter DADPMH2) that would serve the role of anchoring units to the crown ethers (see Scheme 1). 3AmPyH2 has two hydrogen bonding sites viz. the pyridinium N–H bond and the anilinium N–H bonds separated by 120° angular span which can interact with either two crown ethers or with one crown ether and another acceptor species (e.g., counteranion) in the relevant crystal lattice (see Scheme 1). The geometrical situation for the H-bonding interactions of this dication resembles non-protonated or protonated 1,3-phenylenediamine for which 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry between the guest and the hosts (crown ethers) in the solid state has recently been reported.7 Cocrystallization of DADPE and DADPM with the bis-phenol systems has been reported in earlier literature.8 However, to the best of our knowledge, there is no report so far that depicts hydrogen bonded complexes of DADPM and DADPE with the crown ether systems.9 We report herein, the syntheses, crystal structures and supramolecular chemistry of seven host–guest complexes formulated as [3AmPyH2(12C4)2](ClO4)2 (1), [3AmPyH2(B15C5)]2(ClO4)4·2H2O (2), [3AmPyH2(DB21C7)]3(ClO4)6·5H2O·0.5CH3CN (3), [DADPMH2(12C4)]2(ClO4)4·H2O (4), [DADPMH2(15C5)2](ClO4)2 (5), [DADPMH2(18C6)2]2(ClO4)4·6.75H2O·CH3CN (6) and [DADPEH2(18C6)2](ClO4)2·2CH3CN (7).
2 stoichiometry between the guest and the hosts (crown ethers) in the solid state has recently been reported.7 Cocrystallization of DADPE and DADPM with the bis-phenol systems has been reported in earlier literature.8 However, to the best of our knowledge, there is no report so far that depicts hydrogen bonded complexes of DADPM and DADPE with the crown ether systems.9 We report herein, the syntheses, crystal structures and supramolecular chemistry of seven host–guest complexes formulated as [3AmPyH2(12C4)2](ClO4)2 (1), [3AmPyH2(B15C5)]2(ClO4)4·2H2O (2), [3AmPyH2(DB21C7)]3(ClO4)6·5H2O·0.5CH3CN (3), [DADPMH2(12C4)]2(ClO4)4·H2O (4), [DADPMH2(15C5)2](ClO4)2 (5), [DADPMH2(18C6)2]2(ClO4)4·6.75H2O·CH3CN (6) and [DADPEH2(18C6)2](ClO4)2·2CH3CN (7).
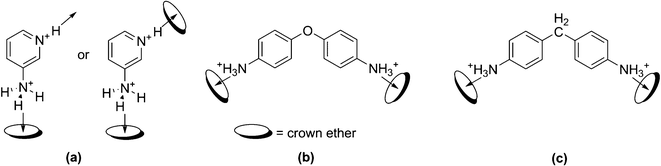 | ||
| Scheme 1 Probable binding modes between the studied guests with the crown ether hosts, (a) protonated 3-aminopyridine, (b) protonated DADPE and (c) protonated DADPM. The arrows indicate the direction of the H-bonding interactions. | ||
The solid-state stoichiometry between the host and the guest is found to vary with the cavity size of the crown ether. For example, 3AmPyH2 undergoes adduct formation in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio with the smaller crown ether 12C4 in compound 1 but with larger crown ethers, B15C5 and DB21C7 in compounds 2 and 3 respectively, it maintains 1
2 ratio with the smaller crown ether 12C4 in compound 1 but with larger crown ethers, B15C5 and DB21C7 in compounds 2 and 3 respectively, it maintains 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Extensive non-covalent interactions i.e. hydrogen-bonding, π-stacking etc. play a major role in space association of the various molecular counterparts in the relevant crystals, the extent of these supramolecular cements being governed by the type of host and guest molecules.
1 stoichiometry. Extensive non-covalent interactions i.e. hydrogen-bonding, π-stacking etc. play a major role in space association of the various molecular counterparts in the relevant crystals, the extent of these supramolecular cements being governed by the type of host and guest molecules.
Experimental section
General methods
All the reactions have been carried out under aerobic conditions. All the chemicals have been procured from commercial sources and used as received. Infrared spectra have been recorded as KBr pellets on a JASCO-5300 FT-IR spectrophotometer at 298 K. The relevant IR bands are represented as follows: s = strong, m = medium, w = weak, sh = sharp and br = broad.Synthesis and characterization data
Appropriate crown ethers (2.5 equivalents) have been added to an acetonitrile solution containing 3-aminopyridine or the angular diamines (DADPE or DADPM) (1 equivalent) and 60% perchloric acid (2.5 equivalents). Colorless single crystals suitable for X-ray diffraction have been grown through slow evaporation of the solvent in cold condition over a period of 1–3 weeks. Apart from compound 1, the solids 2–7 tend to get moistened within minutes after they have been removed from the solution which has precluded optimization of the yield of the relevant compounds. The characterization data for the relevant compounds have been presented below. Only the most prominent and characteristic IR bands have been listed.X-Ray data collection and structure determination
Crystals suitable for X-ray diffraction have been picked up from the mother solution and mounted on a Bruker SMART-APEX three-circle platform diffractometer equipped with a CCD area detector system and a collimator of 0.5 mm. Data have been collected at 100 K using the graphite monochromated Mo Kα radiation through the ϕ and ω scan strategy with 0.3° frame width and crystal to detector distance of 60 mm. The collected raw data have been corrected over background noise and integrated with the aid of the Bruker-SAINT program. Semi empirical absorption correction on the equivalent reflections have been performed by Bruker-SADABS, structure solution by direct method as implemented in the program SHELXS-97 and full-matrix least-square refinement by SHELXL-97.10 All the non-hydrogen atoms have been refined anisotropically after their successful location in the difference Fourier map. Hydrogen atoms attached to the carbon atoms have been introduced at the calculated positions to ride on their respective parents and refined freely. The ammonium, pyridinium and in some cases, water hydrogen atoms have been located from the difference Fourier map and their positions have been refined. No attempts have been made to fix their positions by applying suitable restraints. In some cases, hydrogen atoms of lattice water molecules have not been located although they have been included in the molecular formulae and mass. The disordered components of the crown ethers, where observed, have been refined isotropically. Table 1 summarizes the structural data and refinement parameters of the studied crystals. The crystal structure of compound 3 contains one acetonitrile solvent molecule per unit cell and the crystal structure of compound 6 contains three solvent water molecules per unit cell which have been considered as the diffused contribution to the overall scattering without specific atom positions by SQUEEZE/PLATON.11| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Chemical formula | C21H40Cl2N2O16 | C38H60Cl4N4O28 | C82H119.5Cl6N6.5O50 | C42H66Cl4N4O25 | C33H56Cl2N2O18 | C76H144.5Cl4N5O46.75 | C40H68Cl2N4O21 |
