Shape-controlled synthesis of Au@Pd core-shell nanoparticles and their corresponding electrochemical properties†
Hyon Min
Song
a,
Dalaver H.
Anjum
b and
Niveen M.
Khashab
*ac
aControlled Release and Delivery Lab (CRD), 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bNanofabrication, Imaging & Chracterization Corelab, 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia
cCenter for Advanced Membranes and Porous Materials, 4700 King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia
First published on 28th February 2012
Abstract
The shape-controlled synthesis of Au@Pd core-shell nanoparticles (NPs) was successfully achieved through the emulsion phase generated during the phase transfer from organic to aqueous medium. Contrary to conventional epitaxial growth for obtaining core-shell structures, this method does not require high temperatures and does not have shape restrictions.
One of the characteristics of noble metals is their high reduction potential, which makes their oxidized forms easily reduced even with a mild reducing agent. Along with this inertness, their size and shape dependent properties in the nanoscale draw a lot of interest, such as the surface plasmon resonance effect of gold and silver NPs. Regarded as equally important as each noble metal nanoparticles are hybrid NPs due to their rich electronic properties and ability to form alloy structures. Enhanced catalytic activity among others has been intensely studied for both core-shell1,2 and mixed alloy structures,3 and the search for advanced catalysts spans towards hybrid noble metal NPs.4 Au–Pd3–5 and Pt–Pd6,7 systems are best known in this respect due to their excellent catalytic activity and easy synthesis.
There are two representative methods to synthesize Au@Pd core-shell NPs. One is a simple mixing of two precursors (usually, HAuCl4 and H2PdCl4) which are kept at a high temperature for two days.8 Since Au ions are more prone to be reduced with their higher reduction potential, Au NPs are produced first, followed by Pd coating on the surface of Au. The other method is to use Au NPs as seeds for subsequent Pd growth. Au nanocubes,9 nanorods,10 nanooctahedrons,11 and nanospheres12 are used for the shape-directing cores. Pd shells with interesting high index planes have been prepared in this method and showed better catalytic activity.13 It was found that Au core is more than a simple shape directing material as it is capable of affecting the electronic structure of the Pd shell, which makes Au–Pd NPs adaptable catalysts for reactions otherwise unachievable by conventional Pd catalysts. Examples of such reactions include the electrooxidation of formic acid for the generation of hydrogen,14 solvent-free oxidation of alcohols to aldehydes,15 and working as a green catalyst for converting toluene to benzyl benzoate.3
Epitaxial growth is a relatively easy method to obtain core-shell structures, especially in nanomaterials because of their high surface energy and deviation from the equilibrium status.16 The most contributing factors to the epitaxial growth stem from the thermodynamic process, in that the growth process is sometimes very slow and requires heat for a faster reaction. Another limitation is that the shape of the core directs the overall shape of the shell in most cases. To overcome these two limitations, we herein report the synthesis of Pd shells with different shapes starting from spherical Au NPs at room temperature using the emulsion phase around Au NPs. Benefits of the aqueous medium synthesis at room temperature are the adaptation of NPs to the well-known C–C bond coupling reactions afterwards, and the versatility to further modify the surfaces of Au@Pd core-shell NPs. The Au seed NPs in this study were synthesized in an organic solvent at 280 °C, and were then transferred to the aqueous medium by mixing with CHCl3/cetyltrimethylammonium bromide (CTAB). Micellar mixture has been known to be very effective for phase transfer,17 but few studies have utilized this emulsion phase for the subsequent synthesis.
