Heteroditopic p-tert-butyl thiacalix[4]arenes for creating supramolecular self-assembles by cascade or commutative mechanisms†
Elena A.
Yushkova
a,
Ivan I.
Stoikov
*a,
Arkadiy Yu.
Zhukov
a,
Joshua B.
Puplampu
a,
Ildar Kh.
Rizvanov
b,
Igor S.
Antipin
a and
Alexander
Konovalov
a
aKazan (Volga Region) Federal University, A.M. Butlerov Chemical Institute, 420008, Kremlevskaya, 18, Kazan, Russian Federation. E-mail: Ivan.Stoikov@mail.ru; Tel: +7-8432-337463; Fax: +7-8432-752253
bRussian Academy of Sciences, A.E. Arbuzov Institute of Organic and Physical Chemistry, Kazan, Russian Federation
First published on 12th March 2012
Abstract
New p-tert-butylthiacalix[4]arenes functionalized with hydrazides of nicotinic, isonicotinic, 3-nitrobenzoic acids, 2-hydrazinopyridine, phenylhydrazine, benzotriazole groups at the lower rim in cone, partial cone and 1,3-alternate conformation have been synthesized. The mechanism of self-assembly of supramolecular nanosized particles based on functionalized p-tert-butylthiacalix[4]arenes with silver nitrate and or dicarboxylic acids (oxalic, malonic, succinic acid) has been determined by dynamic light scattering . For the first time, it has been shown that nanoscale particles based on p-tert-butylthiacalix[4]arenes, capable of recognizing metal cations and dicarboxylic acids can form cascade or commutative three-component supramolecular systems. Also for the first time, it has been shown that p-tert-butylthiacalix[4]arenes containing N-substituted hydrazide and heterocyclic fragments are coreceptors, capable of simultaneously binding silver (I) cations and dicarboxylic acids. The formation of cascade systems: “macrocycle-silver (I) nitrate-dicarboxylic acid” is a characteristic of p-tert-butyl thiacalix[4]arenes containing N-substituted hydrazide fragments.
Introduction
The development of self-assembled micro- and nano-sized supramolecular systems with a given property is one of the most rapidly developing fields in organic and supramolecular chemistry.1–4 The structure and properties of supermolecules and supramolecular assembles can be predetermined during the covalent receptor synthesis.3–12 Designed synthetic receptors capable of recognizing different types of “guests” have been employed in the construction of sensors, catalysts, biomimetic systems, selective extractants, drug delivery systems and programmable materials.12–20Various macrocyclic “building” platforms e.g., crown ethers, cyclodextrins and calixarenes, are currently used in the development of synthetic receptors.3,4,16,21,22 The uniqueness of thiacalix[4]arenes, analogues of classical calixarenes, is that: (1) the initial macrocycles are easily obtained by one-step synthesis,21–23 (2) they have sulfide bridged fragments capable of coordinating transition metal cations,24 (3) the upper and lower rims of the macrocycle can be modified by various functional groups,21,22 (4) they exist in multiple conformations that can position binding sites in desired spatial orientations25–27 and, (5) allosteric effect can be realized during the binding of “guests.”28,29
Recently, Lotak's research group demonstrated that thiacalix[4]arenes containing alkoxy groups (n-PrO– and MeO–) formed solid-state assembles during their interaction with silver triflate (I).24 The aggregation of p-tert-butylthiacalix[4]arene stereoisomers with alkali metal nitrate, p- and d-elements in dichloromethane has been studied by dynamic light scattering.30–32 The hydrodynamic diameters of nanoaggregates, polydispersity index (PDI, particle size distribution), and molecular weights (for the determination of the number of structural fragments, N, i.e. p-tert-butylthiacalix[4]arene molecules and metal cations, which form nanoscale associates) have been determined.33,34 It has been shown that depending on the conformation of the macrocycle, the nature of binding sites and the nature of metal cations, p-tert-butylthiacalix[4]arenes and metal nitrates are capable of forming not only “guest–host” complexes, but also dimers, spherical, ellipsoidal and elongated nanosized particles.33,34
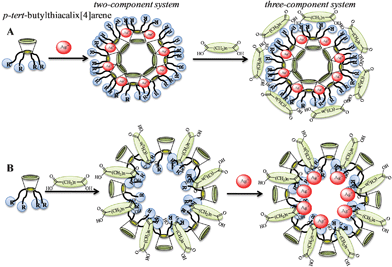
However, there has been no report on the possibility of supramolecular assemblies forming during the interaction of cationic, anionic and neutral substrates with the simultaneous participation of both the sulfide bridges and the functional groups of p-tert-butylthiacalix[4]arenes. Thus, we propose that the ability of the thiacalix[4]arene platform to form nanoscale aggregates with metal cations and dicarboxylic acids with the participation of different coordination sites, and the realization of allosteric effect during binding of “guests” should be exploited in the development of cascade and commutative (three-component) nanoscale systems (Fig. 1).
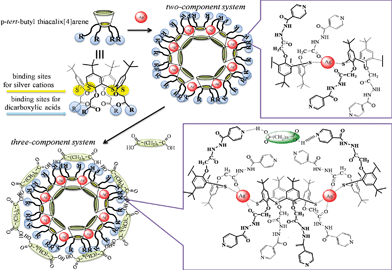 | ||
| Fig. 1 The possible ways of forming two- and three-component systems. | ||
During the synthesis of receptors based on thiacalix[4]arene platform containing the same substituents with different coordination centers (electron donor and or electron acceptor, proton donor and or proton acceptor groups), different binding sites can be introduced into the macrocycle.
In this work, we describe the synthesis of p-tert-butylthiacalix[4]arenes tetrasubstituted by N-substituted hydrazide and heterocyclic fragments at the lower rim and the design of self-assembled supramolecular nanoparticles based on these macrocycles with silver nitrate and or dicarboxylic acids. Also, the mechanism of self-assembly and aggregation which led to the formation of supramolecular assembles in solution has been discussed.
Results and discussion
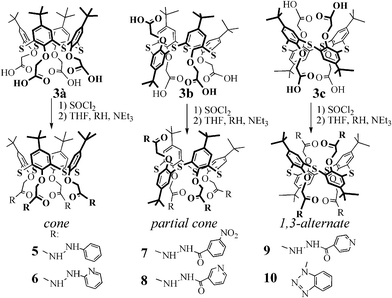
Synthesis of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim containing fragments capable of binding metal cations and dicarboxylic acids
One of the possible approaches to the design of polytopic coreceptors for specific substrates is the use of a combination of binding sites in the macrocyclic system.3–6 Thiacalix[4]arenes containing amide, hydrazide, acyl hydrazide and heterocyclic fragments at the lower rim in cone, partial cone and 1,3-alternate conformations, contain several potential coordination centers for metal cations (bridging sulfur atoms, oxygen atoms of oxymethylene fragments, acyl hydrazide groups and nitrogen atoms of heterocycles,35Fig. 2, A and B), and for carboxylic acids (protons of hydrazide groups and heterocyclic fragments,36,37Fig. 2, C and D). Obviously, these macrocycles are promising supramolecular building blocks capable of forming complexes such as “guest–host” and or extended associates with different substrates.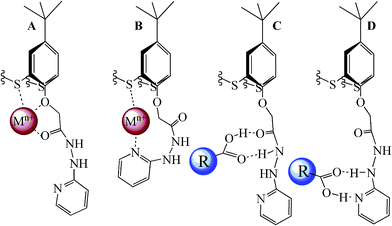 | ||
| Fig. 2 The possible coordination patterns of the “hard” (A), “soft” (B) metal cations and dicarboxylic acids (C, D). | ||
Tetraesters based on p-tert-butylthiacalix[4]arene stereoisomers 2a–c were chosen as the initial reagents for the synthesis of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim by N-substituted hydrazide fragments in three conformations (cone, partial cone and 1,3-alternate). Different methods for the synthesis of target products have been studied: (a) the reaction of hydrazine with tetraesters based on p-tert-butylthiacalix[4]arene stereoisomers 2a–c33 and (b) hydrolysis of the stereoisomeric tetraesters 2a–c to the appropriate tetraacids 3a–c, with subsequent conversion to acylating derivatives (activated esters, anhydrides, chlorides) followed by their interaction with the corresponding hydrazines.38–40
The first approach significantly shortens the synthetic path to the target compound. As a rule, high yields of target products are obtained during hydrazinolysis of ester groups at high temperatures (about 150 °C) with an excess of a sterically unhindered N-substituted hydrazine.38–40 However, under these conditions, the reagent used may take part in adverse reactions: oxidation and polymerization. Thus, for selective acylation of N-substituted hydrazines, activated ester and anhydride fragments are used.38–42 Using the highly reactive acyl chlorides, side reactions with the N-substituted hydrazine functional groups may occur. Tetraesters cone-2a, partial cone-2b and 1,3-alternate-2c conformations were synthesized by preliminarily alkylating the initial p-tert-butylthiacalix[4]arenes with ethyl bromoacetate in the presence of alkali metal carbonates.23 Using phenylhydrazine as an example of a model compound, the hydrazinolysis of stereoisomeric tetraesters based on p-tert-butylthiacalix[4]arenes 2a–c has been studied. Stereoisomers of the p-tert-butylthiacalix[4]arenes tetrasubstituted by phenylhydrazide groups were not obtained under the conditions of hydrazinolysis studied. Moreover, depending on the reaction conditions, the initial tetraesters 2a–c, or a difficult to separate mixture of p-tert-butylthiacalix[4]arenes differently substituted at the lower rim, were identified.
