DBSA catalyzed, one-pot three-component “on water” green protocol for the synthesis of 2,3-disubstituted 4-thiazolidinones†
Davinder
Prasad
,
Amreeta
Preetam
and
Mahendra
Nath
*
Department of Chemistry, University of Delhi, Delhi 110 007, India. E-mail: mnath@chemistry.du.ac.in; Fax: +91 11 27666605
First published on 30th January 2012
Abstract
A simple and environmentally benign one-pot three-component tandem synthesis of a series of novel 4-thiazolidinones has been accomplished “on water” by reacting aliphatic or aromatic primary amines with thioglycolic acid and a wide range of aromatic aldehydes at ambient temperature using p-dodecylbenzenesulfonic acid (DBSA) as a Brønsted acid-surfactant combined catalyst. The unique ability of the catalyst has been utilised to form emulsion droplets with organic substrates in aqueous medium whose interior core is hydrophobic enough to exclude the water molecules generated during the rate limiting dehydration step of the reaction. This protocol worked well with a variety of substrates and the desired products were obtained in fairly good yields.
Introduction
4-Thiazolidinones are of considerable interest due to their diverse applications in the field of medicinal chemistry. They exhibit a wide range of pharmacological properties including antibacterial,1anti-HIV,2 antimalarial,3 nematicidal agents4etc. Moreover, their derivatives are also chemically important precursors for the synthesis of various pharmaceutically useful compounds such as pyrazolothiazole derivatives,5 monofluoro β-lactams6 and polymethine cyanine dyes.7 Consequently, a number of methods have been reported for the synthesis of 4-thiazolidinones either via a one-step or two steps process.8 However, the most widely used approach to synthesize this ring system is through one-pot three-component tandem reaction between a primary amine, carbonyl compound and mercapto-carboxylic acid. The reaction proceeds via the initial formation of a Schiff base intermediate, which undergoes attack by a sulfur nucleophile, mercapto-carboxylic acid, followed by intramolecular cyclization with the expulsion of water to yield the desired product.9 It is generally believed that the last step i.e. removal of the water molecule is rate determining and seems to be critical for obtaining 4-thiazolidinones in high yields. Therefore, a number of strategies have been developed to remove the water molecules formed during the reaction in order to obtain a high yield of the desired product. The most common approach to remove water from the reaction medium involves azeotropic distillation using a Dean Stark trap either with benzene or toluene as a solvent medium.10Alternatively, various desiccants such as Na2SO4,11 molecular sieves,12 dehydrating agents like DCC,13 HBTU14 and Lewis acid catalysts such as anhydrous ZnCl215 and SnCl216 have also been utilised to assist the formation of 4-thiazolidinones in high yields. However, most of these protocols are associated with several drawbacks including the use of hazardous solvents, expensive catalysts, harsh reaction conditions, tedious work-up procedures and the occurrence of toxic byproducts. Hence, there has been an urgent need to develop an eco-friendly, simple, mild and economical protocol for the synthesis of 4-thiazolidinone analogues.
Recently, notable attention has been focussed on the concept of green chemistry for long term sustainability of the environment.17 The build-up pressure against the use of hazardous organic solvents or reagents for carrying out various organic reactions has alarmed researchers due to health, safety and environmental concerns and thereby initiated the search of eco-friendly approaches18 for the synthesis of various biologically useful molecules. The organic reactions in aqueous medium have gained significant importance because of various advantages associated with water such as low cost, safety and environmentally bengin nature as compared to the toxic organic solvents.19 Though, water has been used as a solvent for a wide range of chemical transformations, its application in organic synthesis is still limited as most organic substrates have poor solubility in water.20 Thus, a number of strategies21 has been devised to solve this problem including the use of organic co-solvents, hydrophobic auxiliaries or surfactant molecules. Over recent years, surfactant combined catalysts have occupied an important place in the realm of modern synthetic organic chemistry as they assist in constituting environmentally benign protocols.22 Among the surfactant aided catalysts, DBSA has emerged as an efficient Brønsted-acid surfactant combined catalyst for carrying out diverse water-sensitive organic reactions in water.23 In this paper, we describe a mild and eco-friendly route for the synthesis of 4-thiazolidinones in aqueous medium in the presence of a catalytic amount of DBSA. The attractiveness of this protocol lies in the fact that the target products were obtained in good yields (60–91%) using an aqueous medium in spite of the adverse effect of water on the formation of 4-thiazolidinones. This can be explained on the basis of the unique ability of DBSA to form emulsion droplets with organic substrates in the aqueous phase, having hydrophobic interior and hydrophilic exterior core.23c The reaction is believed to occur in the hydrophobic core of the catalyst that helps in the removal of water molecules generated during the rate determining dehydration step to form the desired products in good yields.
