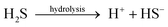DOI:
10.1039/C2RA01313D
(Paper)
RSC Adv., 2012,
2, 3123-3132
Indirect hydrodesulfurization of gasoline via sodium borohydride reduction with nickel catalysis under ambient conditions
Received
19th December 2011
, Accepted 11th January 2012
First published on 12th January 2012
Abstract
The present work introduced a novel indirect hydrodesulfurization (HDS) method for gasoline fuel at room temperature using an excellent reductant of sodium borohydride (NaBH4), which produces active hydrogen with Ni–B catalysis. Then the active hydrogen can convert S-compounds into hydrogen sulfide (H2S) or S2−, released from the gasoline. Under the optimized conditions the maximal desulfurization efficiencies of original and experimental gasoline can reach about 36.6% and 88%, respectively. It is a relatively clean and moderately indirect HDS method; after the process the Ni–B catalyst produced in the filtrate can be reused for the further desulfurization work. Moreover, the realization of the filtrate recycling, the evaluation of the hydrogen releasing and the exploration of catalyst development will be further helpful in cost reduction in future work.
1. Introduction
Sulfur in transportation fuels (i.e. diesel, gasoline) remains a major source of air pollution and engine corrosion. Because of government mandates worldwide, increasingly stringent regulations are being imposed to reduce the S-content to a very low level, such as 10–20 ppm.1,2 So refiners must put forward new requirements to produce increasingly cleaner fuels.3 The primary focus of the new regulations is the reduction of sulfur in gasoline and diesel.4 Removal of sulfur-containing compounds is an important operation in petroleum refining, and is achieved by catalytic processes operated at elevated temperatures (300–340 °C) and pressures (20–100 atm of H2) by using the Co–Mo/Al2O3 or the Ni–Mo/Al2O3 catalyst.5
Hydrodesulfurization (HDS) is the essential process for removing sulfur-containing impurities from crude petroleum in order to obtain (i) cleaner fuels that minimize environmental pollution and (ii) cleaner chemical feedstocks that are less likely to poison the catalysts that are used for subsequent transformations.6–8 Conventional desulfurization methods of transportation fuels are always catalytic HDS under the conditions of high temperature and high pressure. As such, costly reductant and catalysts, strict equipments and operation conditions have deeply restricted the development and utilization of them.9,10
Recently, the renewed oxidative desulfurization (ODS) methods were studied, where ionic liquids (ILs) were used as extractants instead of organic solvents. It was found that the thiophenic S-compounds in fuels can be oxidized to their corresponding sulfone compounds, for example, dibenzothiophene sulfone (Scheme 1, DBTO2).11–22 The proposed method relies on adsorbents; however, the desulfurization function is not due to the adsorption of sulfur compounds, but to the conversion of them via a characteristic reaction occurring in adsorbents. However, ILs and adsorbents are so precious and excessive that they are difficult to be utilized practically on a large scale. Besides, the ODS method, which may destroy the heat value of fuels, becomes disabled in fuels containing too many aromatic components and/or dissolved water.23
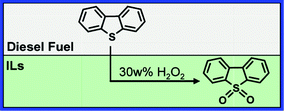 |
| | Scheme 1 Desulfurization of diesel fuel using ionic liquids. | |
In the previous studies, we have found that sodium borohydride (NaBH4), owing to its excellent reductive property, appears to give a promising desulfurization efficiency of coal under ambient conditions.9,24 If fine catalysts used, the hydrogen production and desulfurization efficiency can be significantly enhanced. However, indirect catalytic HDS via NaBH4 reduction is rarely employed for transportation fuels (gasoline, diesel, etc.) desulfurization. In this sense, it is the first time a substitute for the conventional HDS methods has been investigated for gasoline using NaBH4 catalytic (Ni) reduction under ambient conditions.