Different approaches for the synthesis of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim have been studied. Hydrolysis of tetraesters 2a–c in the presence of excess lithium hydroxide in aqueous tetrahydrofuran yielded tetra acids based on p-tert-butylthiacalix[4]arene in cone-3a, partial cone-3b and 1,3-alternate-3c conformations. Thiacalix[4]arenes containing ester fragments with n-nitrophenyl groups and macrocycles containing the mixed anhydride fragments were then obtained in situ. However, both the interaction of acyl chlorides with p-nitrophenol and subsequent reaction of the acylation products with phenylhydrazine, and the interaction of tetraacids with methyl chloroformate and subsequent reaction of the mixture of anhydrides with phenylhydrazine, resulted in a difficult to separate mixture of products. The use of phenylhydrazine in the acylation of p-tert-butylthiacalix[4]arenes with ester, activated ester and anhydride fragments did not lead to the target products. In order to increase the reactivity of the acylating reagents, the three isomeric acids 3a–c were converted into their respective acyl chlorides by boiling in SOCl2. The interaction of the N-substituted hydrazines, hydrazides and benzotriazole with acyl chlorides based on stereoisomers of p-tert-butylthiacalix[4]arenes 3a–c in tetrahydrofuran was studied.
In order to avoid the possible oxidation of phenylhydrazine during acylation by acyl chlorides of tetraacids 3a–c, the reaction was carried out in tetrahydrofuran at room temperature. In the case of the cone and partial cone stereoisomers, target compounds could not be identified due to the formation of a difficult to separate mixture of products (according to 1H NMR spectroscopy). The acylation of hydrazides of nicotinic, isonicotinic, 3-nitrobenzoic acid, 2-hydrazinopyridine and benzotriazole by acyl chlorides of tetraacids 3a–c was carried out in tetrahydrofuran. p-tert-Butylthiacalix[4]arenes 5–10 tetrasubstituted by the respective hydrazides at the lower rim in cone, partial cone and 1,3-alternate conformations were obtained with 41–95% yields (Table 1).
| Compound | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|
| (a) cone | — | 93 | 90 | 57 | 80 | 77 |
| (b) partial cone | — | 90 | 95 | 50 | 50 | 70 |
| (c) 1,3-alternate | 93 | 91 | 41 | 57 | 89 | 75 |
The structure and composition of the synthesized p-tert-butylthiacalix[4]arenes 5–10 tetrasubstituted at the lower rim were determined by physical methods: 1H and 13C NMR, IR spectroscopy, mass spectrometry. The conformations of the macrocycles were established by one-dimensional 1H and two-dimensional 1H–1H NOESY NMR spectroscopy.
The conformation (cone, partial cone, 1,3-alternate) of p-tert-butylthiacalix[4]arenes 5–10 tetrasubstituted at the lower rim was confirmed by 1H NMR spectroscopy. The stereoisomers of p-tert-butylthiacalix[4]arene, cone and 1,3-alternate have a highly symmetric structure compared to the partial cone conformation. Thus, the structure of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim in partial cone conformation can be undoubtedly determined by 1H NMR spectroscopy (Fig. 3). The cone and 1,3-alternate stereoisomers have the same set of proton signals in the 1H NMR spectra (Fig. 4 and 5).
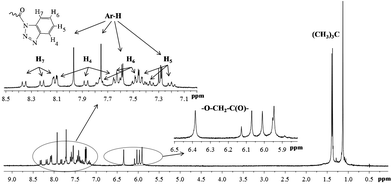 | ||
| Fig. 3 1H NMR spectra of the compound 10b (in CDCl3, 25 °C, 300 MHz). | ||
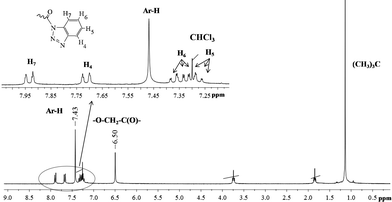 | ||
| Fig. 4 1H NMR spectra of the compound 10a (in CDCl3, 25 °C, 300 MHz). | ||
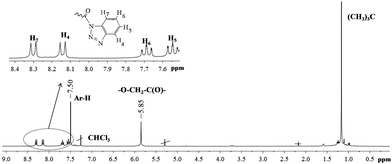 | ||
| Fig. 5 1H NMR spectra of the compound 10c (in CDCl3, 25 °C, 300 MHz). | ||
Nevertheless, the conformational differentiation of cone and 1,3-alternate stereoisomers of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim can be achieved by comparing the chemical shifts of the oxymethylene protons and aryl fragments of macrocycles in 1H NMR spectra.23,43–45 From the literature, we know that the oxymethylene proton signals of the cone stereoisomer of p-tert-butylthiacalix[4]arene derivatives are found in weaker fields and that of the aromatic protons in stronger fields compared to those of the 1,3-alternate, hence the structure of compounds 10a (cone) and 10c (1,3-alternate) were established as such.
Analysis of the 1H NMR spectra of the synthesized macrocycles (Table 2) showed that the chemical shifts of the oxymethylene proton signals of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim strongly depend on the nature of functional groups. As can be seen in Table 2, for the 1,3-alternate stereoisomers in CDCl3, a shift of oxymethylene proton signals of these macrocycles to weaker fields is observed during transition to a more electronegative substituent in the range: N-phenylhydrazide, 2-pyridylhydrazide, 3-nitrobenzylhydrazide, hydrazides of nicotinic, isonicotinic acids and benzotriazole.
| Compound | C(CH3)3 | OCH2C(O) | Ar–H | |||
|---|---|---|---|---|---|---|
| a Low solubility of the compound in the solvent. | ||||||
| CDCl3 | (CD3)2SO | CDCl3 | (CD3)2SO | CDCl3 | (CD3)2SO | |
| 5c | 1.20 | 1.15 | 4.41 | 4.49 | 7.53 | 7.63 |
| 6a | a | 1.11 | a | 5.01 | a | 7.40 |
| 6c | 1.09 | 1.15 | 4.45 | 4.53 | 7.46 | 7.65 |
| 7a | a | 1.11 | a | 5.14 | a | 7.47 |
| 7c | 1.18 | 1.23 | 4.53 | 4.65 | 7.52 | 7.70 |
| 8a | 1.12 | 1.11 | 4.84 | 5.13 | 7.39 | 7.46 |
| 8c | 1.13 | 1.22 | 4.54 | 4.64 | 7.49 | 7.69 |
| 9a | a | 1.08 | a | 5.08 | a | 7.39 |
| 9c | 1.15 | 1.19 | 4.61 | 4.55 | 7.50 | 7.64 |
| 10a | 1.14 | a | 6.50 | a | 7.43 | a |
| 10c | 1.16 | a | 5.85 | a | 7.50 | a |
The study of the influence of the solvent on the proton chemical shifts of synthesized p-tert-butylthiacalix[4]arenes (Table 2) showed a similar tendency for the transition from CDCl3 to (CD3)2SO. As in the case of the 1,3-alternate stereoisomers, for the macrocycle in cone conformation in (CD3)2SO, the proton chemical shifts of tert-butyl groups of p-tert-butylthiacalix[4]arenes does not depend on the nature of the substituents. The aromatic and oxymethylene proton chemical shifts of the macrocycle in cone conformation change during the transition to a more electron acceptor functional group in contrast to 1,3-alternate conformation of stereoisomers (Table 2). An exception is the compound 9c (Table 2).
As was expected, the nature of solvent affected the chemical shifts of aromatic protons of the macrocycle, the tert-butyl groups and the oxymethylene protons of the studied p-tert-butylthiacalix[4]arenes. The transition from CDCl3 to (CD3)2SO resulted in a shift of proton signals in the weaker fields. The greatest changes were observed for the aromatic protons of the macrocycle ((CH3)3C < –OCH2– < ArH) (Table 2).
In the case of macrocycles 8a and 8c, containing the nicotinic acid hydrazide fragment, great differences in chemical shifts were observed for the tert-butyl, oxymethylene and aromatic protons in relation to the cone and 1,3-alternate stereoisomers in (CD3)2SO: Δδ = δcone−δ1,3-alternate, Δδ, ppm: (CH3)3C −0.01 (CDCl3), −0.12 ((CD3)2SO); –OCH2 −0.31 (CDCl3), −0.48 ((CD3)2SO); ArH −0.10 (CDCl3), −0.24 ((CD3)2SO). It should be noted that for all the investigated macrocycles containing hydrazide fragments, a strong proton shift of the –NHNH– group is observed in weaker fields during the transition from CDCl3 to (CD3)2SO. Obviously, this is attributed to the high proton acceptor ability of (CD3)2SO.
The spatial structure of synthesized compounds was also confirmed by two-dimensional 1H–1H NOESY spectroscopy. The observed cross-peaks clearly indicate the presence of p-tert-butylthiacalix[4]arenes in cone, partial cone and 1,3-alternate conformations.