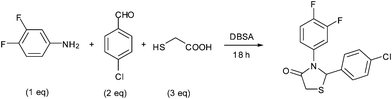
In view of the emerging interest to perform reactions in aqueous media and in continuation of our ongoing work to develop environmentally benign methodolgies24 for the synthesis of biologically useful heterocycles, we wish to report herein the DBSA catalyzed green methodology for the synthesis of a series of novel 4-thiazolidinone derivatives in good yields via a one-pot three-component cascade reaction of aliphatic or aromatic primary amines with various aromatic aldehydes and thioglycolic acid in aqueous medium at 25 °C (Scheme 1).
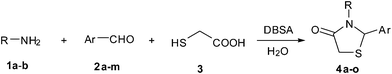 | ||
| Scheme 1 Synthesis of 4-thiazolidinones via one-pot three-component tandem reaction of primary amines, aryl aldehydes and thioglycolic acid. | ||
Results and discussion
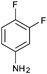
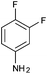
Recently, the synthesis of spirothiazolidiones was carried out in an aqueous medium by using a phase transfer catalyst under ultrasound irradiation, however, the reported protocol25 is associated with limitations of using elevated temperature (45–80 °C), a high load of the catalyst (20 mol%) and requirement of an ultrasonic bath. In contrast, the present methodology is mild e.g. the reactions were carried out at room temperature using 10 mol% of DBSA and do not require any special apparatus.We initiated our investigation by reacting 3,4-difluoroaniline with p-chlorobenzaldehyde and thioglycolic acid as model substrates in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively in the presence of 20 mol% DBSA as a catalyst in water at 25 °C for 18 h (Table 1, entry 1). To our delight, DBSA efficiently catalyzed the reaction and the desired product, 2-(4-chlorophenyl)-3-(3,4-difluorophenyl)thiazolidin-4-one (4c) was obtained as a brown gummy solid in 75% yield. On increasing the load of the catalyst from 20 to 50 mol%, the yield of desired product (4c) decreased to 68% (Table 1, entry 2). In contrast, the use of 10 mol% DBSA under same reaction conditions afforded the corresponding product (4c) in 85% isolated yield within 18 h (Table 1, entry 3). Interestingly, when the same reaction was performed in the absence of the catalyst under identical conditions, only a trace amount of product was observed after 24 h (Table 1, entry 7), demonstrating the catalytic role of DBSA in the synthesis of 4-thiazolidinone derivative (4c). Encouraged by these results and to optimize the reaction conditions, various experimental parameters were examined including the effect of molar ratio of substrates, nature of solvents and the reaction temperature. A number of reactions were carried out by varying the molar ratio of model substrates in the presence of 10 mol% DBSA using water as a solvent medium at ambient temperature. It was observed that the reaction proceeded well when 3,4-difluoroaniline, p-chlorobenzaldehyde and thioglycolic acid were reacted in the molar ratio 1
3, respectively in the presence of 20 mol% DBSA as a catalyst in water at 25 °C for 18 h (Table 1, entry 1). To our delight, DBSA efficiently catalyzed the reaction and the desired product, 2-(4-chlorophenyl)-3-(3,4-difluorophenyl)thiazolidin-4-one (4c) was obtained as a brown gummy solid in 75% yield. On increasing the load of the catalyst from 20 to 50 mol%, the yield of desired product (4c) decreased to 68% (Table 1, entry 2). In contrast, the use of 10 mol% DBSA under same reaction conditions afforded the corresponding product (4c) in 85% isolated yield within 18 h (Table 1, entry 3). Interestingly, when the same reaction was performed in the absence of the catalyst under identical conditions, only a trace amount of product was observed after 24 h (Table 1, entry 7), demonstrating the catalytic role of DBSA in the synthesis of 4-thiazolidinone derivative (4c). Encouraged by these results and to optimize the reaction conditions, various experimental parameters were examined including the effect of molar ratio of substrates, nature of solvents and the reaction temperature. A number of reactions were carried out by varying the molar ratio of model substrates in the presence of 10 mol% DBSA using water as a solvent medium at ambient temperature. It was observed that the reaction proceeded well when 3,4-difluoroaniline, p-chlorobenzaldehyde and thioglycolic acid were reacted in the molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively, and produced the product (4c) in good yield. Instead, the rate of reaction was adversely affected with the change in molar ratio of these