2. Experimental section
2.1. Gasoline and chemicals
Original gasoline (S-content: 40∼50 ppm) was obtained from an oil refinery in Shanghai, China. Organic sulfur (OS) exists in the real gasoline mainly in the forms of mercaptan, thioether, disulfide, thiophene and its derivates, which are shown in Fig. 1A. Mercaptan, thioether and benzothiophene (i.e. DBT) are not detected in the original gasoline. Since some other OS is omitted, the S-content of original gasoline is uniformly set as 40 ppm so that it is convenient for calculation in the following investigation. The sequence of hydrogenation activity about different types sulfides is:25 thiophene < thioether < disulfide < mercaptan. Sodium borohydride (NaBH4, 96%), NiCl2·6H2O (AR, >98%), NaOH (AR, >96%), commercial activated alumina (γ-Al2O3), and agar powder (BR) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), and stored in a dryer. Butyl sulfide (C8H18S, >95%, GC) used for preparing the higher S-content gasoline (experimental gasoline) was purchased from Fluka. All chemicals were used for experiments without any further purification.
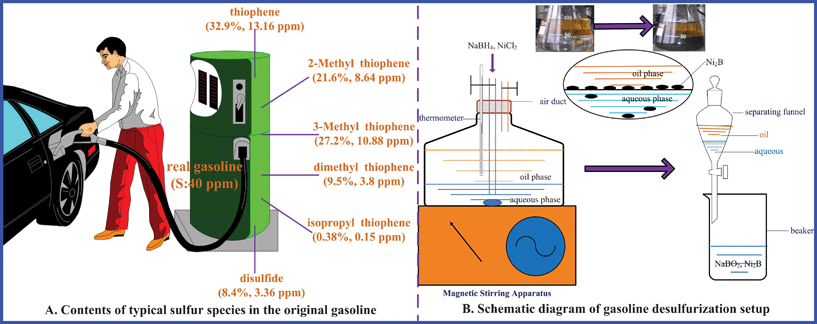 |
| | Fig. 1 Schematic diagram of gasoline S-contents (A) and desulfurization setup (B). | |
Preparation of the KCl salt bridge.
3 g agar powder was added into 97 ml distilled water, and heated up to dissolve completely. Then 30 g KCl was added into the agar solution. Finally, the mixed solution was added dropwise or siphoned into the U-shaped glass tube. The salt-bridge can be used after agar condensation.
2.2. Apparatus and procedure
The reactions were carried out in a simple setup in Fig. 1B, which mainly consisted of the magnetic stirring apparatus, reactor (Erlenmeyer flask) and thermometer. Experimental reagents: aqueous solution/gasoline = 1/2 (volume ratio), NaBH4/NiCl2·6H2O = 20 (mass ratio).
General procedure.
Firstly, isopycnic distilled water (100 ml) and gasoline (100 ml) were added into the reactor in sequence. Then, the chemicals of NaBH4 and NiCl2·6H2O were put into the reactor from the feedstock inlet. After that, the feedstock inlet and gas outlet were closed. With operating the magnetic stirring apparatus at a speed of 300 rpm, the reaction and timing were started. After 5 min the gas outlet was opened. The reaction was not finished until only a small amount of air bubbles were produced. And the produced tail gas (H2S, etc.) was adsorbed by the alkaline solution (NaOH, etc.). The immediate formation of nickel boride (Ni2B, black homogeneous precipitates) was observed, accompanied by the vigorous evolution of hydrogen (H2, H*). Desulfurization occurred rapidly and the spent nickel boride was easily removed by filtration. Finally, the cleaner gasoline could be obtained by a separation method using the separating funnel. The separated aqueous solution containing Ni–B catalysts can be reused in the following experiments.
2.3. Analysis methods
Total sulfur (TS) in the gasoline and elements in the aqueous solution were determined by Inductively Coupled Plasma (Iris Advantage 1000, Thermo king-cord Co., USA). Various organic sulfurs (OS) were analyzed by Gas Chromatography (Shimadzu GC2010, Japan). GC operating conditions (injector temperature: 250 °C; carrier gas: helium; capillary column: SPB-5, bonded 5%, 30 m × 0.25 mm i.d. × 0.25 μm film thickness; flow rate: 1.5 ml min−1; column oven: 50∼130 °C, 5 °C min−1; detector type: FID; detector temperature: 250 °C; detecting time: 30 min) are given. And each GC analysis was repeated three times to check reproducibility. The desulfurization efficiency was calculated by the following equation,
where S0 was the initial S-content and S1 was the final S-content after HDS. And the data were presented as the average of two replicates in each treatment.