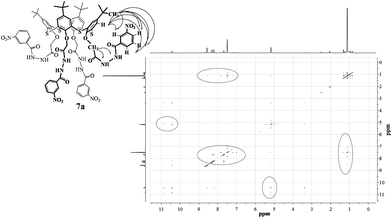
It is interesting to note that apart from the cross-peaks, which are attributed to dipole–dipole interactions between protons of the aromatic rings of the macrocycle with protons of tert-butyl groups and aromatic protons of neighboring rings, cross-peaks due to dipole–dipole interactions between the oxymethylene protons and protons of the aromatic substituents in the ortho or meta position, as well as between the protons of tert-butyl groups and aryl fragments of p-tert-butylthiacalix[4]arenes and the aromatic protons of the substituents are also observed for the cone stereoisomer in two-dimensional 1H–1H NOESY NMR spectra (thiacalix[4]arene 7a, Fig. 6). The observed cross-peaks suggest that macrocycle 7a exists in a pinched cone conformation, in which there is convergence of the heterocyclic substituents with tert-butyl groups and the aryl fragments of the macrocycle. Thus the conformational behavior of the macrocyclic ring of synthesized thiacalix[4]arenes in the cone conformation is due to the structure of the substituents at the lower rim.
Analysis of the 1H–1H NOESY NMR spectra of cone stereoisomers (6a–10a) showed that as a result of the increased length and the conformational mobility (heterocyclic-acyclic fragment) of substituents, the macrocycle in the pinched cone conformation becomes the most preferred during the transition from benzotriazole (10a) and 2-hydrazinopyridine (6a) fragments to 3-nitrobenzoic hydrazides (7a), hydrazides of nicotinic (8a) and isonicotinic (9a) acids. However, the conformational interconversion between the two pinched cone conformers of p-tert-butylthiacalix[4]arene derivatives possessing C2V symmetry via the cone conformation with C4V symmetry (Fig. 7) occur quite fast in the 1H NMR time interval. Hence registration of the individual conformers of p-tert-butylthiacalix[4]arene derivatives by this method is not possible.26
![Conformational interconversion: pinched cone–cone–pinched cone of the p-tert-butylthiacalix[4]arene derivatives.](/image/article/2012/RA/c2ra01255c/c2ra01255c-f7.gif) | ||
| Fig. 7 Conformational interconversion: pinched cone–cone–pinched cone of the p-tert-butylthiacalix[4]arene derivatives. | ||
From the 1H NMR spectra of compounds 5–9, the hydrazide proton signals of cone, partial cone and 1,3 alternate stereoisomers were observed in a weaker field (at 8.21–11.57 ppm) because of the formation of hydrogen bonds between the hydrazide groups. It well corresponds to the infrared spectra of macrocycles 5–9 in the solid state (KBr pellets at the wavenumber range from 400 to 4000 cm−1) in which the absorption bands of associated hydrazide groups (3385–3210 cm−1) are observed.
Thus, the polyfunctional receptors based on p-tert-butylthiacalix[4]arene platform containing similar fragments with multiple coordinated centers for both metal cations (bridging sulfur atoms, oxygen atoms of oxymethylene fragments, acyl hydrazide groups and nitrogen atoms of heterocycles) and carboxylic acids (hydrazide groups and heterocyclic fragments) were obtained. The presence of several different binding sites: electron donor and or electron acceptor, proton donor and or proton acceptor groups, in the structure of p-tert-butylthiacalix[4]arene derivatives capable of cooperatively interacting with multiple substrates, as well as the existence of different conformations of macrocycles with different possible spatial orientations of binding sites for each individual stereoisomer allows p-tert-butylthiacalix[4]arenes 5–10 to be used as components of self-assembled supramolecular aggregates.
The molecular recognition of dicarboxylic acids and silver cation (I) by p-tert-butyl thiacalix[4]arene derivatives
The presence of several types of binding sites (carboxyl, hydrazide, heterocyclic fragments) in the structure of the receptor allows the p-tert-butylthiacalix[4]arene to interact not only with metal cations, but also with dicarboxylic acids. Thus, it was proposed that as a result of competitive binding of either the metal cation or the other substrate (dicarboxylic acid) to one of the binding centers (hydrazide, heterocyclic fragment), an additional possibility of forming a cascade or commutative three-component supramolecular systems arises (Fig. 8).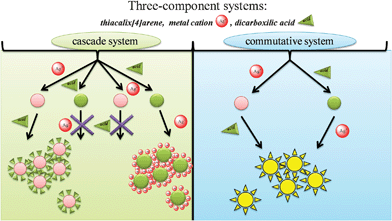 | ||
| Fig. 8 The possible ways for formation of the cascade and commutative three-component systems. | ||
Commutative systems or cosystems are those in which the complexation of several substrates is interconnected (commutative). In commutative systems, it does not matter which of the substrates bind in the first place46 (Fig. 8). In contrast to cascade systems, the binding of multiple substrates must occur in a specific order46 (Fig. 8).
p-tert-Butylthiacalix[4]arenes 5–10 in three conformations containing the same substituents with multiple coordination centers at the lower rim were selected for the formation of three-component supramolecular systems.
The stability constants, the stoichiometry of the complexes, substrate-p-tert-butylthiacalix[4]arene, formed in the organic system, methanol–dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the ability of the p-tert-butylthiacalix[4]arene derivatives 5–10 to recognize silver ions, oxalic, malonic and succinic acids, have been quantitatively determined by UV spectroscopy (Table 3).
1) and the ability of the p-tert-butylthiacalix[4]arene derivatives 5–10 to recognize silver ions, oxalic, malonic and succinic acids, have been quantitatively determined by UV spectroscopy (Table 3).
| System | n | lgKass |
|---|---|---|
| a [L]init = 10−5 M, [AgNO3]init. = 10−3 M. | ||
| 5c | 1.09 ± 0.08 | 4.19 ± 0.37 |
| 6a | 0.44 ± 0.01 | 1.88 ± 0.07 |
| 6b | 0.46 ± 0.03 | 2.31 ± 0.17 |
| 6c | 0.57 ± 0.05 | 3.53 ± 0.26 |
| 7a | 0.98 ± 0.25 | 3.76 ± 1.15 |
| 7b | 0.95 ± 0.06 | 3.48 ± 0.28 |
| 7c | 0.50 ± 0.06 | 1.37 ± 0.28 |
| 8a | 0.94 ± 0.05 | 4.90 ± 0.23 |
| 8b | 1.07 ±.0.06 | 5.97 ± 0.31 |
| 8c | 0.88 ± 0.05 | 5.05 ± 0.25 |
| 9a | 1.15 ± 0.06 | 6.33 ± 0.33 |
| 9b | 0.88 ± 0.04 | 5.00 ± 0.20 |
| 9c | 0.90 ± 0.05 | 5.11 ± 0.24 |
| 10a | 1.19 ± 0.07 | 6.47 ± 0.35 |
| 10b | 1.01 ± 0.11 | 5.44 ± 0.56 |
| 10c | 1.04 ± 0.15 | 5.46 ± 0.75 |
As expected, the stoichiometry of the formed associates, thiacalix[4]arene 5–10 with a silver cation, depends on the macrocycle conformation (Table 3). Thus, in the case of cone stereoisomers (6a–10a), the interaction with silver cations is only possible on one side of the macrocyclic platform with the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (Table 3).
1 complexes (Table 3).
A similar stoichiometry with this substrate was also obtained for partial cone stereoisomers (6b–10b), although the binding fragments are located on both sides of the macrocyclic platform. Hence the ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of substrate–p-tert-butyl thiacalix[4]arene indicates that a single functional group located on one side of macrocyclic platform cannot adequately bind silver cations. However, in the case of 1,3-alternate stereoisomer, with two binding fragments located on both sides of the macrocycle, one receptor molecule is capable of binding two cations with a 2
1) of substrate–p-tert-butyl thiacalix[4]arene indicates that a single functional group located on one side of macrocyclic platform cannot adequately bind silver cations. However, in the case of 1,3-alternate stereoisomer, with two binding fragments located on both sides of the macrocycle, one receptor molecule is capable of binding two cations with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Also, the macrocycles in the 1,3-alternate conformation had a stoichiometry (“guest”
1 stoichiometry. Also, the macrocycles in the 1,3-alternate conformation had a stoichiometry (“guest”![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) macrocycle) of 1
macrocycle) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (6b, 7b) and 1
2 (6b, 7b) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5c, 8c–10c). For complexes based on p-tert-butyl thiacalix[4]arene 7, 9, 10 containing hydrazide and heterocyclic fragments with the same values of stoichiometric coefficients, efficiency of interaction of macrocycles with silver (I) nitrate decreases in the order, cone, partial cone, 1,3-alternate, with an opposite tendency being observed for the macrocycle 6, containing 2-hydrazinopyridine fragments.
1 (5c, 8c–10c). For complexes based on p-tert-butyl thiacalix[4]arene 7, 9, 10 containing hydrazide and heterocyclic fragments with the same values of stoichiometric coefficients, efficiency of interaction of macrocycles with silver (I) nitrate decreases in the order, cone, partial cone, 1,3-alternate, with an opposite tendency being observed for the macrocycle 6, containing 2-hydrazinopyridine fragments.
