A fine line between unimolecular and bimolecular pores formed from poly(choloyl) conjugates with a rigid core†
Wen-Hua
Chen
*
School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, 510515, P. R. China. E-mail: whchen@smu.edu.cn
First published on 28th May 2012
Abstract
A hexa(choloyl) conjugate bearing a rigid p-phenylenediamine linker was synthesized and shown to produce dimer-based pores in POPC bilayers. This is in sharp contrast to a previous finding that its octa(choloyl) analog formed monomer-based pores, and supplies a fine line between unimolecular and bimolecular pores formed from poly(choloyl) conjugates of this type.
During the past decades, considerable endeavors have been devoted toward the creation of pore-forming amphiphiles.1,2 Such amphiphiles can find a multitude of applications. For example, pore-forming amphiphiles that can selectively insert into the plasma membranes of bacterial and fungal cells relative to mammalian cells may lead to new classes of antibiotics with minimal susceptibility toward drug resistance.3 Primarily, the synthetic approaches to create pore-forming amphiphiles are aimed at identifying the structural requirements for ion flow across a membrane, and at acquiring the skills of regulating the pore-forming properties of the amphiphiles as well. Notable among them are the strategies that are based on the assembly of monomers to form so-called supramolecular pores, and the use of a single molecule capable of spanning the entire thickness of a lipid bilayer to form unimolecular pores. Because the formation of unimolecular pores does not rely on supramolecular behavior (i.e., aggregation), their activities are expected to be more controllable than those of the pores formed through aggregation.
To construct pore-forming molecules, various building motifs have been used among which steroids, for example, cholic acid, appear to be a promising class of compounds.4 The flat lipophilic choloyl nucleus provides a rigid framework for interacting with the alkyl chains of lipids, leading to stable pores, whereas the inward-directed hydroxyl groups define a relatively hydrophilic pathway for ions, leading to large ion flux. As a consequence, a number of pore-forming molecules showing interesting properties, such as anion selective transport5 and thermal gating,6,7 have been created based on the use of cholic acid. However, most of them form supramolecular pores whose composition and activities are sensitive to the surrounding microenvironments.
In a previous study we have shown that an octa(choloyl) conjugate with a rigid p-phenylenediamine linker, i.e., conjugate 1 (Fig. 1), produces monomer-based pores in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) bilayers,8 which strongly argues against the common recognition that incorporation of macrocycles is a prerequisite for the formation of monomolecular pores.9 Based on this, each molecule of conjugate 1 contributes, in effect, four choloyl units per monolayer, in part in accordance with Kobuke's proposal that three or four molecules of a transmembrane choloyl dimer linked by m-xylylene dicarbamate were assembled to form the channels.10 This, in turn, provides a strong incentive for further investigation of the minimal structural requirements for the formation of unimolecular pores from poly(choloyl) conjugates with a rigid core, such as conjugate 1. In other words, we believe that a “fine line” exists between unimolecular and bimolecular pores formed by poly(choloyl) conjugates of this type.
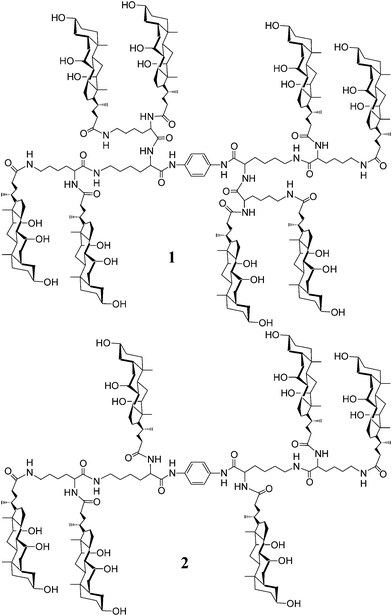 | ||
| Fig. 1 Structures of poly(choloyl) conjugates 1 and 2 bearing a rigid p-phenylenediamine linker. | ||
To clarify this, herein we describe the synthesis and the pore-forming properties of an analog of conjugate 1, i.e., conjugate 2 that has three choloyl units at each end of the p-phenylenediamine linker (Fig. 1). Comparison of the activities of conjugates 1 and 2 provides a means for judging the impact that fine tuning of the structures has on the pore formation.