3. Results and discussions
3.1. Effect of reaction time
Time is an important technical parameter for chemical reactions, which reflects the chemical reaction efficiency and determines the experimental time. As a whole, the S-content in the original gasoline decreases with the increase of reaction time in Fig. 2(a). After 2 h, the S-content of real gasoline reduces from 40 ppm to 30 ppm (w/o Ni) and 25 ppm (with Ni), accompanied by the desulfurization efficiency increases by 25.5% and 36.6%, respectively (Fig. 2b). In the previous work, we have proposed the hydrolysis of NaBH4 basically determining the treatment time.9 In Fig. 2(c), the desulfurization efficiency of real gasoline gradually increases with increase in reaction time as well, which accords with the linear eqn (1) (w/o Ni) and the first-order reaction eqn (2) (with Ni). Likewise, the S-content of experimental gasoline greatly reduces from 400 ppm to 190 ppm (w/o Ni) and 56 ppm (with Ni), accompanied by the desulfurization efficiency increases by 52% (w/o Ni) and 86% (w/o Ni), respectively. And the desulfurization efficiency increases with the increase of reaction time, which can also be fitted as eqn (III) (linear) and eqn (IV) (first-order) in Fig. 2(d). From the results, it is suggested that the increasing trend of S-removal recedes after 60 min, which may be caused by the hydrolysis rate slowing down. Addition of Ni catalyst can convert the hydrolysis kinetics of NaBH4 from the linear reaction into a first-order reaction. Preliminarily, the catalytic reaction kinetics may be accorded with Langmuir–Hinshelwood kinetics. In the following investigation we use 60 min as the experimental time.| | | y = 0.2071x + 4.159 (linear, RI2 = 0.9089); | (I) |
| | | y = −37.30exp(−x/19.92) + 36.96 (first-order, RII2 = 0.998); | (II) |
| | | y = 0.3845x + 11.70 (linear, RIII2 = 0.8637); | (III) |
| | | y = −79.99exp(−x/14.61) + 80.84 (first-order, RIV2 = 0.9896), | (IV) |
where y is the desulfurization efficiency (%), x is the reaction time (0∼120 min), and R is the regression coefficient.
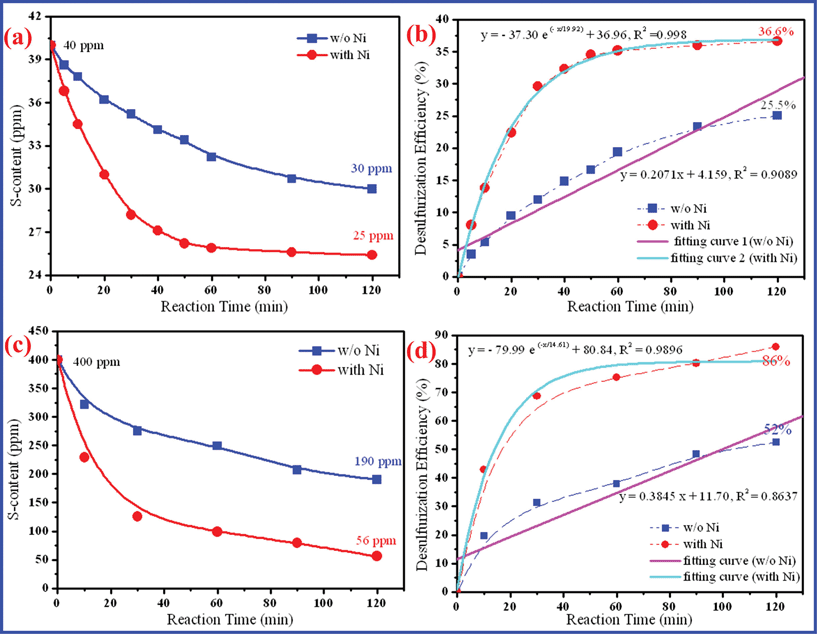 |
| | Fig. 2 Sulfur removal of gasoline (NaBH4: 1 g L−1, NiCl2·6H2O: 0.05 g L−1). | |
3.2. Effect of NaBH4 concentration
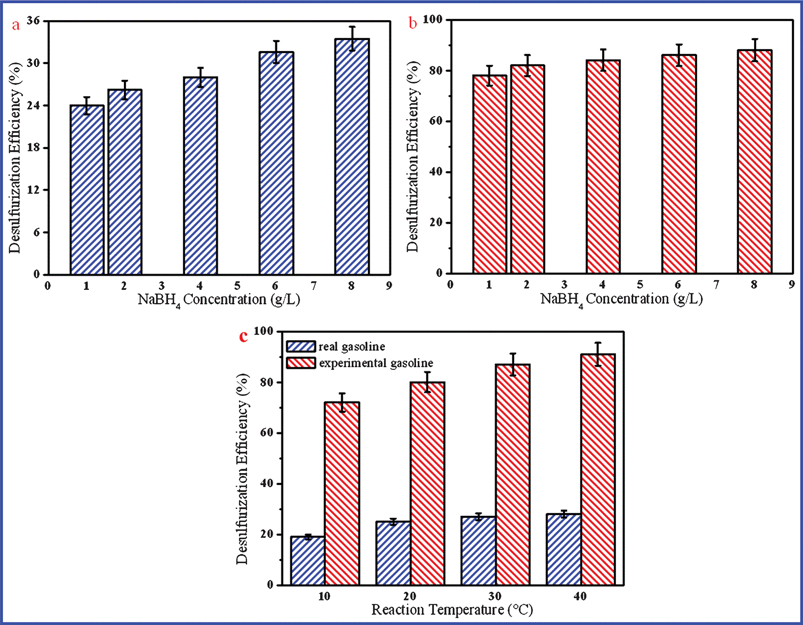