Gold seeds were synthesized with long chain alkylamines as both capping and reducing agents. A mixture of oleylamine and octadecylamine in the ratio of 7 to 1 (molar ratio) produces gold NPs with an average diameter of 11.3 (± 1.9) nm (Fig. 1a). Gold NPs were then transferred to the aqueous medium by mixing with chloroform and 0.1 M of CTAB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, volume ratio). They were treated with ascorbic acid first, and under vigorous stirring, immediately changed color from purple to black upon dropwise addition of a Pd source. The resulting Au@Pd core-shell NPs with 11 nm Au seeds adopt a flower shape (Fig. 1b). The gold core and palladium shell were visible through Scanning Transmission Electron Microscopy (STEM) (Fig. 1c) where the gold cores had a higher intensity . Energy dispersive spectroscopy (EDS) in the core region proves the existence of both elements with 2.18 KeV M edge, 9.7 KeV L edge of Au, and 2.8 KeV L edge of Pd (Fig. 1e). In the high resolution TEM (HRTEM) image, a twin boundary was observed along the tip growth direction (Fig. 1d), which is similar to the growth of branched or multipod Pt NPs obtained by polyol reduction method.18 Centered to the twin boundary, a [1–21] direction along the (111) plane was identified.
3, volume ratio). They were treated with ascorbic acid first, and under vigorous stirring, immediately changed color from purple to black upon dropwise addition of a Pd source. The resulting Au@Pd core-shell NPs with 11 nm Au seeds adopt a flower shape (Fig. 1b). The gold core and palladium shell were visible through Scanning Transmission Electron Microscopy (STEM) (Fig. 1c) where the gold cores had a higher intensity . Energy dispersive spectroscopy (EDS) in the core region proves the existence of both elements with 2.18 KeV M edge, 9.7 KeV L edge of Au, and 2.8 KeV L edge of Pd (Fig. 1e). In the high resolution TEM (HRTEM) image, a twin boundary was observed along the tip growth direction (Fig. 1d), which is similar to the growth of branched or multipod Pt NPs obtained by polyol reduction method.18 Centered to the twin boundary, a [1–21] direction along the (111) plane was identified.
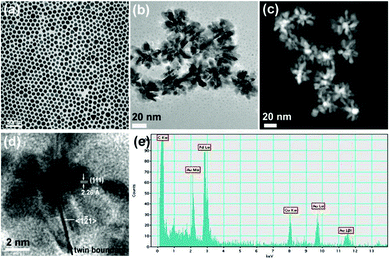 | ||
| Fig. 1 (a) : TEM image of Au NPs with an average diameter of 11.3 (± 1.9) nm, (b) TEM image, (c) STEM image, and (d) HRTEM image of flower shape Au@Pd core-shell NPs. (e) EDS of Au@Pd core-shell NPs with an electron beam at the core Au region. | ||
With larger Au NPs as starting seeds, fully covered spherical shapes, nanospheres (Fig. 2c), or branched shape Au@Pd core-shell NPs, nanobranches (Fig. 2d), were produced. Au NPs with an average diameter of 32.8 (± 4.9) nm were obtained at 280 °C, but with oleylamine as the only capping and reducing agent (Fig. 2a). Although the size distribution is broad compared to the seeded growth approach,19 the advantages of this high temperature method are, i) a one step synthesis with high yield and ii) the simplicity of producing an emulsion phase around Au NPs. The HRTEM of 33 nm Au NP reveals that it has irregular multiple twin boundaries (Fig. 2b). The crystallinity and the size of seed are thought to be important factors in determining the shape of the grown structure,20 with single crystalline gold NPs (2–3 nm) responsible for the nanorod growth and with twinned particles for the growth of bipyramids in the presence of Ag ions.21 Immediate color change from purple to black upon the addition of PdCl2 in the presence of ascorbic acid was also observed in the larger Au@Pd core-shell NPs. The final structure is mostly spherical with an average diameter of 100 (±11) nm (Fig. 2c). After centrifugation of Au@Pd core-shell NPs at 4000 rpm for 5 min, we observed that supernatant remained black, and was isolated as Pd nanocubes after centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 7 min (Fig. 2e). When a control experiment was conducted by reacting Pd source without Au NPs in the presence of CTAB or in the mixture of CTAB and chloroform, well-defined nanocubes were not obtained but aggregated particles with mostly {100} bound planes were produced (ESI, Figs. S3, S4†). Cubes as side products are produced through the emulsion phase only in the presence of 33 nm Au seeds. In the selected area electron diffraction (SAED) pattern of spherical Au@Pd core-shell NPs, a face-centered-cubic (fcc) phase of Pd appears with no obvious pattern from Au cores (Fig. 2f) .