Replacing the spherical silver cation (I) with dicarboxylic acid, the picture of molecular recognition by p-tert-butyl thiacalix[4]arenes 5–10 significantly changes. The study of the interaction of dicarboxylic acids (oxalic, malonic, succinic) as model substrates with macrocycles 5–10 by UV spectroscopy (Table 4) showed that the efficiency of substrate binding depends on the nature and conformation of the receptor. It has been shown that most of the p-tert-butyl thiacalix[4]arenes are able to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (Table 4). However, stoichiometric coefficients of 2
1 complexes (Table 4). However, stoichiometric coefficients of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with various acids have also been obtained (Table 4). In the case of cone stereoisomers (6a, 8a), the 1
2 with various acids have also been obtained (Table 4). In the case of cone stereoisomers (6a, 8a), the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Table 4) is typical of oxalic, malonic and succinic acids. The efficiency of the interaction of the macrocycle with the “guest” decreased according to acidity of acids: oxalic > malonic > succinic. It should be noted that only the interaction of p-tert-butyl thiacalix[4]arenes 9a in a cone conformation with malonic and succinic acids leads to the formation of complexes with 1
1 stoichiometry (Table 4) is typical of oxalic, malonic and succinic acids. The efficiency of the interaction of the macrocycle with the “guest” decreased according to acidity of acids: oxalic > malonic > succinic. It should be noted that only the interaction of p-tert-butyl thiacalix[4]arenes 9a in a cone conformation with malonic and succinic acids leads to the formation of complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, and logarithms of association constants not exceeding 2.
2 stoichiometry, and logarithms of association constants not exceeding 2.
| Oxalic acid | Malonic acid | Succinic acid | ||||
|---|---|---|---|---|---|---|
| Macrocycles | N | lgKass | N | lgKass | N | lgKass |
| a [L]init = 10−5 M, [acid]init. = 10−3 M. | ||||||
| 5c | 0.68 ± 0.08 | 2.20 ± 0.37 | 0.89 ± 0.09 | 3.17 ± 0.43 | 0.70 ± 0.05 | 2.41 ± 0.23 |
| 6a | 1.07 ± 0.06 | 5.38 ± 0.32 | 0.98 ± 0.05 | 5.00 ± 0.26 | 1.07 ± 0.03 | 5.33 ± 0.14 |
| 6b | 1.03 ± 0.09 | 4.18 ± 0.48 | — | — | — | — |
| 6c | 0.44 ± 0.02 | 0.43 ± 0.11 | — | — | — | — |
| 7c | 0.82 ± 0.12 | 3.01 ± 0.55 | 0.91 ± 0.15 | 3.10 ± 0.70 | 0.82 ± 0.11 | 2.90 ± 0.53 |
| 8a | 1.06 ± 0.05 | 4.10 ± 0.24 | 0.93 ± 0.05 | 3.24 ± 0.27 | 0.87 ± 0.07 | 2.43 ± 0.35 |
| 8b | 1.11 ± 0.06 | 4.63 ± 0.30 | — | — | — | — |
| 8c | 0.99 ± 0.05 | 4.32 ± 0.28 | 1.07 ± 0.11 | 4.40 ± 0.52 | — | — |
| 9a | 0.90 ± 0.08 | 4.30 ± 0.43 | 0.42 ± 0.03 | 1.47 ± 0.13 | 0.40 ± 0.02 | 1.16 ± 0.10 |
| 9b | 0.63 ± 0.08 | 2.62 ± 0.43 | — | — | — | — |
| 9c | 0.88 ± 0.06 | 3.91 ± 0.31 | 0.72 ± 0.05 | 2.85 ± 0.25 | — | — |
| 10a | 1.03 ± 0.09 | 4.16 ± 0.45 | — | — | — | — |
| 10b | 0.93 ± 0.05 | 3.47 ± 0.24 | 1.02 ± 0.10 | 3.35 ± 0.47 | — | — |
| 10c | 1.52 ± 0.12 | 6.15 ± 0.61 | — | — | — | — |
For the macrocycles 6b, 8b, 10b in partial cone conformation (Table 4), the stability of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes decreases in the range of acids; oxalic, malonic, succinic. Obviously, as it is with the case of cone stereoisomers, it can be attributed to the decrease in acidity and the increase in size of acids. Compounds 7a, 7b, containing 3-nitrobenzoic acid fragments do not bind to these dicarboxylic acids. In contrast to macrocycles in cone and partial cone conformations, the efficiency of interaction of the 1,3-alternate stereoisomers (Table 4) with oxalic, malonic, succinic acids does not change, and the stoichiometry of the associates, acid
1 complexes decreases in the range of acids; oxalic, malonic, succinic. Obviously, as it is with the case of cone stereoisomers, it can be attributed to the decrease in acidity and the increase in size of acids. Compounds 7a, 7b, containing 3-nitrobenzoic acid fragments do not bind to these dicarboxylic acids. In contrast to macrocycles in cone and partial cone conformations, the efficiency of interaction of the 1,3-alternate stereoisomers (Table 4) with oxalic, malonic, succinic acids does not change, and the stoichiometry of the associates, acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-tert-butyl thiacalix[4]arenes, in most cases is 1
p-tert-butyl thiacalix[4]arenes, in most cases is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The formation of 1
2. The formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes (acid
2 complexes (acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-tert-butyl thiacalix[4]arenes) is characteristic for only p-tert-butyl thiacalix[4]arenes 6c in 1,3-alternate conformation. Interestingly, only p-tert-butyl thiacalix[4]arene 5c, containing phenylhydrazide fragments and 7c, containing hydrazide of 3-nitrobenzoic acid are able to interact with dicarboxylic acids in the absence of additional binding sites (the nitrogen atoms of heterocyclic fragments) apparently due to protons of hydrazide groups.
p-tert-butyl thiacalix[4]arenes) is characteristic for only p-tert-butyl thiacalix[4]arenes 6c in 1,3-alternate conformation. Interestingly, only p-tert-butyl thiacalix[4]arene 5c, containing phenylhydrazide fragments and 7c, containing hydrazide of 3-nitrobenzoic acid are able to interact with dicarboxylic acids in the absence of additional binding sites (the nitrogen atoms of heterocyclic fragments) apparently due to protons of hydrazide groups.
The conformation of the studied p-tert-butyl thiacalix[4]arenes affects the efficiency of interaction not only with silver (I) nitrate but also with dicarboxylic acids: the stability of the complexes based on macrocycles 8 increases in the range for stereoisomers 1,3-alternate < cone < partial cone.
Self-assembly of aggregates consisting of p-tert-butyl thiacalix[4]arene derivatives with silver (I) nitrate and dicarboxylic acids in solution
The interaction of p-tert-butylthiacalix[4]arenes 5–10 and dicarboxylic acids with the formation of complexes with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric coefficients, and the formation of aggregates based on macrocycles containing secondary and tertiary amide fragments with metal cations in dichloromethane30,31 indicates the possibility of the p-tert-butylthiacalix[4]arenes 5–10 containing different binding sites (electron donor and or electron acceptor, proton donor and or proton acceptor groups), to form supramolecular associates with silver cations (I) and dicarboxylic acids in solution. Hence, the aggregation of thiacalix[4]arenes with silver cation (I), and dicarboxylic acids was studied by dynamic light scattering (DLS) in dichloromethane (Table S1, ESI†) since the use of binary solvent mixtures of dichloromethane–methanol, as in the case of UV-titration to determine the size of aggregates by DLS is not desirable.
2 stoichiometric coefficients, and the formation of aggregates based on macrocycles containing secondary and tertiary amide fragments with metal cations in dichloromethane30,31 indicates the possibility of the p-tert-butylthiacalix[4]arenes 5–10 containing different binding sites (electron donor and or electron acceptor, proton donor and or proton acceptor groups), to form supramolecular associates with silver cations (I) and dicarboxylic acids in solution. Hence, the aggregation of thiacalix[4]arenes with silver cation (I), and dicarboxylic acids was studied by dynamic light scattering (DLS) in dichloromethane (Table S1, ESI†) since the use of binary solvent mixtures of dichloromethane–methanol, as in the case of UV-titration to determine the size of aggregates by DLS is not desirable.
The hydrodynamic particle size and polydispersity index of the system was measured 28 h after the solution was prepared at 20 °C.
The study of the processes of self-assembly of p-tert-butylthiacalix[4]arenes 5–10 showed that the formation of aggregates with hydrodynamic diameters from 8 to 482 nm (Table S1) occurs only in the case of macrocycles 6, 8, 9 containing heterocyclic fragments. The ability to self-associate is expressed most in the macrocycles with the cone conformation.
The aggregation of compounds 5–10 with silver (I) nitrate and dicarboxylic acids has been studied in CH2Cl2 by dynamic light scattering. It was found that p-tert-butylthiacalix[4]arenes 5–10, containing hydrazide, amide and heterocyclic fragments are able to form nanosized particles with AgNO3 and dicarboxylic acids (Table S1, ESI†).
Aggregates of p-tert-butylthiacalix[4]arenes 5–10 and silver (I) nitrate showed a general tendency of increasing particle size in the range partial cone, cone, 1,3-alternate (Table S1, ESI†) (Fig. 9). The opposite tendency is observed for macrocycles 10 containing benzotriazol fragments; the hydrodynamic diameter of particles decreases in the range cone > partial cone and 1,3-alternate.
![Effect of p-tert-butylthiacalix[4]arene conformations on the sizes of self-assembled aggregates with silver (1) nitrate.](/image/article/2012/RA/c2ra01255c/c2ra01255c-f9.gif) | ||
| Fig. 9 Effect of p-tert-butylthiacalix[4]arene conformations on the sizes of self-assembled aggregates with silver (1) nitrate. | ||
Only cone stereoisomers (8a and 9a) with silver (I) nitrate or dicarboxylic acid are characterized by the formation supramolecular aggregates with hydrodynamic diameters of about 8–12 nm in addition to particles of about 80–120 nm.