In the present work, NaBH4 is the most important reactive chemical substance, which is the main resource of providing the protons. It can react with H2O to produce amounts of active hydrogen. Meanwhile, the suitable concentration of NaBH4 can reduce the excessive utilization. In that case, it is very necessary to investigate the NaBH4 concentration in this reaction system. In Fig. 3(a), the desulfurization efficiency of gasoline increases with the increase of NaBH4 concentration, while it is not evident. It can be concluded that the desulfurization efficiency is determined by the active hydrogen produced. However, the active hydrogen is determined by the two significant factors of NaBH4 and catalyst (Ni) concentrations. If the ratio of C(NaBH4)/C(Ni) is coordinating, it will produce more active hydrogen. Otherwise, it will bring about excessive NaBH4 concentration. From Fig. 3(b), it can be found that the desulfurization efficiency is almost unchanged when the NaBH4 concentration exceeds 2 g L−1. Thus, the optimized condition of NaBH4 concentration is 2 g L−1.
 |
| | Fig. 3 Effect of NaBH4 concentration and reaction temperature on desulfurization efficiency. | |
3.3. Effect of reaction temperature
The previous experiments were carried on under room temperature (∼20 °C) conditions. However, reaction temperature has a significant effect on the chemical reaction. On one hand, the reaction temperature can affect the catalytic reaction rate. One the other hand, it can enhance the pressure of the reaction system, accordingly increasing the contact probability of S-compounds in gasoline with the reductants and catalysts in the aqueous phase. While gasoline is a volatile organic compound, whose boiling point is around 40–200 °C, a higher temperature can possibly lead to the volatilization of gasoline. Herein the effect of reaction temperature on the S-removal efficiency between 10 and 40 °C was investigated in this work. As Fig. 3(c) shows, the desulfurization efficiency gradually increases with increasing the reaction temperature from 10 to 40 °C. When the reaction temperature gets close to 40 °C, the maximal desulfurization efficiency of experimental gasoline can reach about 90%, while the desulfurization efficiency of real gasoline is almost unchanged. It presents that this indirect catalytic HDS is very difficult to remove sulfur in gasoline with an ultra-low S-content, i.e. below 15∼20 ppm. Since it is hard to control the temperature around so small a range, the desulfurization process could be carried out at room temperature.