The synthesis of conjugate 2 is outlined in Scheme 1. Thus, condensation of compound 38 with cholic acid that was activated with N-hydroxysuccinimide (NHS) afforded conjugate 2 in 14%. Its structure was identified by 1H NMR and MALDI-TOF MS (see supporting information†).11 The purity was confirmed by the clean NMR and also by TLC developed with various solvent systems.
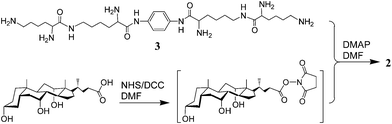 | ||
| Scheme 1 Synthesis of conjugate 2. | ||
The pore-forming properties of conjugate 2 were examined by measuring its ability to promote Na+ transport across POPC-based liposomes (ca. 200 nm diameter, unilamellar), as determined by 23Na+ NMR spectroscopy, using methods similar to those previously described.7,8
As shown in Fig. 2a, the entry of Na+ into the aqueous compartment of the liposomes obeyed pseudo-first-order kinetics. The concentration dependence experiments (Table 1) indicated that the pseudo-first-order rate constants (kobsd) had a strong dependence on the concentration of conjugate 2 in the membrane. Such a dependence lends strong support for a model in which (i) monomers of conjugate 2 are in equilibrium with dimers, (ii) monomers are thermodynamically favored and their concentration can be approximated by the total concentration of conjugate 2 that is present, and (iii) aggregates are responsible for ion transport.7,8 Thus, for the general case of transport-active aggregates, kobsd is expected to obey eqn (1), wherein k2 and k0 are the rate constants for the transport-active species and the background, respectively, K is the dissociation constant that defines the aggregate-monomer equilibrium and n is the aggregation number.
| kobsd = k0 + k2[monomer]n/K | (1) |
![(a) Na+ influx across POPC liposomes in the presence of conjugate 2 (0.2 mol%) at 35 °C. The solid line is the non-linear fitting to pseudo-first-order kinetics. (b) Plot of kobsdversus (mol% conjugate 2) in POPC liposomes at 35 °C. The solid line is a non-linear least-squares fit of the data according to kobsd = k0 + k2[mol% conjugate 2]n/K, where n = 2.16. The inset represents a similar plot where n has been fixed at 2.0.](/image/article/2012/RA/c2ra20121f/c2ra20121f-f2.gif) | ||
| Fig. 2 (a) Na+ influx across POPC liposomes in the presence of conjugate 2 (0.2 mol%) at 35 °C. The solid line is the non-linear fitting to pseudo-first-order kinetics. (b) Plot of kobsdversus (mol% conjugate 2) in POPC liposomes at 35 °C. The solid line is a non-linear least-squares fit of the data according to kobsd = k0 + k2[mol% conjugate 2]n/K, where n = 2.16. The inset represents a similar plot where n has been fixed at 2.0. | ||
Thus, non-linear fitting of the experimental data to eqn (1) afforded the n value of 2.16 (Fig. 2b), revealing a second-order dependence of kobsd on the mol% of conjugate 2 in POPC bilayers. As described previously,8 similar measurements on conjugate 1 afforded the n value of 0.87, showing a first-order dependence of kobsd on the mol% of conjugate 1. Such dependences indicated that monomer and dimer were the active species for conjugates 1 and 2, respectively. In other words, conjugate 1 formed monomer-based pores in POPC bilayers, whereas conjugate 2 formed dimer-based pores. This result shows that a “fine line” exists between unimolecular and bimolecular pores formed by poly(choloyl) conjugates of this type—a line that must be crossed if a conjugate is to be used to form unimolecular pores. The difference in the pore-forming properties of conjugates 1 and 2 is a likely consequence of their structural compactness.8 The four choloyl units at each end of the rigid p-phenylenediamine linker of conjugate 1 became aligned across the bilayer to form a pore structure that was less deformable due to a higher degree of compactness. In contrast, the greater conformational flexibility of conjugate 2 required more than three choloyl units to assemble into a stable pore, because the pores formed by choloyl components are thought to be stabilized by the strong interactions among the components with aid of water molecules, in which the number of choloyl components may have significant influences on the mode of interaction in the most stable form.10