substrates. Further, the effect of temperature on the rate of this domino reaction was also studied. On increasing the reaction temperature, the yield of the desired product (4c) gradually decreased and the best results were obtained at 25 °C (Table 1, entries 3–6). Besides the aqueous medium, the effect of other preferred green solvents such as ethanol, t-butanol and acetonitrile on the yield of the product (4c) was also investigated. Delightfully, water proved to be the best solvent for this reaction and provided higher yields of the corresponding 4-thiazolidinone (4c) possibly due to the strong hydrophobic interactions between the substrates and the catalyst in the aqueous medium23b as compared to the other solvents (Table 1, entries 3 and 8–10). Hence, 10 mol% DBSA was selected as the optimal catalyst load to synthesize various 2,3-disubstituted 4-thiazolidinones in aqueous medium at ambient temperature by reacting primary amines, aryl aldehydes and thioglycolic acid in a 1
3, respectively, and produced the product (4c) in good yield. Instead, the rate of reaction was adversely affected with the change in molar ratio of these substrates. Further, the effect of temperature on the rate of this domino reaction was also studied. On increasing the reaction temperature, the yield of the desired product (4c) gradually decreased and the best results were obtained at 25 °C (Table 1, entries 3–6). Besides the aqueous medium, the effect of other preferred green solvents such as ethanol, t-butanol and acetonitrile on the yield of the product (4c) was also investigated. Delightfully, water proved to be the best solvent for this reaction and provided higher yields of the corresponding 4-thiazolidinone (4c) possibly due to the strong hydrophobic interactions between the substrates and the catalyst in the aqueous medium23b as compared to the other solvents (Table 1, entries 3 and 8–10). Hence, 10 mol% DBSA was selected as the optimal catalyst load to synthesize various 2,3-disubstituted 4-thiazolidinones in aqueous medium at ambient temperature by reacting primary amines, aryl aldehydes and thioglycolic acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio, respectively.
3 molar ratio, respectively.
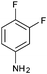
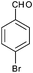
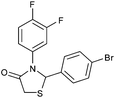
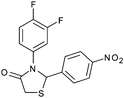
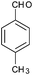
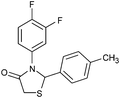
After optimization of the reaction conditions, we focussed our investigation towards the scope and limitations of this tandem reaction. Under standardized reaction conditions, a series of novel 2,3-disubstituted 4-thiazolidinones (4a–o) have been successfully synthesized by reacting various aromatic aldehydes with aliphatic or aromatic primary amines and thioglycolic acid in water at ambient temperature in the presence of 10 mol% of DBSA (Table 2). It is evident from Table 2 that the aryl aldehydes having various functional groups such as halogens, methyl, methoxy, CF3, nitro groups etc. were well tolerated in this condensation–cyclization reaction. The aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents underwent this domino reaction smoothly with varying rates to afford the corresponding 4-thiazolidinones in moderate to high yields. In general, the reaction proceeded well with aromatic aldehydes having electron withdrawing substituents (Table 2, entries 2–9, 13 and 14) while the reaction was slightly disfavoured when aldehydes had electron donating substituents on the aromatic ring (Table 2, entries 10–12 and 15). In the case of aryl aldehydes having halogens at the para-position, the yield of the corresponding products slightly increased with the decrease of the electronegativity of these substituents (Table 2, entries 2–4). On the other hand, the yields of the products were unaffected owing to the position of substituents viz. o-, m- or p- (Table 2, entries 3, 5 and 6). The aldehyde with a strong electron-donating substituent such as –OCH3 at the para-position on the aromatic ring provided the corresponding product (4k) in 60% yield (Table 2, entry 11) while the moderate electron-donating groups such as –CH3 or –C(CH3)3 generated the desired products (4j, 4l and 4o) in 70–86% yields (Table 2, entries 10, 12 and 15). Furthermore, the nature of the primary amines also influenced the rate of reaction. The aliphatic amine produced higher yields (86–91%) of the desired products (4n and 4o) when compared to aromatic amines (Table 2, entries 2 and 14, 12 and 15).