| Formula Mass | 647.45 | 1162.70 | 2209.04 | 1168.79 | 839.70 | 2018.27 | 1011.88 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Monoclinic | Triclinic | Monoclinic | Triclinic |
| a/Å | 8.3389(4) | 17.7353(14) | 11.9802(8) | 17.190(3) | 8.8552(6) | 16.1814(9) | 12.2134(7) |
| b/Å | 14.6212(7) | 15.4444(12) | 12.7041(9) | 16.795(2) | 14.2350(9) | 21.7220(12) | 13.2564(8) |
| c/Å | 12.1621(6) | 19.7642(16) | 34.277(2) | 18.068(3) | 16.8384(11) | 29.3019(17) | 17.0677(10) |
| α (°) | 90.00 | 90.00 | 85.190(1) | 90.00 | 110.236(1) | 90.00 | 75.025(1) |
| β (°) | 104.483(1) | 112.756(1) | 89.215(1) | 92.640(2) | 95.478(1) | 103.233(1) | 71.730(1) |
| γ (°) | 90.00 | 90.00 | 89.297(1) | 90.00 | 90.002(1) | 90.00 | 72.189(1) |
| Unit cell volume/Å3 | 1435.74(12) | 4992.2(7) | 5197.7(6) | 5210.7(13) | 1981.2(2) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 025.9(10) 025.9(10) |
2457.6(3) |
| T/K | 100(2) | 100(2) | 298(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| Space group | P21 | P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Z | 2 | 4 | 2 | 4 | 2 | 4 | 2 |
| No. of reflections measured | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 292 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 928 928 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 319 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 366 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
| No. of independent reflections | 5034 | 8790 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
9128 | 6938 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 646 646 |
8631 |
| R int | 0.0222 | 0.0419 | 0.0345 | 0.0394 | 0.0311 | 0.0517 | 0.0263 |
| Final R1 values (I > 2σ(I)) | 0.0500 | 0.0647 | 0.0659 | 0.0828 | 0.0638 | 0.0759 | 0.0447 |
| Final wR(F2) values (I > 2σ(I)) | 0.1279 | 0.1502 | 0.1578 | 0.1978 | 0.1490 | 0.1842 | 0.1118 |
| Final R1 values (all data) | 0.0512 | 0.0732 | 0.0799 | 0.0863 | 0.0743 | 0.0919 | 0.0551 |
| Final wR(F2) values (all data) | 0.1291 | 0.1559 | 0.1668 | 0.2006 | 0.1567 | 0.1931 | 0.1187 |
| Goodness of fit on F2 | 1.060 | 1.062 | 1.029 | 1.106 | 1.020 | 1.115 | 1.030 |
Results and discussion
Synthesis and characterization
In situ protonation of 3AmPy, DADPM and DADPE generates the relevant cationic (ammonium) guests which on integration with the crown ethers present in solution precipitates as the perchlorate salts of the supramolecular complexes in crystalline form. The usage of more than two equivalents of perchloric acid ensures complete protonation of the neutral guest molecules. Apart from compound 1, which has been isolated as a stable colorless solid, all the other compounds (2–7) are hygroscopic and tend to form a gummy mass after evaporation of the solvent (mother liquor). Several attempts to obtain stable solids for these compounds by means of alteration of the crystallization conditions have ended up without success. The infrared spectra of the compounds 2–7 exhibit broad features in the region 3500–3100 cm−1 indicating the presence of moisture in the compounds. In the IR spectrum of compound 1, the medium intensity bands at 3368 and 3219 cm−1 might be attributable to the N–H(str) vibrational motion of the 3AmPyH2 guest whereas, in all other compounds clarity of this pertinent absorption mode is masked by the absorption due to the high moisture content, present in the solids in this region. However, two IR bands near 1600 and 1500 cm−1 in the spectra of the compounds 1–7 might be due to the asymmetric and symmetric N–H(def) vibrational mode of the guest ammonium cations which indicates the presence of the guest moiety in the relevant solids. The existence of crown ether hosts in compounds 1–7 is elucidated by the presence of weak intensity C–H(str) bands near 3000 cm−1 and strong to medium intensity IR bands in the region of 1300–1050 cm−1 due to the C–C and C–O vibrational modes of the ethereal hosts. Scrutiny of the phase homogeneity of the compounds 2–7 has not been possible because of a lack of powdery sample as required for the PXRD instrumentation.Crystallography
Single crystal X-ray diffraction analysis of the isolated perchlorate salt of 3AmPyH2⋯12C4 supramolecular complex (compound 1) reveals a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry between the 3AmPyH2 guest and the ethereal host rendering the formulation of the relevant compound to be [3AmPyH2(12C4)2](ClO4)2. Among the series of supramolecular complexes demonstrated in this article, compound 1 crystallizes in a chiral space symmetry (P21), the asymmetric unit of which is characterized by the presence of a host–guest complex and two perchlorate counteranions. The 3AmPyH2 guest dication possesses two types of N–H bond viz. the anilinium N–H and the pyridinium N–H bonds. In the crystal structure of compound 1, the tetrahedral anilinium moiety of the guest interacts with the host unit through N2⋯O1 (dN⋯O = 2.911(5) Å) and N2⋯O3 (dN⋯O = 2.815(6) Å) non-covalent interactions and the pyridinium N–H bond of the guest is projected towards the cavity of another host molecule (dN⋯O = 2.913(4)–3.051(4) Å). The two crown ethers are connected by C13⋯O7 (dC⋯O = 3.454(5) Å) interaction. The third N–H bond of the tetrahedral anilinium moiety of the guest dication is involved in N–H⋯O interaction with a perchlorate counteranion (dN⋯O = 2.875(4) Å). The O–C–C–O dihedral angles in both the 12C4 host units in the crystal structure of compound 1 are in the range 60 ± 3° (gauche) and the crowns adopt “– – – –” i.e. all-down conformation. The packing topology in the crystal of compound 1 has been shown in Fig. 1 which reveals a zig-zag chain-like assembly of the host–guest complexes mediated by the C–H⋯O hydrogen bonds between the 3AmPyH2 guests and the perchlorate counteranions, most notably, the C5⋯O11 (dC⋯O = 3.266(5) Å) supramolecular contact.