3.4. Reuse of the filtrate
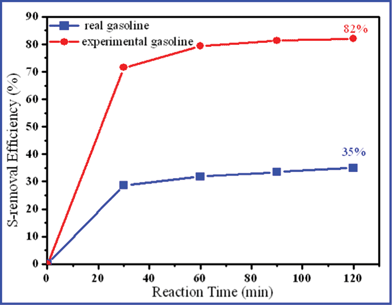
As we know, the catalytic chemical reaction and hydrogen release are happening in the aqueous medium. Namely, most of the chemicals still remain in the aqueous phase. These reaction products, such as Ni2B, stably exist in the aqueous phase. Possibly, the part of the Ni2B can be reused for further experiments. As Table 1 shows, the elemental concentration (i.e. B, Ni, and Na) in the filtrate is almost unchanged. Besides, the slight increase of sulfur (S) may be caused by HDS from the gasoline. In Fig. 4, we investigate the recycling utilization efficiency of the filtrate just by adding the same amount of NaBH4. It can be found that the S-removal efficiencies of gasoline are 82% (initial S-content: 400 ppm) and 35% (initial S-content: 40 ppm) after 120 min. It demonstrates that the Ni2B catalyst in the filtrate can also be reused for further desulfurization process. After the reaction, the pH value of the filtrate is slightly reduced, possibly due to the hydrolysis of H2S.
 |
| | Fig. 4 Sulfur removal efficiency of the gasoline using the filtrates. | |
Table 1 ICP analytical results of the filtratea
| Element |
Before reaction/mg L−1 (pH = 9.80) |
After reaction/mg L−1 |
| Ob (pH = 9.26) |
Ec (pH = 9.48) |
|
Process conditions: 60 min reaction time, 2 g L−1 of NaBH4, and 0.1 g L−1 of NiCl2.
O = original gasoline.
E = experimental gasoline.
|
| S |
0 |
3.62 |
6.80 |
| B |
277.90 |
275.30 |
271. 76 |
| Ni |
24.20 |
23.96 |
24.02 |
| Na |
592.05 |
591.40 |
590.78 |
3.5. GC analysis
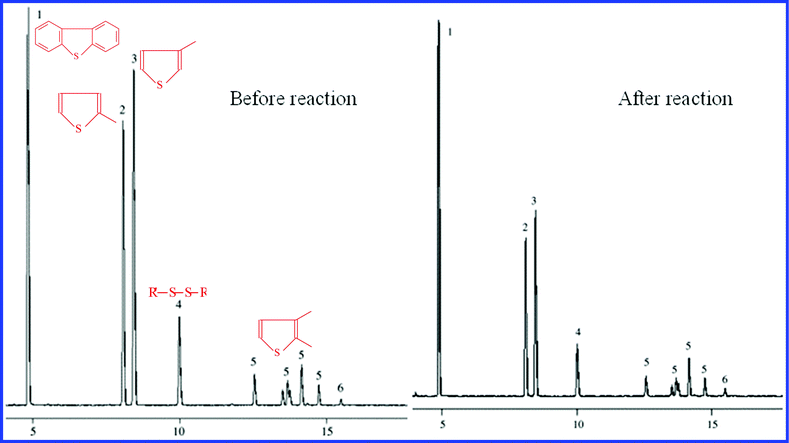
Fig. 5 shows the chromatogram of sulfur species distribution in original gasoline before and after the reaction. Also, the desulfurization performance values for main typical sulfur species are listed in Table 2. It can be found that thiophene and its derivates are significant components of OS in gasoline. This method is beneficial for 2-methylthiophene, 3-methylthiophene, and disulfide, etc. Moreover, the addition of thioether (higher concentration) can be greatly reduced, which is not shown in this figure. According to the peak areas before and after the reaction, we may estimate the approximate value of the S-removal efficiency (24.2%) using the following formula:
The estimated value of the desulfurization efficiency is just approaching the detected value (25.5%) as above. The produced error may be caused by the slightly reduced reaction time and ignoring some other little adsorption peaks of OS, which are not detected by GC.