In addition, to observe a similar level of sodium ion transport activity, the required concentration of conjugate 1 was at least 10-fold lower than that of conjugate 2 (Table 1). From an operational standpoint, therefore, conjugate 1 was at least 10-fold more active in transporting sodium ion than conjugate 2. This is a likely result of the higher ability that conjugate 1 has to form transport-active species in the membrane, because it does not rely on aggregation to form a pore. In other words, we believe that this difference is largely a reflection of the difference in the intrinsic activities of the pores (i.e., k2 is dominant). The k2/K values for conjugates 1 and 2 were 1.71 min−1 mol%−1 and 0.14 min−1 mol%−2, respectively.
As conjugate 1 was more active, its pore-forming properties were further examined by a planar bilayer lipid membrane method.12Fig. 3 shows typical records of single channel currents that were observed at −120.4 mV and + 110.4 mV in a symmetric KCl solution buffered with HEPES-Tris (pH 7.2). It is clear that conjugate 1 showed channel-forming characteristics.13 However, it should be noted that it did not exhibit spontaneous opening and closing.14 The current–voltage relationships indicated that conjugate 1 was capable of transporting potassium and sodium ions with greater conductivity for the former (Table S1 and Fig. S3†).
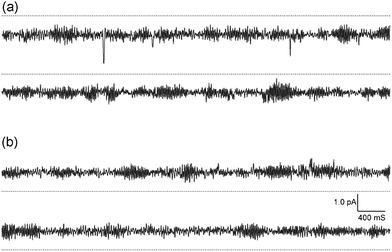 | ||
| Fig. 3 Typical current–time records by conjugate 1 at (a) −120.4 mV and (b) + 110.4 mV, symmetric 0.5 M KCl, pH 7.2. The current increases (a) downward and (b) upward from the dotted line as a closed level of the channel. | ||
The above results indicated that under our measuring conditions, conjugate 1 needed one molecule to contribute, in effect, four choloyl units per monolayer to form a pore, whereas conjugate 2 needed two molecules to contribute totally six choloyl units per monolayer. Thus, one immediate concern we had was whether there was any significant difference in the sizes of the pores created by conjugates 1 and 2. To gain insight into this, we carried out the thermally gated release of carboxyfluorescein (CF) to estimate the size of the pores created by conjugates 1 and 2 (Fig. 4). Thus, using experimental procedures similar to those described previously,7 CF was encapsulated in liposomes made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Tm = 41 °C) containing conjugate 1. By raising the temperature of the liposome dispersion from 37 to 43 °C, a rapid efflux of CF was observed. Similar phenomena were observed in the presence of conjugate 2. Although whether there is any significant difference in the sizes of the pores created by conjugates 1 and 2 remains to be further clarified, these results suggest that both conjugates 1 and 2 are capable of creating pores that are big enough for CF, whose effective diameter is ca. 0.96 nm, to pass through.15
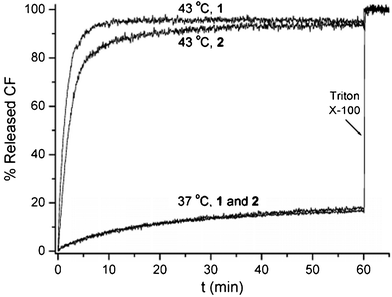 | ||
| Fig. 4 Plots of the percentage of CF release from liposomes made from DPPC containing 0.01 mol% of conjugates 1 and 2 as a function of time at 37 and 43 °C. Complete release of CF was measured after destroying the liposomes with Triton X-100. | ||
In conclusion, a conjugate bearing three choloyl units at each end of the p-phenylenediamine linker has been synthesized and characterized based on 1H NMR and MALDI-TOF MS. Kinetic evidences supported the formation of dimer-based pores across lipid bilayers made from POPC. This is in contrast to a previous finding that its octa(choloyl) analog formed monomer-based pores, and thus supplies a “fine line” between unimolecular and bimolecular pores. Such a fine line raises the possibility to regulate the formation of unimolecular or bimolecular pores by fine tuning the structures of poly(choloyl) conjugates of the type described in this study.