2 stoichiometry between the 3AmPyH2 guest and the ethereal host rendering the formulation of the relevant compound to be [3AmPyH2(12C4)2](ClO4)2. Among the series of supramolecular complexes demonstrated in this article, compound 1 crystallizes in a chiral space symmetry (P21), the asymmetric unit of which is characterized by the presence of a host–guest complex and two perchlorate counteranions. The 3AmPyH2 guest dication possesses two types of N–H bond viz. the anilinium N–H and the pyridinium N–H bonds. In the crystal structure of compound 1, the tetrahedral anilinium moiety of the guest interacts with the host unit through N2⋯O1 (dN⋯O = 2.911(5) Å) and N2⋯O3 (dN⋯O = 2.815(6) Å) non-covalent interactions and the pyridinium N–H bond of the guest is projected towards the cavity of another host molecule (dN⋯O = 2.913(4)–3.051(4) Å). The two crown ethers are connected by C13⋯O7 (dC⋯O = 3.454(5) Å) interaction. The third N–H bond of the tetrahedral anilinium moiety of the guest dication is involved in N–H⋯O interaction with a perchlorate counteranion (dN⋯O = 2.875(4) Å). The O–C–C–O dihedral angles in both the 12C4 host units in the crystal structure of compound 1 are in the range 60 ± 3° (gauche) and the crowns adopt “– – – –” i.e. all-down conformation. The packing topology in the crystal of compound 1 has been shown in Fig. 1 which reveals a zig-zag chain-like assembly of the host–guest complexes mediated by the C–H⋯O hydrogen bonds between the 3AmPyH2 guests and the perchlorate counteranions, most notably, the C5⋯O11 (dC⋯O = 3.266(5) Å) supramolecular contact.
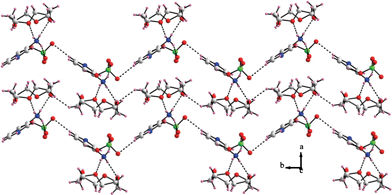 | ||
| Fig. 1 Zig-zag chain-like packing in compound 1. Only one crown ether has been shown for clarity. | ||
Compound 2 crystallizes as a hydrate and its X-ray structural analysis reveals the formulation to be [3AmPyH2(B15C5)]2(ClO4)4·2H2O i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between the guest and the host. Two symmetry independent supramolecular complexes are found in the asymmetric unit of compound 2 with one water molecule per formula unit. Alike compound 1, in the case of compound 2, the guest is integrated with the crown ether cavity through two N–H⋯O contacts (dN⋯O = 2.769(6)–2.980(5) Å) using the Ar–NH3+ functionality and the third N–H bond interacts with the counteranion (dN⋯O = 2.892(4)–2.960(8) Å). The lattice water molecules are also found to be involved in non-covalent interactions and are bonded with the guest dications (N–H⋯O) through the pyridinium fragment (dN⋯O = 2.687(5)–2.813(9) Å) and with two perchlorate counteranions (dO
1 complexation between the guest and the host. Two symmetry independent supramolecular complexes are found in the asymmetric unit of compound 2 with one water molecule per formula unit. Alike compound 1, in the case of compound 2, the guest is integrated with the crown ether cavity through two N–H⋯O contacts (dN⋯O = 2.769(6)–2.980(5) Å) using the Ar–NH3+ functionality and the third N–H bond interacts with the counteranion (dN⋯O = 2.892(4)–2.960(8) Å). The lattice water molecules are also found to be involved in non-covalent interactions and are bonded with the guest dications (N–H⋯O) through the pyridinium fragment (dN⋯O = 2.687(5)–2.813(9) Å) and with two perchlorate counteranions (dO![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O = 2.821(6)–2.936(4) Å) through the O(water)–H⋯O(perchlorate) hydrogen bonds. The guest dication is further connected to a perchlorate counteranion through C–H⋯O interaction (dC⋯O = 3.125(6)–3.289(5) Å). As can be seen in Fig. 2, the packing feature of compound 2 bears a two dimensional sheet-like topology in which two different conformations of the crown ethers are tagged with A and B. Unlike compound 1, in the lattice of compound 2, C–H⋯π stacking interactions are abundant between the crown ethers and between the crown ethers and the pyridine rings.