000 rpm for 7 min (Fig. 2e). When a control experiment was conducted by reacting Pd source without Au NPs in the presence of CTAB or in the mixture of CTAB and chloroform, well-defined nanocubes were not obtained but aggregated particles with mostly {100} bound planes were produced (ESI, Figs. S3, S4†). Cubes as side products are produced through the emulsion phase only in the presence of 33 nm Au seeds. In the selected area electron diffraction (SAED) pattern of spherical Au@Pd core-shell NPs, a face-centered-cubic (fcc) phase of Pd appears with no obvious pattern from Au cores (Fig. 2f) .
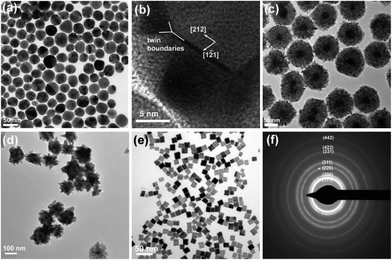 | ||
| Fig. 2 (a) TEM and (b) HRTEM images of Au NPs with an average diameter of 32.8 (± 4.9) nm. TEM images of (c) spherical Au@Pd core-shell NPs, (d) branched Au@Pd core-shell NPs, (e) Pd nanocubes. (f) SAED pattern of spherical Au@Pd core-shell NPs. | ||
When a higher concentration of CTAB (0.2 M) was used for the phase transfer, the resulting Au@Pd core-shell NPs adopt a branched shape with dominant {111} planes of Pd in the branches (Fig. 2d). Cubes with all {100} planes were not observed as a separable side product. For the effect of CTAB concentration on the shape of noble metal NPs, it was suggested that a high concentration of CTAB slows deposition of metal ions on the {111} planes and assists faster deposition along the <100> direction, which results in the formation of octahedrons with all {111} planes. Low concentration of CTAB, however, helps deposition on the {111} planes until all {111} planes disappear and leads to the formation of cubes with all {100} planes.11,22 In this study, we saw similar trend, in that 0.2 M CTAB with Au NPs does not contribute to the formation of {100}-bound cubes, but branched arms with dominant {111} planes, while 0.1 M CTAB helps the formation of cubes with {100} planes.
In the HRTEM image of spherical Au@Pd core-shell NPs, (200) planes (d = 1.95 Å) along the [0–10] direction and (020) planes along [100] direction were observed with zone axis of [001] (Fig. 3b). On the contrary, {111} planes of Pd were observed in the branched shape Au@Pd NPs, and {100} planes were barely found (Fig. 3e). Low concentrations of CTAB (0.1 M) lead to the {100}-bound planes, while higher concentrations of CTAB (0.2 M) precludes the formation of cubes, but produces branched NPs with {111} planes. In the STEM images, dendritic shell formation is clearly seen in spherical Au@Pd core-shell NPs (Fig. 3c), while thicker and continuous Pd shell growth was observed in branched Au@Pd NPs (Fig. 3f).