The author thanks Prof. Yoshiaki Kobuke and Ms. Mika Yamamura at Nara Institute of Science and Technology, Japan for the measurement of single channel currents, and Prof. Steven L. Regen at Lehigh University, USA, for his critical reading of the manuscript. This work was financially supported by the National Natural Science Foundation of China (No. 21072090), the Program for New Century Excellent Talents in University (NCET-11-0920), and the Department of Education of Guangdong Province, China.
References
- (a) Recent reviews on synthetic channels and pores: S. Matile, A. V. Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC; (b) G. W. Gokel, R. Ferdani, J. Liu, R. Pajewski, H. Shabany and P. Uetrecht, Chem.–Eur. J., 2001, 7, 33–39 CrossRef CAS; (c) J. M. Boon and B. D. Smith, Curr. Opin. Chem. Biol., 2002, 6, 749–756 CrossRef CAS; (d) S. Matile, A. Som and N. Sorde, Tetrahedron, 2004, 60, 6405–6435 CrossRef CAS; (e) T. M. Fyles, Chem. Soc. Rev., 2007, 36, 335–347 RSC.
- (a) Some recent examples on pore formation: S. Zhang and Y. Zhao, Org. Biomol. Chem., 2012, 10, 260–266 RSC; (b) A. V. Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati and S. Matile, Angew. Chem., Int. Ed., 2011, 50, 11675–11678 CrossRef; (c) J. M. Moszynski and T. M. Fyles, Org. Biomol. Chem., 2011, 9, 7468–7475 RSC; (d) M. Ma and D. Bong, Langmuir, 2011, 27, 1480–1486 CrossRef CAS; (e) H. Cho, L. Widanapathirana and Y. Zhao, J. Am. Chem. Soc., 2011, 133, 141–147 CrossRef CAS.
- (a) E. Stadler, P. Dedek, K. Yamashita and S. L. Regen, J. Am. Chem. Soc., 1994, 116, 6677–6682 CrossRef CAS; (b) Y. Yamashita, V. Janout, E. M. Bernard, D. Armstrong and S. L. Regen, J. Am. Chem. Soc., 1995, 117, 6249–6253 CrossRef.
- (a) Some examples on synthetic channels and pores based on sterols: F. De Riccardis, M. Di Filippo, D. Garrisi, I. Izzo, F. Mancin, L. Pasquato, P. Scrimin and P. Tecilla, Chem. Commun., 2002, 3066–3067 RSC; (b) P. Bandyopadhyay, P. Bandyopadhyay and S. L. Regen, J. Am. Chem. Soc., 2002, 124, 11254–11255 CrossRef CAS; (c) J. Zhang, B. Jing and S. L. Regen, J. Am. Chem. Soc., 2003, 125, 13984–13987 CrossRef CAS; (d) P. Bandyopadhyay, V. Janout, L.-H. Zhang and S. L. Regen, J. Am. Chem. Soc., 2001, 123, 7691–7696 CrossRef CAS; (e) C. Goto, M. Yamamura, A. Satake and Y. Kobuke, J. Am. Chem. Soc., 2001, 123, 12152–12159 CrossRef CAS; (f) Y. Kobuke and T. Nagatani, J. Org. Chem., 2001, 66, 5094–5101 CrossRef CAS.