O = 2.821(6)–2.936(4) Å) through the O(water)–H⋯O(perchlorate) hydrogen bonds. The guest dication is further connected to a perchlorate counteranion through C–H⋯O interaction (dC⋯O = 3.125(6)–3.289(5) Å). As can be seen in Fig. 2, the packing feature of compound 2 bears a two dimensional sheet-like topology in which two different conformations of the crown ethers are tagged with A and B. Unlike compound 1, in the lattice of compound 2, C–H⋯π stacking interactions are abundant between the crown ethers and between the crown ethers and the pyridine rings.
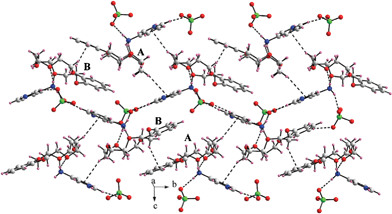 | ||
| Fig. 2 A portion of crystal packing featuring diverse supramolecular contacts in the lattice viewed perpendicular to the bc plane of the unit cell. Hydrogen bonding and the C–H⋯π stacking interactions have been shown by black dashed lines. The lattice water molecules have been omitted for clarity. | ||
To gain insight into the structural outcome upon altering the cavity dimension of the host from a small to a larger and flexible one, we have made use of DB21C7 to be the host for the 3AmPyH2 dication. Under ideal circumstances, this crown polyether possesses a mirror symmetry passing through the oxygen atom in between two catechol units and the opposite C(sp3)–C(sp3) bond. The complexation with tetrahedral guest systems (e.g. ammonium etc.) occurs through the three alternate O heteroatoms of the host, which typically adopts a bowl shape after the molecular recognition process. Crystallographic analysis on compound 3 shows the formation of a hydrate and the formulation of the relevant solid as [3AmPyH2(DB21C7)]3(ClO4)6·5H2O·0.5 CH3CN. The structure is rather complicated and consists of three symmetry independent host–guest adducts, all the three maintaining 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The crown ether essentially houses the anilinium moiety through N–H⋯O hydrogen bonds (dN⋯O = 2.775(4)–2.948(4) Å) involving all the three available anilinium N–H bonds of the guest. Involvement of the perchlorate counteranion to saturate the N–H⋯O hydrogen–bonding possibilities around the guest anilinium moiety, as described for the crystal structures of compounds 1, 2, is therefore irrelevant in the structure of compound 3. In all the three symmetry independent adducts the guest cation orients almost parallel to the mirror symmetry of the crown ether, which in turn attains a quasi-Cs conformation. Similarly, the bending of the crown ether towards the pyridine ring makes the host bowl shaped, the extent of bending being different in the three adducts. Involvement of the lattice water molecules in the hydrogen bonding interactions along with the presence of three symmetry independent host–guest adducts attribute a very complicated three dimensional crystal packing feature. However, a view of the chain-like assembly in the relevant crystal has been shown in Fig. 3. Two inversion symmetry related host–guest complexes form a supramolecular dimer through C–H⋯π stacking interactions between the pyridine rings and the phenyl rings of the crown ethers (dC⋯Cg = 3.304(4), <C–H⋯Cg = 142°). These dimers are further connected by the C–H⋯O interactions with the perchlorate anions (dC⋯O = 3.426(5) Å) parallel to the crystallographic b-axis (see Fig. 3).
1 stoichiometry. The crown ether essentially houses the anilinium moiety through N–H⋯O hydrogen bonds (dN⋯O = 2.775(4)–2.948(4) Å) involving all the three available anilinium N–H bonds of the guest. Involvement of the perchlorate counteranion to saturate the N–H⋯O hydrogen–bonding possibilities around the guest anilinium moiety, as described for the crystal structures of compounds 1, 2, is therefore irrelevant in the structure of compound 3. In all the three symmetry independent adducts the guest cation orients almost parallel to the mirror symmetry of the crown ether, which in turn attains a quasi-Cs conformation. Similarly, the bending of the crown ether towards the pyridine ring makes the host bowl shaped, the extent of bending being different in the three adducts. Involvement of the lattice water molecules in the hydrogen bonding interactions along with the presence of three symmetry independent host–guest adducts attribute a very complicated three dimensional crystal packing feature. However, a view of the chain-like assembly in the relevant crystal has been shown in Fig. 3. Two inversion symmetry related host–guest complexes form a supramolecular dimer through C–H⋯π stacking interactions between the pyridine rings and the phenyl rings of the crown ethers (dC⋯Cg = 3.304(4), <C–H⋯Cg = 142°). These dimers are further connected by the C–H⋯O interactions with the perchlorate anions (dC⋯O = 3.426(5) Å) parallel to the crystallographic b-axis (see Fig. 3).
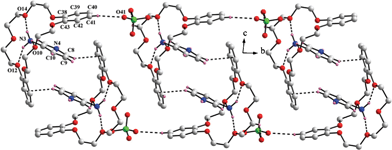 | ||
| Fig. 3 Chain-like packing topology in compound 3 viewed parallel to the crystallographic 011 plane. | ||
The crown ether based host–guest complexes, discussed so far (compounds 1–3), consist of the planar guest molecule 3AmPyH2 in which the two hydrogen bond donor sites i.e. the anilium and pyridinium functionalities are at 120° angular separation. It is observed that the anilinium moiety of the guest interacts with the crown ether cavity, whereas the pyridinium moiety is involved in N–H⋯O hydrogen bonding interaction with other acceptor species in the crystal. The next four compounds consist of doubly protonated DADPE and DADPM i.e. DADPEH2 and DADPMH2 as the guests in which the two anilinium functionalities are connected by an ethereal and a methylene spacer respectively.