| Entry | Amines | Aldehydes | Products | Time (h) | Yield (%)a | |
|---|---|---|---|---|---|---|
| a Isolated yields after column chromatography. | ||||||
| 1 |
|

|
|
4a | 18 | 76 |
| 2 |
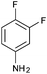
|
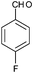
|
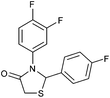
|
4b | 18 | 84 |
| 3 |

|
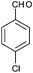
|

|
4c | 18 | 85 |
| 4 |
|
|
|
4d | 18 | 87 |
| 5 |
|
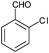
|
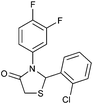
|
4e | 18 | 85 |
| 6 |
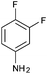
|
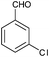
|
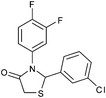
|
4f | 18 | 85 |
| 7 |
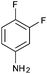
|
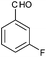
|
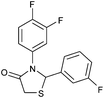
|
4g | 18 | 83 |
| 8 |

|
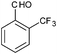
|
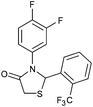
|
4h | 18 | 89 |
| 9 |
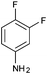
|
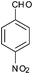
|
|
4i | 18 | 80 |
| 10 |
|
|
|
4j | 18 | 72 |
| 11 |
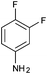
|
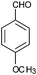
|
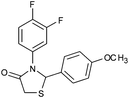
|
4k | 30 | 60 |
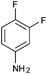
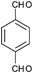
| 12 |
|
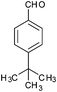
|
|
4l | 18 | 70 |
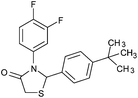
| 13 |
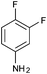
|
|
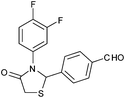
|
4m | 18 | 79 |
| 14 |
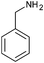
|
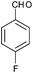
|
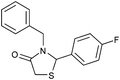
|
4n | 18 | 91 |
| 15 |
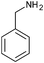
|
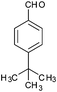
|
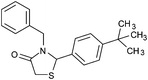
|
4o | 18 | 86 |
All the newly synthesized compounds have been characterized on the basis of IR, 1H NMR, 13C NMR and mass spectral analysis. The IR spectrum of compound 4c indicated the formation of the product by showing a characteristic absorption peak at 1694 cm−1 which corresponds to the carbonyl group of cyclic amide. The 1H NMR spectrum of compound 4c showed one double doublet and a doublet at δ 3.95 and 3.86 ppm respectively, due to the geminal coupling between two hydrogens of S–CH2–CO and a sharp singlet at δ 6.01 ppm corresponding to N–CH–S. The presence of three characteristic carbon signals observed at δ 33.19, 64.66 and 170.83 ppm in the 13C NMR spectrum of 4c owing to S–CH2–CO, N–CH–S and CO groups, respectively, confirmed the presence of a 4-thiazolidinone ring in the product. The mass spectrum further strengthen the formation of 2-(4-chlorophenyl)-3-(3,4-difluorophenyl)thiazolidin-4-one (4c) by showing an [M+H]+ ion peak at m/z 326.0546 for the molecular formula, C15H11ClF2NOS.