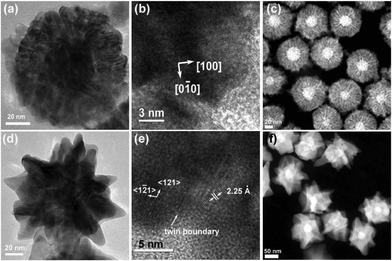 | ||
| Fig. 3 (a), (b) HRTEM images and (c) STEM image of spherical Au@Pd core-shell NPs. (d), (e) HRTEM images and (f) STEM image of branched Au@Pd core-shell NPs. Both types of NPs were grown from the same 32.8 nm Au NPs with different concentration of CTAB. | ||
The seed-mediated approach is a very popular method in metal nanoparticle synthesis, notably in large size nanospheres, one dimensional nanorods, and low symmetry three dimensional structures. The difference is that the Au@Pd core-shell NPs in this study are grown with the help of the emulsion around the Au NPs, and not necessarily by conventional heteroepitaxial growth. Well-defined planes of core NPs are typically necessary for the plane matching growth of the shell, such as Au@Pd core-shell nanocubes grown from gold nanooctahedrons with only {111} planes.11 Other examples also prove that Au@Pd core-shell structures were grown as plane matching heteroepitaxial growth starting from the specific planes of the Au cores, and careful synthetic conditions are required for the core-shell structure growth such as temperature and concentration.10,23 The reaction in this environment leads to the thermodynamic process with slow growth at high temperature. On the contrary, a Au seed (33 nm) in this study has a multiply twinned structure with poorly defined lattice planes. The emulsion around the Au cores, with help from ascorbic acid as a reductant, attracts small size Pd NPs which is followed by continuous growth. The reaction is more likely a kinetic process, which takes one hour of reaction at room temperature.
In the XRD patterns in Fig. 4, both Au and Pd peaks were clearly observed in the Au@Pd core-shell NPs when 32.8 nm Au NPs were used as seeds. The structure is preferred to be a separate core-shell type, rather than an alloy. One step synthesis usually produces an alloy structure with (111) peak between Au and Pd, and d111 values reside between the d111 of Au (2.36 Å) and Pd (2.24 Å).14,24 In this study, the preformed gold NPs were produced and annealed at 280 °C for 2 h and act as the solid cores for Pd coating. When 11 nm Au NPs were used as cores, the peaks of the Au@Pd core-shell NPs are broad which indicates that smaller size cores with low crystallinity lead to low crystallinity in the overall structure.
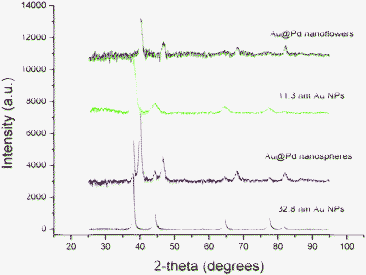 | ||
| Fig. 4 XRD patterns of 11.3 nm (green) and 32.8 nm Au NPs (black), Au@Pd nanoflowers grown from 11.3 nm Au NPs (blue), and Au@Pd nanospheres grown from 32.8 nm Au NPs (red). | ||
In the optical absorbance spectra, 33 nm Au NPs have a red-shifted peak at 534 nm, compared to the resonance peak at 524 nm for 11 nm Au NPs (Fig. 5a). For the larger gold NPs with a diameter of over 25 nm, size-dependent dielectric functions were employed for the Mie scattering. The larger spherical particles have more red-shifted peaks and the stronger multipole mode Au@Pd core-shell NPs grown from 33 nm Au NPs have the resonance peaks (Fig. 5b). Notable red shift of the resonance peaks was observed in the spherical Au@Pd NPs at 473 nm, and at extinction.25 Au@Pd nanoflowers grown from 11 nm Au NPs do not have the strong resonance peak, while other types of branched Au@Pd NPs at 630 nm do. Red shift of the resonance peak in the hetero core-shell structures was observed in Au@Ag,26 and CdS@ZnS core-shell NPs.27 Similar red shifts of the resonance peaks depending on the shell thickness was also studied in Au@Pd core-shell structure grown from Au nanocubes.9