Structural characterization of compound 4 reveals 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between the diammonium guest and the crown ether host (12C4) irrespective of the amount of the reactants used in the synthesis. Although, 1
1 stoichiometry between the diammonium guest and the crown ether host (12C4) irrespective of the amount of the reactants used in the synthesis. Although, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio between the guest and the host has been anticipated, the solid state characterization sets the formulation of the compound 4 as [DADPMH2(12C4)]2(ClO4)4·H2O which crystallizes as a hydrate in P21/c space symmetry. Two symmetry independent host–guest complexes are located in the relevant crystal in which the two guests adopt different conformations as far as the orientation of the two phenyl rings is concerned. The guest cations interact with the crown ethers using two N–H bonds (dN⋯O = 2.835(5)–2.880(6) Å) and the third N–H bond is donated to a perchlorate counteranion (dN⋯O = 2.941(6)–3.016(6) Å). The other ammonium end of the guests interact with the counteranions or with the lattice water molecule (dN⋯O = 2.693(6)–3.099(6) Å). Both the crown ether units in the crystal structure of compound 4 adopt gauche “– – – –” conformation with the O–C–C–O dihedral angles in the range of 60 ± 6° for the macrocycle consisting of O1–O4 heteroatoms and 60 ± 1° for the crown ether unit comprising O5–O8 heteroatoms. A chain-like packing topology is observed in the crystal lattice as shown in Fig. 4.
2 stoichiometric ratio between the guest and the host has been anticipated, the solid state characterization sets the formulation of the compound 4 as [DADPMH2(12C4)]2(ClO4)4·H2O which crystallizes as a hydrate in P21/c space symmetry. Two symmetry independent host–guest complexes are located in the relevant crystal in which the two guests adopt different conformations as far as the orientation of the two phenyl rings is concerned. The guest cations interact with the crown ethers using two N–H bonds (dN⋯O = 2.835(5)–2.880(6) Å) and the third N–H bond is donated to a perchlorate counteranion (dN⋯O = 2.941(6)–3.016(6) Å). The other ammonium end of the guests interact with the counteranions or with the lattice water molecule (dN⋯O = 2.693(6)–3.099(6) Å). Both the crown ether units in the crystal structure of compound 4 adopt gauche “– – – –” conformation with the O–C–C–O dihedral angles in the range of 60 ± 6° for the macrocycle consisting of O1–O4 heteroatoms and 60 ± 1° for the crown ether unit comprising O5–O8 heteroatoms. A chain-like packing topology is observed in the crystal lattice as shown in Fig. 4.
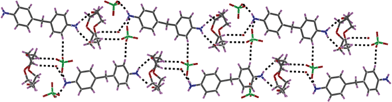 | ||
| Fig. 4 Formation of supramolecular chain through several non-covalent interactions in the lattice of compound 4. | ||
An alteration of the crown ethers from the smaller 12C4 to the larger 15C5 reveal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry between the DADPMH2 guest and the crown ether hosts in compound 5. The crystal structure of compound 5 exhibits the formulation of the concerned solid to be [DADPMH2(15C5)2](ClO4)2i.e. two hydrogen bonded crown ether hosts per formula unit. The compound 5 crystallizes in triclinic centrosymmetric space group i.e. P-1 with one gross diperchlorate salt of the host–guest complex in the asymmetric unit. Each of the two macrocyclic host units in the pertinent solid uses two alternate oxygen acceptor heteroatoms to interact with the ammonium guest moieties and the corresponding donor to acceptor separations (N⋯O) are found to be in the range 2.855–2.948 Å and 2.906–2.972 Å. As the 15C5 host units in the crystal structure of compound 5 is hydrogen bonded with only two N–H bonds of the guest functionalities, involvement of the perchlorate counteranions is required in order to saturate the hydrogen bonding ambience around the guest ammonium cation. The corresponding N(ammonium)⋯O(perchlorate) distances are found to be in the range 2.831–2.881 Å which are shorter than the relevant N(ammonium)⋯O(crown ether) distances. The two host units in the crystal structure of compound 5 are found to be puckered to different extents as indicated by the deviation of the five oxygen atoms of the crown ethers from the mean plane defined by them. The O–C–C–O torsion angles in both the crown ether units are found to be gauche 60 ± 6° and the conformation of the crown ethers are observed to be “+ + + + −” for the O1–O5 host and as “+ + + − −” for the O6–O10 host. Involvement of the various C–H⋯O and N–H⋯O contacts in the crystal lattice leads to a chain-like arrangement as shown in Fig. 5.
2 stoichiometry between the DADPMH2 guest and the crown ether hosts in compound 5. The crystal structure of compound 5 exhibits the formulation of the concerned solid to be [DADPMH2(15C5)2](ClO4)2i.e. two hydrogen bonded crown ether hosts per formula unit. The compound 5 crystallizes in triclinic centrosymmetric space group i.e. P-1 with one gross diperchlorate salt of the host–guest complex in the asymmetric unit. Each of the two macrocyclic host units in the pertinent solid uses two alternate oxygen acceptor heteroatoms to interact with the ammonium guest moieties and the corresponding donor to acceptor separations (N⋯O) are found to be in the range 2.855–2.948 Å and 2.906–2.972 Å. As the 15C5 host units in the crystal structure of compound 5 is hydrogen bonded with only two N–H bonds of the guest functionalities, involvement of the perchlorate counteranions is required in order to saturate the hydrogen bonding ambience around the guest ammonium cation. The corresponding N(ammonium)⋯O(perchlorate) distances are found to be in the range 2.831–2.881 Å which are shorter than the relevant N(ammonium)⋯O(crown ether) distances. The two host units in the crystal structure of compound 5 are found to be puckered to different extents as indicated by the deviation of the five oxygen atoms of the crown ethers from the mean plane defined by them. The O–C–C–O torsion angles in both the crown ether units are found to be gauche 60 ± 6° and the conformation of the crown ethers are observed to be “+ + + + −” for the O1–O5 host and as “+ + + − −” for the O6–O10 host. Involvement of the various C–H⋯O and N–H⋯O contacts in the crystal lattice leads to a chain-like arrangement as shown in Fig. 5.