Conclusions
In summary, DBSA has been successfully used as an efficient Brønsted-acid surfactant combined catalyst for the synthesis of a series of novel 2,3-disubstituted 4-thiazolidinones through one-pot three-component condensation-cyclization reaction of aliphatic or aromatic primary amines with various aromatic aldehydes and thioglycolic acid in an aqueous medium at ambient temperature. This synthetic methodology is found to be mild, operationally simple, economical, environmentally benign and affords the target products in good yields.Experimental
All the chemicals were purchased from Sigma-Aldrich and used without further purification. The progress of the reactions was monitored by thin layer chromatography (TLC) using silica gel 60 F254 (pre-coated aluminium sheets) from Merck. 1H NMR and 13C NMR spectra were obtained in CDCl3 on Jeol ECX 400 MHz NMR spectrometer by using TMS as an internal standard. Chemical shifts are expressed in parts per million (ppm) and coupling constants (J) are reported in Hertz (Hz). Infrared spectra were recorded on Perkin Elmer IR spectrometer and absorption maxima (vmax) are given in cm−1. Mass spectra (ESI-HRMS) were recorded on JEOL-AccuTOF JMS-T100LC mass spectrometer having a DART source. The melting points were determined in open capillary tubes on Buchi M-560 melting point apparatus and are uncorrected.General procedure for the synthesis of 2,3-disubstituted 4-thiazolidinones (4a–o)
To a solution of DBSA (0.1 mmol) in water (3 mL), aliphatic or aromatic primary amine (1 mmol), aromatic aldehyde (2 mmol) and thioglycolic acid (3 mmol) were added successively at 25 °C. The reaction mixture was stirred at same temperature for the time as reported in the Table 2. After completion of the reaction, a saturated NaHCO3 solution (5 mL) was added followed by the addition of saturated brine solution (5 mL). The product was extracted with ethyl acetate (10 mL × 3 times). The organic layers were combined, washed with water, dried over anhydrous sodium sulfate and evaporated under reduced pressure to dryness. The crude product was purified by column chromatography over silica gel (60–120 mesh size) using 5–20% ethyl acetate in heptane as an eluent to furnish the desired product in pure form.Acknowledgements
This work is supported by University of Delhi, India under the scheme to strengthen R&D Doctoral Research Programme. We are thankful to CIF, University of Delhi and SAIF, CDRI, Lucknow, India for providing NMR and mass data. Davinder Prasad and Amreeta Preetam are grateful to CSIR, New Delhi, India for providing SRF and JRF, respectively.References
- P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti and F. Zani, Bioorg. Med. Chem., 2006, 14, 3859 CrossRef CAS.
- (a) R. K. Rawal, R. K. Tripathi, S. B. Katti, C. Pannecouque and E. D. Clercq, Medicinal Chemistry, 2007, 3, 355 CrossRef CAS; (b) J. Balzarini, B. O. Krzesinska, J. K. Maurin and A. Orzeszko, Eur. J. Med. Chem., 2009, 44, 303 CrossRef CAS.
- V. R. Solomon, W. Haq, K. Srivastava, S. K. Puri and S. B. Katti, J. Med. Chem., 2007, 50, 394 CrossRef CAS.
- A. Srinivas, A. Nagaraj and C. S. Reddy, Eur. J. Med. Chem., 2010, 45, 2353 CrossRef CAS.
- Z. Turgut, C. Yolacan, F. Aydogan, E. Bagdatli and N. Ocal, Molecules, 2007, 12, 2151 CrossRef CAS.
- T. Fuchigami, S. Narizuka and A. Konno, J. Org. Chem., 1992, 57, 3755 CrossRef CAS.
- R. M. A. El-Aal, Phosphorus, sulfur and Silicon, 2003, 178, 681 CrossRef CAS.
- (a) A. Verma and S. K. Saraf, Eur. J. Med. Chem., 2008, 43, 897 CrossRef CAS; (b) S. Ebrahimi, A. Mobinikhaldei and H. Eibagi, Phosphorus, Sulfur and Silicon, 2011, 186, 2279 CrossRef CAS; (c) P. D. Neuenfeldt, B. B. Drawanz, G. M. Siqueira, C. R. B. Gomes, S. M. S. V. Wardell, A. F. C. Flores and W. Cunico, Tet. Lett., 2010, 51, 3106 CrossRef CAS; (d) J. R. Mali, U. R. Pratap, P. D. Netankar and R. A. Mane, Tet Lett., 2009, 50, 5025 CrossRef CAS.
- A. Bolognese, G. Correale, M. Manfra, A. Lavecchia, E. Novellino and V. Barone, Org. Biomol. Chem., 2004, 2, 2809 CAS.
- (a) J. Tierney, J. Heterocycl. Chem., 1989, 26, 997 CrossRef CAS; (b) A. R. Surrey, J. Am. Chem. Soc., 1947, 69, 2911 CrossRef CAS; (c) A. A. Fadda;, Indian J. Chem., 1991, 30B, 834 Search PubMed; (d) H. Ma, Y. Hou, Y. Bai, J. Lu and B. Yang, J. Organomet. Chem., 2001, 637-639, 742 CrossRef CAS.