For supramolecular associates based on p-tert-butylthiacalix[4]arenes 8a and 9a with dicarboxylic acids, the tendency of increasing hydrodynamic diameter corresponds to the increasing size of oxalic, malonic and succinic acids (Table S1†) (Fig. 10). p-tert-Butylthiacalix[4]arenes in the cone conformation (6a, 8a, 9a) are characterized by the formation of supramolecular aggregates with oxalic, malonic and succinic acids, in contrast to macrocycles in partial cone (6b, 8b, 9b) and 1,3-alternate (6c, 10c) conformation. In the latter, the formation of nanoscale particles only occurs with oxalic acid. Supramolecular aggregates only form for dicarboxylic acids and macrocycles 6, 8, 9, containing heterocyclic fragments. Systems based on p-tert-butylthiacalix[4]arene 5 tetrasubstituted by phenylhydrazide fragments in 1,3-alternate conformation with oxalic acid are an exception. For most of the systems, the size of particles formed by the macrocycle 6, 8, 9 and the studied dicarboxylic acids increase in the range, cone, partial cone, 1,3-alternate.
![Effect of p-tert-butylthiacalix[4]arene conformation on the sizes of aggregates with dicarboxylic acids.](/image/article/2012/RA/c2ra01255c/c2ra01255c-f10.gif) | ||
| Fig. 10 Effect of p-tert-butylthiacalix[4]arene conformation on the sizes of aggregates with dicarboxylic acids. | ||
p-tert-Butylthiacalix[4]arenes 10 containing benzotriazol fragments selectively form nanoscale aggregates with dicarboxylic acids (Table S1†): partial cone (10b) forms supramolecular associates only with malonic acid and 1,3-alternate (10c) with oxalic acid. The cone stereoisomer (10a) does not form an association with dicarboxylic acids.
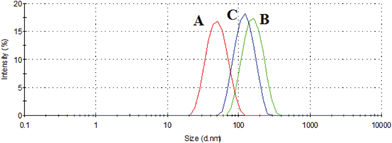
It is interesting to note that most of the systems form various types of nano-sized aggregates (Table S1†) (Fig. 11). Moving from 1,3-alternate stereoisomers to the macrocycles in partial cone and cone conformations, an increase in the number of aggregate types in the system occurs. However, transforming the above distribution from intensity into volume and quantity distributions by Mi theory47 showed a unimodal distribution with the mode being less than the smallest value of the hydrodynamic diameter. For example, for a system consisting of associates based on macrocycle 8a in cone conformation and malonic acids, the main particle with a hydrodynamic diameter of about 11 nm was observed. Aggregates with peaks around 128 nm and 4906 nm were present in small quantities, and with high light scattering intensities (Fig. 11 A) due to the large particle size. The most significant, is the analysis of particle size for all systems in intensity units, measured after 28 h (Table S1†).
![Size distribution by intensity (A), volume (B), and number (C) for the system consisting of thiacalix[4]arene 8a in cone conformation and malonic acid in CH2Cl2.](/image/article/2012/RA/c2ra01255c/c2ra01255c-f11.gif) | ||
| Fig. 11 Size distribution by intensity (A), volume (B), and number (C) for the system consisting of thiacalix[4]arene 8a in cone conformation and malonic acid in CH2Cl2. | ||
Therefore, it has been shown that p-tert-butylthiacalix[4]arenes 5–10, containing substituents with different coordination centers, are able to form nanoscale aggregates with silver (I) nitrate and dicarboxylic acids. It is obvious that the compounds 5–10 are promising supramolecular building blocks.
Three-component systems based on nanoscale aggregates formed by p-tert-butyl thiacalix[4]arenes tetrasubstituted at the lower rim and dicarboxylic acids, silver (I) nitrate
It is well documented that coreceptors can exhibit higher forms of molecular behavior: cooperativity, allosteric binding and regulation.46 Three-component systems based on polytopic coreceptors are usually grouped as cascade or commutative.46p-tert-Butylthiacalix[4]arenes with several binding centers for metal cations (bridging sulfur atoms, oxygen atoms of oxymethylene fragments, acyl hydrazide groups and nitrogen atoms of heterocycles), and for carboxylic acids (protons of hydrazide groups, and heterocyclic fragments) may be used to create three-component cascade or commutative systems.
Previously, it was shown by UV-visible spectroscopy that p-tert-butylthiacalix[4]arenes 5–10 are able to selectively and effectively interact with both AgNO3 and dicarboxylic acids. By dynamic light scattering, it has been shown that macrocycles 5–10 are able to form supramolecular ensembles, two-component systems (p-tert-butylthiacalix[4]arenes-cation or dicarboxylic acid) in the organic phase. The ability of nanoscale particles (two-component systems) to interact with a third component (silver nitrate, or dicarboxylic acid) for the development of three-component cascade or commutative systems was studied. The change in the hydrodynamic particle size resulting from the introduction of a third component into the supramolecular assemble (p-tert-butyl thiacalix[4]arenes + AgNO3 or dicarboxylic acid) was shown by dynamic light scattering. It confirms that three-component systems are formed.
By the change in the hydrodynamic size of particles resulting from the interaction of two-component systems with a third component, the type of three-component system (cascade or commutative) can be determined.
For cascade systems, interactions of individual components occur in a defined order (Fig. 12). We assume that following the formation of aggregates of p-tert-butylthiacalix[4]arenes in cone conformation with silver nitrate, free heterocyclic fragments capable of further interacting with dicarboxylic acids are present on the surface of the assemblies. Thus a three-component system (Fig. 12) can easily be formed. However, the interaction of p-tert-butylthiacalix[4]arenes with dicarboxylic acids either forms nanoparticles with no free coordination centers available for further interacting with silver nitrate, or two-component systems are not formed at all. Hence, this order of interaction of p-tert-butylthiacalix[4]arenes with substrates does not lead to the formation of three-component systems.
 | ||
| Fig. 12 The possible ways of forming cascade systems. | ||
For example, during the interaction of macrocycle 6b with silver (I) nitrate and oxalic acid in dichloromethane, nanoscale particles with hydrodynamic size of about 52 and 168 nm, respectively, are formed (Table 5, S2) (Fig. 13). The interaction of supramolecular associates (compound 6b and oxalic acid) with silver (I) nitrate leads to a change (decrease) in particle size to 124 nm (Table 5, S2) (Fig. 12B, 13). However, during the interaction of supramolecular assemblies (compound 6b and silver (I) nitrate) with oxalic acid, a change in the hydrodynamic diameter of nanoparticles does not occur (Fig. 13). Thus, this is a cascade system.