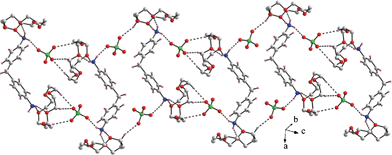 | ||
| Fig. 5 A portion of crystal packing showing various hydrogen bridges in compound 5. | ||
DADPMH2 undergoes complexation with 18C6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio between the guest and the host in compound 6 which crystallizes as a mixed water–acetonitrile solvate in monoclinic centric P21/n space symmetry. The concerned asymmetric unit consists of two DADPMH2(18C6)2 supramolecular complexes, four perchlorate counteranions, six water molecules and an acetonitrile solvent molecule. The ammonium ends of the guest dications incorporate in the crown ether cavity through three N–H···O interactions involving three alternate oxygen acceptor heteroatoms of the host (dN⋯O = 2.826(4)–2.930(4) Å). The four crown ether units in the crystal structure of compound 6 adopt quasi-D3d “+ − + − + −” conformation as revealed by the O–C–C–O torsion of 60 ± 6 − 7° (gauche) and C–O–C–C dihedral angle of ca. 180 ± 3 − 5°. The lattice water molecules are found to be involved in extensive supramolecular interactions with the various counterparts. A view of packing of the host–guest complexes in compound 6 has been shown in Fig. 6.
2 stoichiometric ratio between the guest and the host in compound 6 which crystallizes as a mixed water–acetonitrile solvate in monoclinic centric P21/n space symmetry. The concerned asymmetric unit consists of two DADPMH2(18C6)2 supramolecular complexes, four perchlorate counteranions, six water molecules and an acetonitrile solvent molecule. The ammonium ends of the guest dications incorporate in the crown ether cavity through three N–H···O interactions involving three alternate oxygen acceptor heteroatoms of the host (dN⋯O = 2.826(4)–2.930(4) Å). The four crown ether units in the crystal structure of compound 6 adopt quasi-D3d “+ − + − + −” conformation as revealed by the O–C–C–O torsion of 60 ± 6 − 7° (gauche) and C–O–C–C dihedral angle of ca. 180 ± 3 − 5°. The lattice water molecules are found to be involved in extensive supramolecular interactions with the various counterparts. A view of packing of the host–guest complexes in compound 6 has been shown in Fig. 6.
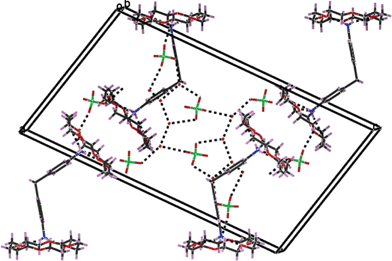 | ||
| Fig. 6 Hydrogen bonded assembly in compound 6. | ||
In compound 7, the guest is DADPEH2—which is similar to DADPMH2—possesses an angular geometry, the only difference being the sp3 ethereal (–O–) spacer between the two phenyl rings in the former dication as compared to the methylene (–CH2–) spacer in the latter. As a result, analogous to compound 6, a similar type of host–guest complexation is expected in case of compound 7, which actually is the case as observed through the X-ray diffraction. Compound 7 crystallizes as an acetonitrile solvate and the crystal structure of the relevant perchlorate salt evaluates the formulation of compound 7 as [DADPEH2(18C6)2](ClO4)2·2CH3CN. This compound maintains packing of the pertinent formula units in a triclinic centrosymmetric space group i.e. P-1 and the asymmetric unit of the crystal structure is comprised of one gross formula unit and two acetonitrile solvent molecules. Both the ammonium ends of the guest di-cation perch into the crown ether cavity through three N–H⋯O hydrogen bonds with the three alternate oxygen atoms of the crown ether. The corresponding N1(ammonium⋯O(crown ether) and N2(ammonium)⋯O(crown ether) distances are observed to be in the range 2.827–2.893 and 2.839–2.925 Å respectively. Similarly, both the crown ethers in the crystal structure of compound 7 adopt very symmetrical “+ − + − + −” conformation with O–C–C–O and C–O–C–C torsions of about 60 ± 8° and 180 ± 9° respectively. Therefore, the basic host–guest scenario in both the crystal structures of compound 6 and 7 is almost identical. Although varying the guest leads to alteration of space symmetry, the issue is not very straightforward as the relevant host–guest complexes (compounds 6 and 7) have been isolated as solvates in different extents. With the intention of getting rid of the solvent molecules to obtain the solvent free X-ray data for compounds 6 and 7, crystals of the relevant solids have been heated in an inert atomosphere (or under vacuum) which results in the loss of crystallinity of the samples. A chain-like packing topology has been observed in the lattice of compound 7 (Fig. 7).