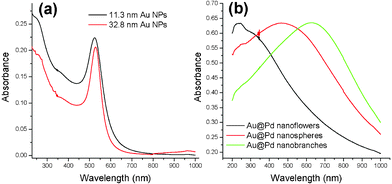 | ||
| Fig. 5 Optical absorbance spectra of (a) gold seed NPs with 11.3 nm diameter (black) and 32.8 nm diameter (red). (b) Au@Pd core-shell nanoflowers (black), nanospheres (red), and nanobranches (green). Absorbance in Fig. 5b was normalized. | ||
The electrochemical properties of Au@Pd core-shell NPs were measured with potentiostat (Fig. 6). Cyclic voltammograms were recorded at the scan rate of 80 mV s−1 from −0.5 to 0.9 V. The shape and peaks of voltammograms are different depending on the types of Au@Pd core-shell NPs. With small size Au@Pd nanoflowers grown from 11 nm Au NPs, the voltammogram has a major oxidation peak at 0.23 V and small minor peaks at 0.39 V and 0.49 V (Fig. 6a). In the voltammogram of spherical Au@Pd NPs grown from 33 nm Au NPs, a major peak was found at 0.34 V with a shoulder peak at 0.23 V (Fig. 6b). This major peak stems from the Pd shell with {100} planes. Usually less reactive planes with low surface energy have oxidation peaks at higher voltage.28,29 Therefore, {111}-dominant planes have higher oxidation voltages than {110} or {100}-bound planes. Branched Au@Pd core-shell NPs grown from 33 nm Au NPs have the major peak at 0.46 V stemming from dominant {111} planes in the core and branches (Fig. 6c). These branched Au@Pd NPs will have better performance with about 3–4 times larger current density than the nanoflowers and spherical Au@Pd core-shell NPs. However, nanoflowers and spheres are more practical catalysts due to their oxidation peaks at low voltage.
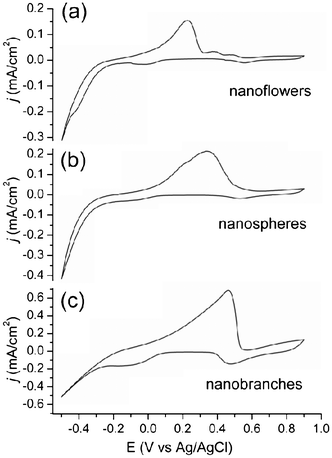 | ||
| Fig. 6 Cyclic voltammograms of (a) nanoflower, (b) nanospherical, and (c) nanobranched Au@Pd core-shell NPs. | ||
In summary, Au@Pd core-shell NPs with different sizes and shapes were obtained in the emulsion phase surrounding core Au NPs. The growth of the Pd shell is driven by the help of the emulsion phase around the Au NPs. Electrochemical properties of three different Au@Pd core-shell NPs (nanoflowers, nanospheres, nanobranches) showed their dependence on the shapes, with smaller nanoflowers or spherical NPs with {100}-bound planes being more practical catalysts. The micellar emulsion method described here can be applied to the preparation of other nonconventional hetero core-shell structures.
We gratefully acknowledge King Abdullah University of Science and Technology (KAUST) for the support of this work.
References
- S. Alayoglu, A. U. Nilekar, M. Mavrikakis and B. Eichhorn, Nat. Mater., 2008, 7, 333–338 CrossRef CAS.
- P.-P. Fang, A. Jutand, Z.-Q. Tian and C. Amatore, Angew. Chem., Int. Ed., 2011, 50, 12184–12188 CrossRef CAS.
- L. Kesavan, R. Tiruvalam, M. H. A. Rahim, M. I. bin Saiman, D. I. Enache, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, S. H. Taylor, D. W. Knight, C. J. Kiely and G. J. Hutchings, Science, 2011, 331, 195–199 CrossRef CAS.
- C.-H. Cui, J.-W. Yu, H.-H. Li, M.-R. Gao, H.-W. Liang and S.-H. Yu, ACS Nano, 2011, 5, 4211–4218 CrossRef CAS.
- L. Zhang, J. Zhang, Q. Kuang, S. Xie, Z. Jiang, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2011, 133, 17114–17117 CrossRef CAS.
- Q. Yuan, Z. Zhou, J. Zhuang and X. Wang, Chem. Commun., 2010, 46, 1491–1493 RSC.