 | ||
| Fig. 13 Size distribution by intensity for the system formed by: (A) macrocycle 6b and silver (I) nitrate, (B) macrocycle 6b and oxalic acid, (C) supramolecular associates (compound 6b and oxalic acid) and silver (I) nitrate in CH2Cl2. | ||
| Three-component system | d1, nm/S1 (%) | PDI | Two-component system | d1, nm/S1 (%) | PDI |
|---|---|---|---|---|---|
| a ±, standard deviation; -, no aggregates are formed. | |||||
| cascade systems | |||||
| [partial cone(6b)+oxalic acid]agr.+AgNO3 | 124.4 ± 25.6/100 | 0.23 ± 0.09 | partial cone (6b)+ oxalic acid | 168.6 ± 16.8/100 | 0.15 ± 0.05 |
| [1,3-alternate(6c)+AgNO3]agr.+ succinic acid | 86.6 ± 4.4/93.9 ± 2.6 | 0.27 ± 0.01 | 1,3-alternate (6c)+AgNO3 | 232.2 ± 72.6/97.3 ± 2.2 | 0.24 ± 0.06 |
| [cone (8a)+AgNO3]agr.+ succinic acid | 40.4 ± 8.8/95.5 ± 4.6 | 0.23 ± 0.12 | cone (8a)+AgNO3 | 81.0 ± 41.0/93.9 ± 5.0 | 0.31 ± 0.14 |
| [1,3-alternate (8c)+ malonic acid]agr.+AgNO3 | 80.8 ± 16.2/98.6 ± 1.9 | 0.21 ± 0.15 | 1,3-alternate (8c)+ malonic acid | 203.4 ± 44.8/100 | 0.10 ± 0.05 |
| [partial cone (9b)+AgNO3]agr.+ oxalic acid | 95.8 ± 6.0/99.4 ± 1.2 | 0.16 ± 0.03 | partial cone (9b)+AgNO3 | 65.2 ± 6.4/96.1 ± 3.3 | 0.22 ± 0.09 |
| [1,3-alternate (9c)+AgNO3]agr.+ oxalic acid | 80.4 ± 5.8/93.2 ± 9.7 | 0.31 ± 0.01 | 1,3-alternate (9c)+AgNO3 | 137.0 ± 27.6/100 | 0.10 ± 0.02 |
| [cone (10a)+AgNO3]agr.+ oxalic acid | 79.2 ± 32.4/98.9 ± 1.5 | 0.30 ± 0.20 | cone (10a)+AgNO3 | 246.4 ± 17.6/100 | 0.20 ± 0.05 |
| [cone (10a)+AgNO3]agr.+ malonic acid | 73.8 ± 31.8/98.8 ± 1.7 | 0.20 ± 0.15 | |||
| [cone (10a)+AgNO3]agr.+ succinic acid | 74.6 ± 18.0/98.0 ± 1.4 | 0.26 ± 0.06 | |||
| [partial cone (10b)+AgNO3]agr.+ oxalic acid | 37.4 ± 11.0/100 | 0.67 ± 0.37 | partial cone (10b)+AgNO3 | 84.4 ± 58.0/94.6 ± 4.7 | 0.19 ± 0.10 |
| [1,3-alternate (10c)+AgNO3]agr.+ malonic acid | 121.6 ± 41.4/84.0 ± 13.4 | 0.18 ± 0.10 | 1,3-alternate (10c)+AgNO3 | 84.8 ± 21.0/96.6 ± 3.1 | 0.22 ± 0.10 |
| intermediate systems | |||||
| [1,3-alternate (8c)+AgNO3]agr.+ oxalic acid | 87.8 ± 10.6/100 | 0.21 ± 0.10 | 1,3-alternate (8c)+AgNO3 | 51.8 ± 9.4/96.6 ± 2.5 | 0.15 ± 0.14 |
| [1,3-alternate (8c)+oxalic acid]agr.+AgNO3 | 131.4 ± 2.4/100 | 0.06 ± 0.02 | 1,3-alternate (8c)+ oxalic acid | 183.0 ± 19.6/100 | 0.14 ± 0.06 |
| [cone (9a)+ succinic acid]agr.+AgNO3 | 54.4 ± 4.6/87.4 ± 0.8 | 0.39 ± 0.14 | cone (9a)+ succinic acid | 174.6 ± 58.4/96.5 ± 2.5 | 0.19 ± 0.10 |
| [cone (9a)+AgNO3]agr.+succinic acid | 86.6 ± 11.0/96.8 ± 4.6 | 0.25 ± 0.08 | cone (9a)+AgNO3 | 157.6 ± 43.0/98.1 ± 3.0 | 0.23 ± 0.09 |
| commutative systems | |||||
| [1,3-alternate (6c)+AgNO3]agr.+ oxalic acid | 87.2 ± 4.2/100 | 0.21 ± 0.05 | 1,3-alternate (6c)+AgNO3 | 232.2 ± 72.6/97.3 ± 2.2 | 0.24 ± 0.06 |
| [1,3-alternate (6c)+oxalic acid]agr.+AgNO3 | 84.8 ± 4.0/100 | 0.23 ± 0.14 | 1,3-alternate (6c)+ oxalic acid | 182.6 ± 13.8/100 | 0.19 ± 0.08 |
| [cone (8a)+ malonic acid]agr.+AgNO3 | 46.4 ± 4.8/85.0 ± 2.4 | 0.38 ± 0.14 | cone (8a)+ malonic acid | 127.8 ± 33.4/94.0 ± 5.5 | 0.18 ± 0.14 |
| [cone (8a)+AgNO3]agr.+ malonic acid | 52.4 ± 2.2/64.2 ± 2.8 | 0.39 ± 0.01 | cone (8a)+AgNO3 | 81.0 ± 41.0/93.9 ± 5.0 | 0.31 ± 0.14 |
| [1,3-alternate (9c)+ malonic acid]agr.+AgNO3 | 95.4 ± 21.4/88.9 ± 2.6 | 0.25 ± 0.14 | 1,3-alternate (9c)+ malonic acid | 140.4 ± 23.2/100 | 0.14 ± 0.02 |
| [1,3-alternate (9c)+AgNO3]agr.+ malonic acid | 94.0 ± 3.4/100 | 0.24 ± 0.01 | 1,3-alternate (9c)+AgNO3 | 137.0 ± 27.6/100 | 0.10 ± 0.02 |
Cascade systems also include examples in which the p-tert-butylthiacalix[4]arenes form supramolecular assembles (two-component system) only with one of the substrates; with silver (I) nitrate or dicarboxylic acid, and that supramolecular assemble must have the ability to interact with a third component. For example, p-tert-butylthiacalix[4]arene 10a does not form nanoparticles with oxalic, malonic and succinic acids but can form nanoscale aggregates with silver (I) nitrate (246 nm). The aggregates (compound 10a and silver (I) nitrate) are then able to interact with dicarboxylic acids to form supramolecular associates with hydrodynamic diameter of about 74 nm (Table 5).
For commutative systems, the interaction of supramolecular assembles (p-tert-butyl thiacalix[4]arenes and silver (I) nitrate or p-tert-butylthiacalix[4]arenes and dicarboxylic acid) with their respective substrates leads to the formation of three component nanoscale particles with the same size (Table 5). For example, the hydrodynamic diameter of supramolecular assembles ([macrocycle 9c + malonic acid] + AgNO3) and ([macrocycle 9c + AgNO3] + malonic acid) does not depend on the sequence of the binding of substrates and is about 94 nm.
In addition to the cascade and commutative systems, intermediate systems exist, in which during the interaction of nanoscale particles (p-tert-butylthiacalix[4]arenes and silver (I) nitrate or p-tert-butylthiacalix[4]arenes and carboxylic acid) with their respective substrates, two three-component systems of nanoscale aggregates with various sizes are formed (Table 5). For example, the hydrodynamic diameter of particles ([macrocycle 8c + oxalic acid] + AgNO3) is 131.4 nm, and in the case of the system [macrocycle 8c + AgNO3] + oxalic acid, the particle size is equal to 87.8 nm.
To verify the existence of a positive or negative allosteric effect during the formation of three-component systems in the complex solvent dichloromethane–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the effectiveness of the interaction of nanoscale aggregates (two-component systems) with the appropriate substrates, dicarboxylic acid and silver (I) nitrate, were determined by UV-visible spectroscopy (Table S2†).
1), the effectiveness of the interaction of nanoscale aggregates (two-component systems) with the appropriate substrates, dicarboxylic acid and silver (I) nitrate, were determined by UV-visible spectroscopy (Table S2†).
Comparing the efficiency of interaction of nanoscale associates (p-tert-butylthiacalix[4]arenes + AgNO3 and p-tert-butylthiacalix[4]arenes + dicarboxylic acid) with a third component, showed that lgKass decreased in the latter case, indicating a negative allosteric effect. However, the interaction of supramolecular aggregates (compound 10a-silver (I) nitrate and compound 10b-silver (I) nitrate) with dicarboxylic acids as compared to p-tert-butylthiacalix[4]arenes 10a and 10b with dicarboxylic acids showed an increase in the stoichiometric coefficients i.e. increase in the number of interacting particles thus indicating a positive allosteric effect.
A positive allosteric effect has been observed during the interaction of supramolecular associates (macrocycle-dicarboxylic acid) with silver (I) nitrate. The logarithms of the association constants, lgKass increase by several orders of magnitude compared with the efficiency of interaction of macrocycles with AgNO3.
Thus, it has been shown that p-tert-butylthiacalix[4]arenes 6b, 6c, 8a, 8b, 9a–c, 10a–c, containing several potential coordination centers for both metal cations (bridging sulfur atoms, the oxymethylene oxygen atoms, acyl hydrazide groups and nitrogen atoms of heterocycles) and carboxylic acids (protons of hydrazide groups and heterocyclic fragments) are coreceptors capable of simultaneously interacting with both silver (I) nitrate and dicarboxylic acids. Moreover, depending on the sequence of substrate binding, macrocycles 6b, 6c, 8a, 8b, 9a–c, 10a–c are able to form cascade or commutative three component systems.
Conclusion
In this work, we have proposed and implemented a step by step strategy for the stereoselective functionalization of p-tert-butylthiacalix[4]arenes with hydrazide fragments at the lower rim for the synthesis of heterotopic receptors capable of binding metal cations and dicarboxylic acids. It has been shown that the activation of carboxyl groups of the studied thiacalix[4]arenes in reactions with N-substituted hydrazides is achieved by converting them to highly reactive acid chlorides. However, the reaction does not take place in the case of esters, activated esters and anhydride fragments. By dynamic light scattering and electron microscopy, several mechanisms for the formation of supramolecular nanosized aggregates based on synthesized p-tert-butylthiacalix[4]arenes with silver (I) nitrate and dicarboxylic acids in the organic phase have been established. It has been shown that p-tert-butylthiacalix[4]arenes containing N-substituted hydrazide and heterocyclic fragments are coreceptors capable of simultaneously interacting with silver (I) cations and dicarboxylic acids. It has also been shown that the formation of cascade systems “macrocycle-silver (I) nitrate-dicarboxylic acid” is characteristic of the thiacalix[4]arenes containing N-substituted pyridine and hydrazide fragments.Experimental
General
Melting points were determined using Boetius Block apparatus. Most chemicals were purchased from Aldrich and used as received without additional purification. Organic solvents were purified by standard procedures. The 1H and 13C NMR spectra were recorded with 300 MHz Varian XL-300 spectrometer. IR spectra (KBr pellets) were recorded with Vector 22 (Bruker) IR spectrometer. MALDI-TOF mass spectra were recorded with MALDI-TOF Dynamo Finnigan and Kratos Compact MALDI-II. Elemental analysis was performed with Perkin–Elmer 2400 Series II instruments.General procedure of the synthesis of compounds 5–10(a–c).