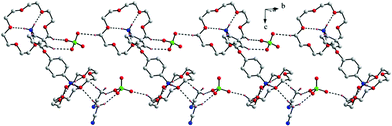 | ||
| Fig. 7 Chain-like packing topology in compound 7. | ||
Thermal analysis
The thermal durability of the synthesized solids have been checked by heating them in the temperature range 30–500 °C under nitrogen atmosphere in a thermogravimetric analyzer. However, among the seven compounds described in this article, only compound 1 exhibits excellent thermal stability. The thermal behavior of compound 1 is shown in Fig. 8, which illustrates almost no weight loss up to 230 °C. The 5 and 10% weight loss of this compound occurs only at 290 and 315 °C respectively and the corresponding DTA graph (not shown in the picture) shows a sharp melting point at 162 °C.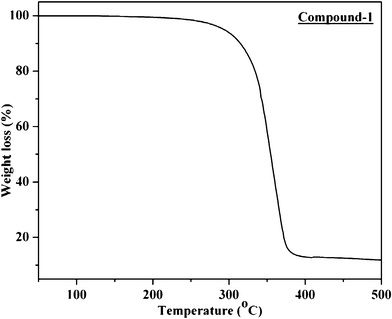 | ||
| Fig. 8 Thermal analysis of compound 1. | ||
Conclusions
In summary, a series of crown ether based host–guest systems have been structurally characterized and the underlying supramolecular chemistry has been presented in this article. Crown ethers are paradigms of supramolecular chemistry and hitherto, a diverse class of crystallographically characterized supramolecular complexes featuring the crown ethers as hosts has been reported. Attempts to cocrystallize 3-aminopyridine or the neutral amines (DADPM and DADPE) with the crown ethers i.e. to isolate the host–guest systems utilizing the pertinent amine compounds in lieu of the ammonium functionalities as the guests have remained unsuccessful although, there are some reports illustrating amine–(crown ether) supramolecular systems. The compounds 1–7 have thus been isolated as their perchlorate salts, the crystallographic analysis of which resembles an established principle i.e. the hydrogen bonding acceptance as well as the donation propensity of crown ethers. While the N–H⋯O hydrogen bridges between the ammonium functionalities and the oxygen acceptor heteroatoms of the crown ethers are responsible for the guest integration within the host cavity, the weak C–H⋯O interaction between the crown ethers and the perchlorate counteranions in the crystal lattice of compounds 1–7 concomitantly play an important role in packing the host–guest complexes. The differing behavior of 12C4 in compounds 1 (chiral packing) and 4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry) is not very clear to us at this level of investigation.
1 stoichiometry) is not very clear to us at this level of investigation.
Acknowledgements
The authors thank Department of Science and Technology (DST), Government of India (Project No. SR/S1/IC-23/2007) and Centre for Nanotechnology at University of Hyderabad for financial support. National single crystal X-ray diffraction facility at the University of Hyderabad by DST is gratefully acknowledged. T.C. and M.S. thank CSIR and UGC, India, respectively for their fellowships.References
- (a) C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017 CrossRef CAS; (b) C. J. Pedersen, J. Am. Chem. Soc., 1970, 92, 391 CrossRef CAS; (c) C. J. Pedersen, J. Am. Chem. Soc., 1970, 92, 386 CrossRef CAS; (d) C. J. Pedersen, Science, 1988, 241, 536 CAS; (e) C. J. Pedersen, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 12, 7 CrossRef.
- (a) J. M. Lehn, In Comprehensive Supramolecular Chemistry; Elsevier: Armsterdam, 1996 Search PubMed; (b) B. Dietrich, P. Viout, J. M. Lehn, In Macrocyclic Chemistry: Aspects of Organic and Inorganic Supramolecular Chemistry; VCH: New York, 1993 Search PubMed; (c) J. M. Lehn, In Nobel Lectures in Chemistry (1981-1990); World Scientific Publishing Co. Pte. Ltd.: Singapore, 1992, p. 444 Search PubMed; (d) J. M. Lehn, In Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, 1995 Search PubMed; (e) J. L. Atwood, J. W. Steed, In Encyclopaedia of Supramolecular Chemistry; Taylor & Francis: New York, 2004 Search PubMed.
- (a) J. W. Steed, Coord. Chem. Rev., 2001, 215, 171 CrossRef CAS and references therein; (b) P. C. Junk, New J. Chem., 2008, 32, 762 RSC and references therein; (c) B. R. Bhogala and A. Nangia, Cryst. Growth Des., 2006, 6, 32 CrossRef CAS; (d) P. C. Junk and J. L. Atwood, J. Chem. Soc., Dalton Trans., 1997, 4393 RSC; (e) M. J. Zaworotko, C. R. Kerr and J. L. Atwood, Organometallics, 1985, 4, 238 CrossRef CAS; (f) V. C. Kravtsov, M. S. Fonari, M. J. Zaworotko and J. Lipkowski, Acta. Crystallogr., 2002, C58, o683 CAS; (g) T. Akutagawa, T. Hasegawa, T. Nakamura, S. Takeda, T. Inabe, K. Sugiura, Y. Sakata and A. E. Underhil, Inorg. Chem., 2000, 39, 2645 CrossRef CAS; (h) M. S. Fonari, Y. A. Simonov, W. -J. Wang, S. -W. Tang and E. V. Ganin, CrystEngComm, 2009, 11, 94 RSC; (i) D. Sato, T. Akutagawa, S. Takeda, S. Noro and T. Nakamura, Inorg. Chem., 2007, 46, 363 CrossRef CAS; (j) S. Nishihara, T. Akutagawa, T. Hasegawa and T. Nakamura, Chem. Commun., 2002, 408 RSC.