 |
| | Fig. 5 Chromatogram of sulfur species distribution in gasoline. | |
Table 2 Desulfurization performance for typical sulfur species in gasolinea
| Peak no. |
Sulfur species |
Before reaction |
After reaction |
| Peak area, P(a) |
Percent. (%, total: 100) |
Peak area, P(b) |
Percent. (%) |
Removal (%) |
|
Process conditions: 60 min of reaction time, 2 g L−1 of NaBH4, and 0.1 g L−1 of NiCl2.
|
| 1 |
Dibenzothiophene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
32.9 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 111 |
31.2 |
5.2 |
| 2 |
2-Methylthiophene |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382 382![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634 |
21.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 902 |
12.8 |
40.7 |
| 3 |
3-Methylthiophene |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995 995![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 981 981 |
27.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
16.3 |
40.1 |
| 4 |
Disulfide |
924![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 349 |
8.4 |
586![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631 631 |
5.3 |
36.9 |
| 5 |
Dimethylthiophene |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 046![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 519 |
9.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 051![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 124 |
9.5 |
0 |
| 6 |
Isopropylthiophene |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 276 |
0.38 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 062 062 |
0.65 |
— |
3.6. Possible mechanism of the indirect catalytic HDS
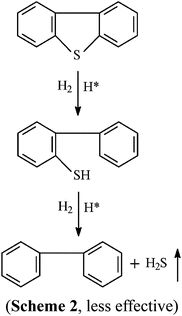
From the above analytical results, we herein can find that the increase of desulfurization efficiency and the decrease of treatment time are attributed to the addition of Ni catalyst. It is possible that the S atom in gasoline contains lone-pair electrons, and Ni2+ has an empty “d” orbital, so S react easily with Ni2+. In the Ni based catalyst, the stabilizing agent (NaOH) promoted the production of hydrogen. Furthermore, NaBH4 can undergo fast reaction with NiCl2 in the transformation of Ni2B at the beginning, dispersing in the aqueous solution. Under Ni2B catalysis, NaBH4 can immediately react with H2O, producing amounts of H2 and active hydrogen. In the reaction, the activated hydrogen is adsorbed on the surface of nickel boride (Ni–B), forming into a kind of Ni–MH intermediate product. Then high activated hydrogen attacks S bonded with α-C, resulting in C–S bond cleavage. Meanwhile, the Ni–B produced by the reaction of NiCl2 and deoxidizer has a high Ni–B/Ni rate, so it is beneficial for the activated adsorption of in situ hydrogen and organic sulfides (OS), which possess a higher desulfurization activity to aromatic organic sulfides.26–30 Nickel boride (Ni2B) is a fine and stable black solid that is easily prepared by the reduction of Ni2+ salts with NaBH4, usually in protic solvents as shown in eqn (1).27 Using Ni catalysts can greatly improve the desulfurization efficiency of organic sulfurs. The possible desulfurization mechanisms are presented as the following eqn (1)–(11).