- (a) W.-H. Chen, J. Zhou and Y.-M. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 4010–4013 CrossRef CAS; (b) C. Chhun and A. R. Schmitzer, Med. Chem. Commun., 2011, 2, 987–990 RSC.
- R. R. Petrov, W.-H. Chen and S. L. Regen, Bioconjugate Chem., 2009, 20, 1037–1043 CrossRef CAS.
- W.-H. Chen and S. L. Regen, J. Am. Chem. Soc., 2005, 127, 6538–6539 CrossRef CAS.
- W.-H. Chen, X.-B. Shao and S. L. Regen, J. Am. Chem. Soc., 2005, 127, 12727–12735 CrossRef CAS.
- M. S. Gin, E. G. Schmidt and P. Talukdar, Self-assembled artificial transmembrane ion channels, in Nanobiotechnology II – More concepts and applications, ed. C. A. Mirkin and C. M. Niemeyer, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007, 3–15 Search PubMed.
- M. Yoshii, M. Yamamura, A. Satake and Y. Kobuke, Org. Biomol. Chem., 2004, 2, 2619–2623 CAS.
- Synthetic procedures for conjugate 2: To a solution of cholic acid (250 mg, 0.61 mmol) and NHS (80 mg, 0.70 mmol) in anhydrous DMF (2 mL) was added DCC (160 mg, 0.78 mmol). After the mixture was stirred at room temperature for 3 h, a solution of 3 (51 mg, 0.08 mmol) and 4-dimethylaminopyridine (DMAP, 300 mg, 2.46 mmol) in anhydrous DMF (2.0 mL) was added. The reaction mixture was stirred at room temperature for 24 h, and then concentrated and poured into diluted hydrochloride acid (1 M, 200 mL). The resulting precipitates were collected by filtration and purified by chromatography on a silica-gel column, eluted with CHCl3/CH3OH/H2O (40/10/1, v/v/v) to give conjugate 2 (35 mg, 14%) with 1H NMR (500 MHz, CD3OD, 318 K) δ 7.50 (s, 4H), 4.44 (t, J = 6.68 Hz, 2 H), 4.22 (t, J = 6.69 Hz, 2 H), 3.93 (s, 6 H), 3.78 (s, 6 H), 3.36–3.34 (m, 6 H), 3.14 (m, 8 H), 2.25–0.91 (m, 192 H), 0.69 (s, 18 H) and MALDI-TOF MS m/z: 2987 ([M+Na]+), 3003 ([M+K]+.
- (a) A recent review on ionic conductance of synthetic channels: J. K. W. Chui and T. M. Fyles, Chem. Soc. Rev., 2012, 41, 148–175 RSC; (b) One example on channel current measurement: W.-H. Chen, K. Nishikawa, S.-D. Tan, M. Yamamura, A. Satake and Y. Kobuke, Chem. Commun., 2004, 872–873 RSC.
- In view of either conductance level or open-closed time behavior, the channel-forming characteristics of conjugate 1 were not specific. Therefore, we did not carry out channel current measurements on conjugate 2.
- For an example of spontaneous channel gating, see: G. A. Woolley and B. A. Wallace, J. Membr. Biol., 1992, 129, 109–136 CAS.
- D. E. Rudnick, J. S. Noonan, D. H. Geroski, M. R. Prausnitz and H. F. Edelbauser, Invest. Ophth. Vis. Sci., 1999, 40, 3054–3058 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of conjugate 2, experimental procedures for sodium transport, channel current measurement and CF release. See DOI: 10.1039/c2ra20121f |
| This journal is © The Royal Society of Chemistry 2012 |