Acids 2(a–c) (2 g, 2.10 × 10−3 mol) were put into a round-bottom flask and SOCl2 (10 mL, 0.084 mol) was added. The mixture was refluxed for 1.5 h, excess of SOCl2 was removed; the remainder was dried under reduced pressure for 2 h. A solution of hydrazides of nicotinic, isonicotinic, 3-nitrobenzoic acids, 2-hydrazinopyridine, phenylhydrazine, benzotriazole (27.02 × 10−3 mol) and triethylamine (1.54 ml, 7.86 × 10−3 mol) in 50 mL of tetrahydrofuran was added. The mixture was stirred at rt overnight, the remainder was separated, organic layer was evaporated in vacuo. The remainder was crystallized from ethanol–dichloromethane.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2929 (ArH); 3332 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1313.5, [M+Na]+m/z = 1335.5, [M+K]+m/z = 1357.5, found m/z = 1314.8, 1335.6, 1351.7. El. Anal. Calcd for C72H80N8O8S4: C, 65.83; H, 6.14; N, 8.53; S, 9.76. Found: C, 66.71; H, 6.60; N, 8.53; S, 11.12.
O); 2854, 2929 (ArH); 3332 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1313.5, [M+Na]+m/z = 1335.5, [M+K]+m/z = 1357.5, found m/z = 1314.8, 1335.6, 1351.7. El. Anal. Calcd for C72H80N8O8S4: C, 65.83; H, 6.14; N, 8.53; S, 9.76. Found: C, 66.71; H, 6.60; N, 8.53; S, 11.12.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3229 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, found m/z = 1317.5, 1339.6. . El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, 61.44; H, 6.32; N, 12.68; S, 10.31.
O); 2854, 2924 (ArH); 3229 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, found m/z = 1317.5, 1339.6. . El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, 61.44; H, 6.32; N, 12.68; S, 10.31.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3294 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, found m/z = 1317.5, 1339.5. El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, 61.69; H, 6.06; N, 12.37; S, 10.75.
O); 2854, 2924 (ArH); 3294 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, found m/z = 1317.5, 1339.5. El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, 61.69; H, 6.06; N, 12.37; S, 10.75.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3299 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, [M+K]+m/z = 1355.5,found m/z = 1317.9, 1339.6, 1355.7. El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, , 61.71; H, 5.99; N, 12.75; S, 10.25.
O); 2854, 2924 (ArH); 3299 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1317.5, [M+Na]+m/z = 1339.5, [M+K]+m/z = 1355.5,found m/z = 1317.9, 1339.6, 1355.7. El. Anal. Calcd for C68H76N12O8S4: C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, , 61.71; H, 5.99; N, 12.75; S, 10.25.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3230, 3337 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.6, 1627.6, 1643.5. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 56.82; H, 4.70; N, 12.47; S, 8.63.
O); 2854, 2924 (ArH); 3230, 3337 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.6, 1627.6, 1643.5. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 56.82; H, 4.70; N, 12.47; S, 8.63.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3240 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.7, 1627.7, 1643.8. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 56.60; H, 4.81; N, 11.57; S, 8.86.
O); 2854, 2924 (ArH); 3240 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.7, 1627.7, 1643.8. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 56.60; H, 4.81; N, 11.57; S, 8.86.
![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 10.94 (br.s, 4H, Ar-C(O)NH). 13C NMR (125 MHz, (CD3)2SO) δ 30.9, 33.9, 69.5, 122.3, 126.4, 126.7, 130.3, 133.5, 133.7, 133.8, 145.9, 147.7, 157.7, 163.3, 166.3. 1H–1H NOESY NMR (NOE) (the most important cross-peaks): H1/H3, H1/H4, H1/H5, H1/H6, H1/H7, H1/H8, H1/H9, H2/H3, H2/H4, H2/H5, H2/H7, H2/H8, H2/H9. IR (KBr)νmax 1263 (COC); 1350, 1535 (NO2); 1679 (C
), 10.94 (br.s, 4H, Ar-C(O)NH). 13C NMR (125 MHz, (CD3)2SO) δ 30.9, 33.9, 69.5, 122.3, 126.4, 126.7, 130.3, 133.5, 133.7, 133.8, 145.9, 147.7, 157.7, 163.3, 166.3. 1H–1H NOESY NMR (NOE) (the most important cross-peaks): H1/H3, H1/H4, H1/H5, H1/H6, H1/H7, H1/H8, H1/H9, H2/H3, H2/H4, H2/H5, H2/H7, H2/H8, H2/H9. IR (KBr)νmax 1263 (COC); 1350, 1535 (NO2); 1679 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2922 (ArH); 3256, 3627 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.6, 1627.6, 1643.7. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 57.02; H, 4.98; N, 10.25; S, 7.93.
O); 2854, 2922 (ArH); 3256, 3627 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1605.4, [M+Na]+m/z = 1627.4, [M+K]+m/z = 1643.4,found m/z = 1605.6, 1627.6, 1643.7. El. Anal. Calcd for C76H76N12O20S4: C, 56.85; H, 4.77; N, 10.47; S, 7.99. Found: C, 57.02; H, 4.98; N, 10.25; S, 7.93.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3230 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1429.5, 1451.4, 1467.4. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.53; H, 6.18; N, 13.93; S, 7.48.
O); 2854, 2924 (ArH); 3230 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1429.5, 1451.4, 1467.4. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.53; H, 6.18; N, 13.93; S, 7.48.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2912 (ArH); 3230 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, found m/z = 1430.9, 1453.0. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.12; H, 5.60; N, 11.14; S, 9.70.
O); 2854, 2912 (ArH); 3230 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, found m/z = 1430.9, 1453.0. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.12; H, 5.60; N, 11.14; S, 9.70.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH); 3225, 3384 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1430.1, 1452.2, 1468.0. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.41; H, 5.15; N, 11.38; S, 10.30.
O); 2854, 2924 (ArH); 3225, 3384 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1430.1, 1452.2, 1468.0. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.41; H, 5.15; N, 11.38; S, 10.30.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2853, 2920 (ArH); 3209 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1429.9, 1451.8, 1467.8. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.32; H, 5.56; N, 12.51; S, 8.98.
O); 2853, 2920 (ArH); 3209 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1429.9, 1451.8, 1467.8. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 60.32; H, 5.56; N, 12.51; S, 8.98.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2852, 2933 (ArH); 3232 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1430.0, 1452.0, 1467.9. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 59.32; H, 5.56; N, 11.77; S, 9.00.
O); 2852, 2933 (ArH); 3232 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, [M+K]+m/z = 1467.5, found m/z = 1430.0, 1452.0, 1467.9. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 59.32; H, 5.56; N, 11.77; S, 9.00.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2849, 2923 (ArH); 3283 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, found m/z = 1429.6, 1451.6. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 59.65; H, 5.52; N, 11.71; S, 8.70.
O); 2849, 2923 (ArH); 3283 (NH). MALDI-TOF: calcd for [M+H]+m/z = 1429.5, [M+Na]+m/z = 1451.5, found m/z = 1429.6, 1451.6. El. Anal. Calcd for C72H76N12O12S4: C, 60.49; H, 5.36; N, 11.76; S, 8.97. Found: C, 59.65; H, 5.52; N, 11.71; S, 8.70.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2853, 2923 (ArH). MALDI-TOF: calcd for [M+Na]+m/z = 1379.4, [M+K]+m/z = 1395.4, found m/z = 1379.4, 1495.4. El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 64.03; H, 5.06; N, 10.71; S, 9.29.
O); 2853, 2923 (ArH). MALDI-TOF: calcd for [M+Na]+m/z = 1379.4, [M+K]+m/z = 1395.4, found m/z = 1379.4, 1495.4. El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 64.03; H, 5.06; N, 10.71; S, 9.29.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2853, 2923 (ArH). El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 63.47; H, 4.98; N, 12.41; S, 7.21.
O); 2853, 2923 (ArH). El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 63.47; H, 4.98; N, 12.41; S, 7.21.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 2854, 2924 (ArH). MALDI-TOF: calcd for [M+H]+m/z = 1357.4, [M+Na]+m/z = 1379.4, [M+K]+m/z = 1395.4, found m/z = 1357.3, 1379.4, 1495.4. El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 63.40; H, 4.70; N, 11.28; S, 10.67.
O); 2854, 2924 (ArH). MALDI-TOF: calcd for [M+H]+m/z = 1357.4, [M+Na]+m/z = 1379.4, [M+K]+m/z = 1395.4, found m/z = 1357.3, 1379.4, 1495.4. El. Anal. Calcd for C72H68N12O8S4: C, 63.70; H, 5.05; N, 12.38; S, 9.45. Found: C, 63.40; H, 4.70; N, 11.28; S, 10.67.
The system equilibrium is described by eqn (1), where H, G, and GnH denote the ligands (receptor 5–10 or nanoparticles based on macrocycles 5–10 and substrates), substrates (silver nitrate and carboxylic acid (oxalic, malonic, succinic, glycolic, tartaric, maleic, fumaric acids)), and complex with substrate.
| nG + H ⇔ GnH | (1) |
The association constant, Kass, is defined by eqn (2).
| Kass = [GnH]/[G]n [H] | (2) |
To determine the stoichiometry coefficient, n of the complexes formed in the organic phase, eqn (2) was converted to eqn (3).
| lgKass = lg [GnH] − n lg [G] − lg [H] | (3) |
The absorbance A, a sum of those related to the complex, ligand and substrate (AGnH, AH and AG, respectevly) is equal to:
| A = AGnH + AH+ AG | (4) |
Assuming that the Beer–Lambert law is obeyed for all the components considered, the absorbance A is expressed as
| Ai = Ciεi l | (5) |
| A = AGnH + AH | (6) |
Concentration of the complex [GnH] in the system is calculated according to eqn (5) and (6).