Step 1 (catalyst production)
| | | 4NaBH4 + 9H2O + 2NiCl2 → Ni2B↓ (black precipitates) + 3H3BO3 + 4NaCl + 12.5H2↑ | (1) |
Step 2 (active hydrogen production)
| | | NaBH4 + 2H2O → NaBO2 + 4H2↑ (w/o Ni catalysis, slow reaction) | (2) |
| | | NaBH4 + 2H2O → NaBO2 + 4H2↑ (8H*) (with Ni catalysis, fast reaction) | (3) |
| | | 4NaBO2 + 11H2O → Na2B4O5 (OH)4·8H2O (sodium borate) + 2NaOH | (4) |
Step 3 (S-removal)
| | | 2H* + R–SH → R–H + H2S↑ | (5) |
| | | 4H* + R–S–R¢ → R–H + R¢–H + H2S↑ | (6) |
| | | 6H* + R–S–S–R¢ → R–H + R¢–H + 2H2S↑ | (7) |
Special example
| | | 4H* + C4H9–S–C4H9 → 2C4H10 + H2S↑ (Fig. 6A, very effective) | (8) |
| |  | (9) |
| | | H2S + 2NaOH → Na2S + 2H2O | (10) |
| |
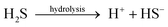
| (11) |
Also, the schematic diagram of indirect catalytic HDS is shown in
Fig. 6.
 |
| | Fig. 6 Schematic diagram of indirect catalytic HDS. | |
3.7. Further R&D for indirect catalytic HDS
3.7.1. Catalyst (Ni–B/γ-Al2O3) for indirect HDS.
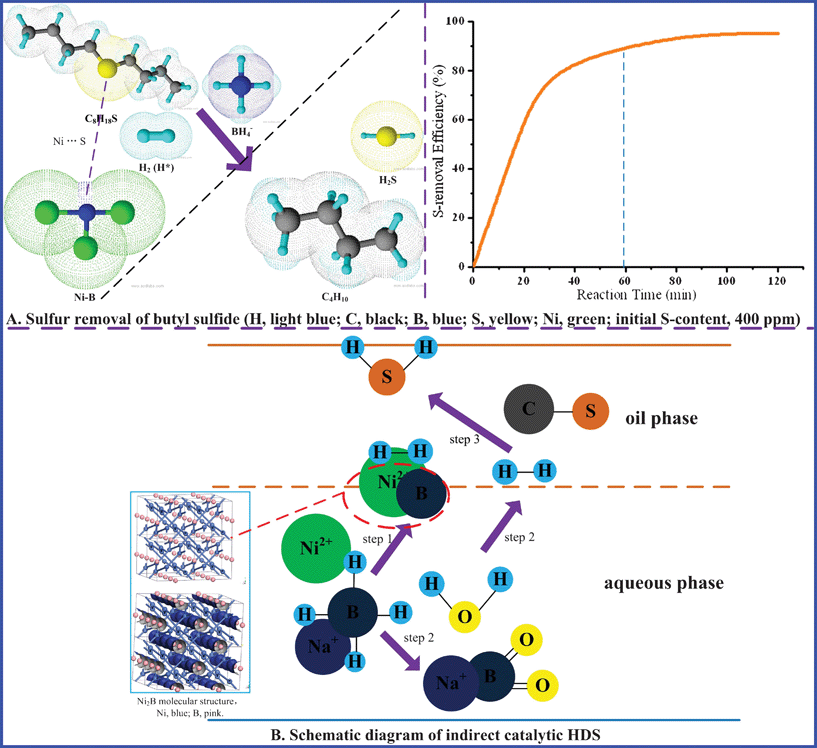
Since the Ni–B catalyst is beneficial for hydrogen production by NaBH4 reduction and S removal from gasoline, it can be loaded on the surface of activated aluminium oxide (γ-Al2O3),5 mesoporous silica (SiO2)31,32 or TiO2 mesocrystals33 in the form of the Ni–B/γ-Al2O3 supported amorphous catalyst, Ni–B/SiO2 or Ni–B/TiO2 (converted from dispersion to non-dispersion), which is easier to be prepared and recycled. Meanwhile, metallic compounds loaded in the surface pores of γ-Al2O3, just like Co–Mo/Al2O3 or Ni–Mo/Al2O3 catalyst,5 can enhance the catalytic activity of HDS. Fig. 7A presents the general preparation process of the Ni–B/γ-Al2O3 supported catalyst. And catalyst samples before and after reaction are given in Fig. 7B. From the XPS analysis, one can conclude that Ni in the Ni–B amorphous alloy is electron-rich and that the number of active centers decreases after reaction (Fig. 7C). It is suggested that loaded Ni–B is slightly reduced due to hydrogen (H2) production and the HDS process. As Fig. 7D shows, the S-removal efficiency and H2 yield increase rapidly with increasing the reaction time under the certain optimized conditions (details of optimizing experiments not shown here), while the increase trend becomes weaken after 120 min.