- R. C. Sharma and D. Kumar, J. Indian Chem. Soc., 2000, 77, 492 CAS.
- C. P. Holmes, J. P. Chinn, G. C. Look, E. M. Gorden and M. A. Gallop, J. Org. Chem., 1995, 60, 7328 CrossRef CAS.
- T. Srivastava, W. Haq and S. B. Katti, Tetrahedron, 2002, 58, 7619 CrossRef CAS.
- R. K. Rawal, T. Srivastava, W. Haq and S. B. Katti, J. Chem. Res., 2004, 5, 368 CrossRef.
- (a) S. K. Srivastava, S. L. Srivastava and S. D. Srivastava, J. Indian Chem. Soc., 2000, 77, 104 CAS; (b) P. M. Shah and M. P. Patel, Indian J. Chem., 2011, 50B, 310 CAS.
- A. S. Nagarajan, S. Kamalraj, J. Muthumary and B. S. R. Reddy, Indian J. Chem., 2009, 48B, 1577 CAS.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807 CrossRef CAS.
- C. Capello, U. Fischer and K. Hungerbuhler, Green Chem., 2007, 9, 927 RSC.
- C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC.
- N. Shapiro and A. Vigalok, Angew. Chem., 2008, 120, 2891 CrossRef.
- U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef.
- (a) S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176 RSC; (b) L. Zhang and J. Wu, Adv. Synth. Catal., 2007, 349, 1047 CrossRef CAS; (c) S. S. Weng, S. C. Chang, T. H. Chang, J. P. Chyn, S. W. Lee, C. A. Lin and F. K. Chen, Synthesis, 2010, 9, 1493 CrossRef; (d) L. Gang, L. Xinzong and W. Eli, New J. Chem., 2007, 31, 348 RSC; (e) A. Takasu, A. Takemoto and T. Hirabayashi, Biomacromolecules, 2006, 7, 6 CrossRef CAS; (f) A. Saito, J. Numaguchi and Y. Hanzawa, Tet. Lett., 2007, 48, 835 CrossRef CAS; (g) K. Deleersnyder, D. Shi, K. Binnemans and T. N. Parac-Vogt, J. Alloys Compd., 2008, 451, 418 CrossRef CAS; (h) K. Manabe, Y. Mori, S. Nagayama, K. Odashima and S. Kobayashi, Inorg. Chimica Acta, 1999, 296, 158 CrossRef CAS; (i) K. Manabe and S. Kobayashi, Tet. Lett., 1999, 40, 3773 CrossRef CAS; (j) S. Kobayashi, Y. Mori, S. Nagayama and K. Manabe, Green Chem., 1999, 1, 175 RSC.
- (a) K. Manabe, Y. Mori, T. Wakabayashi, S. Nagayama and S. Kobayashi, J. Am. Chem. Soc., 2000, 122, 7202 CrossRef CAS; (b) K. Manabe and S. Kobayashi, Org. Lett., 1999, 1, 1965 CrossRef CAS; (c) K. Manabe, S. Limura, X. M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2002, 124, 11971 CrossRef CAS; (d) T. S. Jin, J. S. Zhang, T. T. Guo, A. Q. Wang and T. S. Li, Synthesis, 2004, 2001 CrossRef CAS; (e) K. Manabe, Y. Mori and S. Kobayashi, Tetrahedron, 2001, 57, 2537 CrossRef CAS.
- (a) B. P. Mathew and M. Nath, J. Heterocycl. Chem., 2009, 46, 1003 CrossRef CAS; (b) B. P. Mathew, A. Kumar, S. Sharma, P. K. Shukla and M. Nath, Eur. J. Med. Chem., 2010, 45, 1502 CrossRef CAS; (c) D. Prasad and M. Nath, Catal. Sci. Technol., 2012, 2, 93 RSC.
- A. Dandia, R. Singh, S. Bhaskaran and S. D. Samant, Green Chem., 2011, 13, 1852 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: 1H and 13C NMR spectra of the synthesized products. See DOI: 10.1039/c2ra20171b/ |
| This journal is © The Royal Society of Chemistry 2012 |