The plot of lg [GnH]–lg [H] versus lg [G] (Fig. 14) presents a straight line, slope of which equals to n. Association constants Kass are calculated using the intercept values (b).
![Plot of lg [GnH]–lg [H] versus lg [G].](/image/article/2012/RA/c2ra01255c/c2ra01255c-f14.gif) | ||
| Fig. 14 Plot of lg [GnH]–lg [H] versus lg [G]. | ||
| b = lg Kass | (7) |
Dynamic light scattering (DLS)
The particle sizes were determined by Zetasizer Nano ZS instrument at 20 °C. The instrument contains a 4 mW He–Ne laser operating at a wavelength of 633 nm and incorporates non-invasive backscatter optics (NIBS). The measurements were performed at a detection angle of 173° and the measurement position within the quartz cuvette was automatically determined by the software. The solutions of the investigated systems were prepared by addition of 1000 excess of silver nitrate or carboxylic acid (oxalic, malonic, succinic acids) to 10 mL of 10−5 M solution of thiacalixarene derivatives 5–10 or nanoparticles based on macrocycles 5–10 and substrates in CH2Cl2 (HPLC). The mixture was mechanically shaken for 3 h and then magnetically stirred in a thermostated water bath at 20 °C for 1 h. Three independent experiments were carried out for each combination of a ligand and metal nitrate. Student's t-test was used in statistical data processing.Acknowledgements
The financial support from the Federal Program “Research and scientific-pedagogical personnel of innovative Russia” for 2009-2013 (No. 16.740.11.0472 on 13 May 2011), RFBR (09-03-00426-a) and the Program of the President of the Russian Federation for the State support of young Russian scientists-Doctors of Sciences (MD-2747.2010.3) is gratefully acknowledged.References
- J. M. Lehn, Science, 2002, 295, 2400 CrossRef CAS.
- J. M. Lehn, Supramolecular Chemistry: Concepts and Perspectives; Wiley-VCH: New York, NY, 1995 Search PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry. 2nd ed. John Wiley Hoboken, NJ, 2009 Search PubMed.
- J. L. Atwood and J. W. Steed, Organic Nanostructures. Wiley-VCH, Weinheim, 2008 Search PubMed.
- R. L. Fresenius, J. Anal. Chem., 2000, 367, 103 Search PubMed.
- J. L. Atwood, J. E. D. avies, D. D. acNicol, F. ogtle, J. M. Lehn and G. W. Gokel, Comprehensive Supramolecular Chemistry; Pergamon: London, 1996 Search PubMed.
- Z. Asfari, V. Bohmer, J. Harrowfield and J. Vicens, Calixarenes, 2001; Kluwer: Dordrecht, The Netherlands, 2001 Search PubMed.
- H. Akdas, E. Graf, A. Ciana and J. Harrowfield, Chem. Commun., 2000, 2219 RSC.
- N. Kon, N. Iki, T. Kajiwara, T. Ito and S. Miyano, Chem. Lett., 2006, 35, 430 CrossRef CAS.
- P. Ball, Nanotechnology, 2002, 13, 15 CrossRef.
- M. T. Frank, S. R. Seidel, A. M. Arif and P. J. Stang, J. Am. Chem. Soc., 2001, 123, 7740 CrossRef.
- H. Miyaji, M. Dudic, J. H. R. Tucker, I. Prokes, M. E. Light, M. B. Hursthouse, I. Stibor and P. Lhotak, Tetrahedron Lett., 2002, 43, 873 CrossRef CAS.
- J. C. Lockhart and G. W. Gokel, Chemical sensors. In Comprehensive Supramolecular Chemistry, Washington University School of Medicine: St. Louis, MO, 1996, 605 Search PubMed.
- D. Diamond and M. A. McKervey, Chem. Soc. Rev., 1996, 25, 15 RSC.
- T. Fricke, J. Hamann, M. Bahadir and B. Konig, Anal. Bioanal. Chem., 2002, 374, 148 CrossRef CAS.
- J. Vicens, J. Harrowfield, Calixarenes in the Nanoworld, Springer: London, 2007 Search PubMed.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. J. Peng, J. Natl. Cancer Inst., 1998, 90, 889 CrossRef CAS.
- T. Nabeshima, T. Saiki, K. Sumitomo and S. Akine, Tetrahedron Lett., 2004, 45, 4719 CrossRef CAS.
- T. Nabeshima, T. Saiki and K. Sumitomo, Org. Lett., 2002, 4, 3207 CrossRef CAS.
- C. P. Poole Jr., and F. J. Owens, Introduction to Nanotechnology, John Wiley & Sons: Hoboken, NJ, 2003, Chapter 10 Search PubMed.
- C. D. Gutsche, Calixarenes Revisited. In Monographs in Supramolecular Chemistry; RSC: London, 1998 Search PubMed.
- C. D. Gutsche, Calixarenes. An Introduction. 2nd ed. RSC Cambridge, 2008.
- N. Iki, F. Narumi, T. Fujimoto, N. Morohashi and S. Miyano, J. Chem. Soc., Perkin Trans. 2, 1998, 2745 RSC.
- J. Sykora, M. Himl, I. Stibor, I. Cısarovac and P. Lhotak, Tetrahedron, 2007, 63, 2244 CrossRef CAS.
- N. Iki and S. Miyano, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 99 CrossRef CAS.
- P. Lhotak, Eur. J. Org. Chem., 2004, 1675 CrossRef CAS.
- N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291 CrossRef CAS.
- F. Corbellini, R. Fiammengo, P. Nimmerman, M. Crego-Calama, K. Versiuis, A. J. R. Heck, I. Luyten and D. N. Reinhoudt, J. Am. Chem. Soc., 2002, 124, 6569 CrossRef CAS.
- M. Fujita, M. Tominaga and B. Therrien, Acc. Chem. Res., 2005, 38, 369 CrossRef CAS.
- I. I. Stoikov, E. A. Yushkova, A. Yu. Zhukov, I. Zharov, I. S. Antipin and A. I. Konovalov, Tetrahedron, 2008, 64, 7112 CrossRef CAS.
- I. I. Stoikov, E. A. Yushkova, A. Yu. Zhukov, I. Zharov, I. S. Antipin and A. I. Konovalov, Tetrahedron, 2008, 64, 7489 CrossRef CAS.
- E. A. Yushkova and I. I. Stoikov, Langmuir, 2009, 25, 4919 CrossRef CAS.
- I. I. Stoikov, E. A. Yushkova, I. Zharov, I. S. Antipin and A. I. Konovalov, Tetrahedron, 2009, 65, 7109 CrossRef CAS.
- I. I. Stoikov, E. A. Yushkova, A. A. Bukharaev, D. A. Biziaev, S. A. Ziganshina and I. Zharov, J. Phys. Chem. C, 2009, 113, 15838 CAS.
- P. Lhotak and S. Shinkai, Tetrahedron Lett., 1995, 36, 4829 CAS.
- B. L. Poh and C. M. Tan, Tetrahedron, 1994, 50, 3453 CrossRef CAS.
- D. Ranganathan, V. Haridas and I. L. Karle, J. Am. Chem. Soc., 1998, 120, 2695 CrossRef CAS.
- V. Stastny, I. Stibor, H. Petrıckova, J. Sykora and P. Lhotak, Tetrahedron, 2005, 61, 9990 CrossRef CAS.
- V. Stastny, I. Stibor, I. Cısarova, J. Sykora, M. Pojarova and P. Lhotak, J. Org. Chem., 2006, 71, 5404 CrossRef CAS.
- P. Zlatuskova, I. Stibor, M. Tkadlecova and P. Lhotak, Tetrahedron Lett., 2004, 50, 11383 Search PubMed.
- M. Ashram, N. Al-Ghezawi, I. Awaad and G. Asman, Turk J Chem., 2009, 33, 647 CAS.
- E. Metay, M. Duclos, S. Pellet-Rostaing, M. Lemaire, J. Schulz, R. Kannappan, Ch. Bucher, E. Saint-Aman and C. Chaix, Supramol. Chem., 2009, 21, 68 CrossRef CAS.
- S. E. Solovieva, M. Gruner, A. O. Omran, A. T. Gubaidullin, I. A. Litvinov, W. D. Habicher, I. S. Antipin and A. I. Konovalov, Russ. Chem. Bull., 2005, 54, 2104 CrossRef CAS.
- L. I. Gafiullina, I. S. Vershinina, I. I. Stoikov, I. S. Antipin and A. I. Konovalov, J. Struct. Chem., 2005, 46, 25 CrossRef.
- I. I. Stoikov, O. A. Omran, S. E. Solovieva, S. K. Latypov, K. M. Enikeev, A. T. Gubaidullin, I. S. Antipin and A. I. Konovalov, Tetrahedron, 2003, 59, 1469 CrossRef CAS.
- H. Dodziuk, Introduction to Supramolecular Chemistry, Kluwer Academic: Dordrecht, 2002 Search PubMed.
- G. Mie, Ann. Phys., 1908, 25, 377 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Table S1, S2, 1H NMR, 13C NMR 1H–1H NOESY NMR and MALDI-TOF mass spectra. See DOI: 10.1039/c2ra01255c/ |
| This journal is © The Royal Society of Chemistry 2012 |