- X. Huang, H. Zhang, C. Guo, Z. Zhou and N. Zheng, Angew. Chem., Int. Ed., 2009, 48, 4808–4812 CrossRef CAS.
- Y. W. Lee, M. Kim, Z. H. Kim and S. W. Han, J. Am. Chem. Soc., 2009, 131, 17036–17037 CrossRef CAS.
- C.-L. Lu, K. S. Prasad, H.-L. Wu, J.-a. A. Ho and M. H. Huang, J. Am. Chem. Soc., 2010, 132, 14546–14553 CrossRef CAS.
- W. Annan, P. Qing and L. Yadong, Chem. Mater., 2011, 23, 3217–3222 CrossRef.
- F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie, Z.-Y. Jiang and Z.-Q. Tian, J. Am. Chem. Soc., 2008, 130, 6949–6951 CrossRef CAS.
- J. Xu, A. R. Wilson, A. R. Rathmell, J. Howe, M. Chi and B. J. Wiley, ACS Nano, 2011, 5, 6119–6127 CrossRef CAS.
- F. Wang, C. Li, L.-D. Sun, H. Wu, T. Ming, J. Wang, J. C. Yu and C.-H. Yan, J. Am. Chem. Soc., 2010, 133, 1106–1111 CrossRef.
- J. Chai, F. Li, Y. Hu, Q. Zhang, D. Han and L. Niu, J. Mater. Chem., 2011, 21, 17922–17929 RSC.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef CAS.
- G. B. Stringfellow, in Crystal growth: from fundamentals to technology, ed. G. Müller, J.-J. Métois and P. Rudolph, Elsevier Science, 2004, pp. 1–7 Search PubMed.
- H. Fan, E. W. Leve, C. Scullin, J. Gabaldon, D. Tallant, S. Bunge, T. Boyle, M. C. Wilson and C. J. Brinker, Nano Lett., 2005, 5, 645–648 CrossRef CAS.
- M. Tsuji, P. Jiang, S. Hikino, S. Lim, R. Yano, S.-M. Jang, S.-H. Yoon, N. Ishigami, X. Tang and K. S. N. Kamarudin, Colloids Surf., A, 2008, 317, 23–31 CrossRef CAS.
- C. Ziegler and A. Eychmüller, J. Phys. Chem. C, 2011, 115, 4502–4506 CAS.
- M. Grzelczak, J. Pérez-Juste, P. Mulvaney and L. M. Liz-Marzán, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192–22200 CrossRef CAS.
- E. Dovgolevsky and H. Haick, Small, 2008, 4, 2059–2066 CrossRef CAS.
- L. Carbone and P. D. Cozzoli, Nano Today, 2010, 5, 449–493 CrossRef CAS.
- D. Jana, A. Dandapat and G. De, J. Phys. Chem. C, 2009, 113, 9101–9107 CAS.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 4212–4217 CrossRef CAS.
- H. Huang, C. Qu, X. Liu, S. Huang, Z. Xu, B. Liao, Y. Zeng and P. K. Chu, ACS Appl. Mater. Interfaces, 2011, 3, 183–190 CAS.
- J. S. Steckel, J. P. Zimmer, S. Coe-Sullivan, N. E. Stott, V. Bulović and M. G. Bawendi, Angew. Chem., Int. Ed., 2004, 43, 2154–2158 CrossRef CAS.
- K. Sashikata, Y. Matsui, K. Itaya and M. P. Soriaga, J. Phys. Chem., 1996, 100, 20027–20034 CrossRef CAS.
- J. Solla-Gullon, F. J. Vidal-Iglesias, A. Lopez-Cudero, E. Garnier, J. M. Feliu and A. Aldaz, Phys. Chem. Chem. Phys., 2008, 10, 3689–3698 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, TEM images and size analysis of Au nanoparticles and Au@Pd core-shell nanoparticles are available. See DOI: 10.1039/c2ra20152f/ |
| This journal is © The Royal Society of Chemistry 2012 |