- (a) D. Braga, M. Gandolfi, M. Lusi, D. Paolucci, M. Polito, K. Rubini and F. Grepioni, Chem.–Eur. J., 2007, 13, 5249 CrossRef CAS; (b) D. Braga, M. Curzi, M. Lusi and F. Grepioni, CrystEngComm, 2005, 7, 276 RSC; (c) C. W. Yoon, P. J. Carroll and L. G. Sneddon, J. Am. Chem. Soc., 2009, 131, 855 CrossRef CAS; (d) T. Akutagawa, D. Sato, Q. Ye, S. Noro and T. Nakamura, Dalton Trans., 2010, 39, 2191 RSC; (e) M. S. Fonari, E. V. Ganin, S. S. Basok, K. A. Lyssenko, M. J. Zaworotko and V. Ch. Kravtsov, Cryst. Growth Des., 2010, 10, 5210 CrossRef CAS; (f) D. Braga, S. D'Agostino, F. Grepioni, M. Gandolfi and K. Rubini, Dalton Trans., 2011, 40, 4765 RSC and references therein; (g) D. Braga, S. d'Agostino, M. Polito, K. Rubini and F. Grepioni, CrystEngComm, 2009, 11, 1994 RSC; (h) E. V. Ganin, S. S. Basok, A. A. Yavolovskii, M. M. Botoshanskyc and M. S. Fonari, CrystEngComm, 2011, 13, 674 RSC; (i) M. Calleja, S. A. Mason, P. D. Prince, J. W. Steed and C. Wilkinson, New J. Chem., 2003, 27, 28 RSC; (j) J. W. Steed, E. Sakellariou, P. C. Junk and M. K. Smith, Chem.–Eur. J., 2001, 7, 1240 CrossRef CAS; (k) C. W. Yoon, P. J. Carroll and L. G. Sneddon, J. Am. Chem. Soc., 2009, 131, 855 CrossRef CAS; (l) Y. Li, N. Hao, E. Wang, M. Yuan, C. Hu, N. Hu and H. Jia, Inorg. Chem., 2003, 42, 2729 CrossRef CAS; (m) W. You, E. Wang, Y. Xu, Y. Li, L. Xu and C. Hu, Inorg. Chem., 2001, 40, 5468 CrossRef CAS; (n) S. Kiviniemi, A. Sillanpää, M. Nissinen, K. Rissanen, M. T. Lämsä and J. Pursiainen, Chem. Commun., 1999, 897 RSC; (o) S. Kiviniemi, M. Nissinen, M. T. Lämsä, J. Jalonen, K. Rissanen and J. Pursiainen, New J. Chem., 2000, 24, 47 RSC.
- (a) R. D. Rogers and A. N. Rollins, J. Chem. Crystallogr., 1994, 24, 531 CrossRef CAS; (b) R. D. Rogers, A. N. Rollins, R. F. Henry, J. S. Murdoch, R. D. Etzenhouser, S. E. Huggins and L. Nunez, Inorg. Chem., 1991, 30, 4946 CrossRef CAS; (c) R. D. Rogers, A. H. Bond, W. G. Hipple, A. N. Rollins and R. F. Henry, Inorg. Chem., 1991, 30, 2671 CrossRef CAS; (d) H. Hassaballa, J. W. Steed, P. C. Junk and M. R. J. Elsegood, Inorg. Chem., 1998, 37, 4666 CrossRef CAS; (e) H. Hassaballa, J. W. Steed and P. C. Junk, Chem. Commun., 1998, 577 RSC; (f) Z. P. Ji and R. D. Rogers, J. Chem. Crystallogr., 1994, 24, 415 CrossRef CAS; (g) R. D. Rogers, A. H. Bond, S. Aguinaga and A. Reyes, J. Am. Chem. Soc., 1992, 114, 2967 CrossRef CAS; (h) M. Calleja, S. A. Mason, P. D. Prince, J. W. Steed and C. Wilkinson, New J. Chem., 2001, 25, 1475 CAS; (i) K. Johnson and J. W. Steed, Chem. Commun., 1998, 1479 RSC; (j) P. C. Junk and J. W. Steed, J. Chem. Soc., Dalton Trans., 1999, 407 RSC; (k) R. Luboradzki, J. Lipkowski, Y. A. Simonov, M. S. Fonari, E. V. Ganin and A. A. Yavolovskii, J. Inclusion Phenom. Macrocyclic Chem., 2001, 40, 59 CrossRef CAS; (l) R. D. Rogers, L. K. Kurihara and M. M. Benning, Inorg. Chem., 1987, 26, 4346 CrossRef CAS.
- (a) T. Chatterjee, M. Sarma and S. K. Das, Cryst. Growth Des., 2010, 10, 3149 CrossRef CAS; (b) T. Chatterjee, M. Sarma and S. K. Das, J. Mol. Struct., 2010, 981, 34 CrossRef CAS.
- T. Akutagawa, D. Endo, H. Imai, S. Noro, L. Cronin and T. Nakamura, Inorg. Chem., 2006, 45, 8628 CrossRef CAS.
- (a) V. R. Vangala, R. Mondal, C. K. Broder, J. A. K. Howard and G. R. Desiraju, Cryst. Growth Des., 2005, 5, 99 CrossRef CAS; (b) A. Dey, G. R. Desiraju, R. Mondal and J. A. K. Howard, Chem. Commun., 2004, 2528 RSC.
- Based on Cambridge Structural Database (CSD) version 5.32, November 2010 at University of Hyderabad.
- (a) SAINT: Software for the CCD Detector System; Bruker Analytical X-ray Systems, Inc.: Madison, WI, 1998 Search PubMed; (b) SADABSProgram for absorption correction; Sheldrick, G. M.University of Göttingen: Göttingen, Germany, 1997 Search PubMed; (c) SHELXS-97: Program for structure solution; Sheldrick, G. M.University of Göttingen: Göttingen, Germany, 1997 Search PubMed; (d) SHELXL-97: Program for Crystal Structure Analysis; Sheldrick, G. M.University of Göttingen: Göttingen, Germany, 1997 Search PubMed.
- P. Van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194 CrossRef.
Footnote |
| † CCDC reference numbers 846227–846233. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20109g |
| This journal is © The Royal Society of Chemistry 2012 |