 |
| | Fig. 7 Catalyst (Ni–B/γ-Al2O3) test for indirect HDS of gasoline. | |
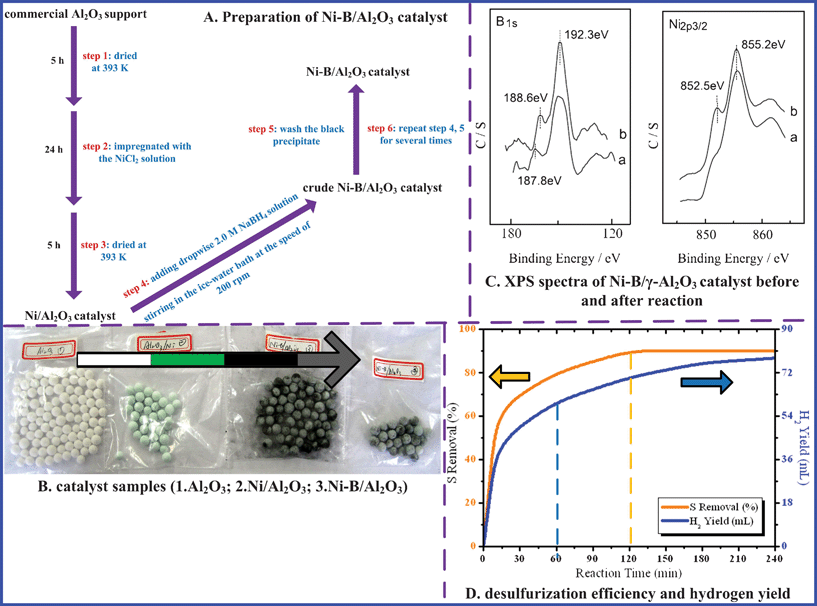
3.7.2. Electrochemical hydrodesulfurization (EHDS) and electrochemical oxidative desulfurization (EODS).
According to previous reports in the literatures and experimental theory analysis,34,35 the ideal equation can be got as Fig. 8A. It is noteworthy that the removed S from gasoline in the form of gaseous H2S in the cathodic slot can be further directly electrochemically oxidized into the element sulfur or absorbed by sodium hydroxide (NaOH) in the anodic slot. So it may be a good conception to combine chemical with electrochemical methods of NaBH4 reduction for the desulfurization of fuels. It can resolve the problems of deoxidizer costs and realize the filtrate recycling. Besides, this kind of clean reduction method only uses electricity and green solutions, so it reduces the additional pollution, realizing reasonable application and sustainable development of resources. Fig. 8B shows the schematic diagram of the EHDS process for gasoline. General procedure: step 1. EHDS process in the cathodic slot; step 2. EODS process in the anodic slot. Fig. 8C presents the FT-IR analysis of gasoline before and after treatment, and gives the removal mechanism of OS (i.e. dibenzothiophene, DBT) in the anode. Meanwhile, employing of the optimized conditions (details of optimizing experiments and reductive mechanism not shown here), the desulfurization efficiency rapidly increases with increasing of the electrolytic time from 4 to 8 h (Fig. 8D). Also, the hydrogen yield during the EDS process improves remarkably with the electrolytic time as well from 1 to 5 h, according with first-order reaction (Fig. 8E). The further investigations of filtrate recycling and hydrogen yield will be helpful for cost reduction and sustainable development. Significantly, the developed method makes the most of electric energy to realize the cathodic reduction and anodic oxidation, converting OS into the inorganic S from gasoline to the aqueous phase.
 |
| | Fig. 8 Combination EHDS with EODS for gasoline. | |
4. Conclusions
Herein, the presented indirect catalytic HDS process for gasoline fuel, which is an efficient and convenient method, can be operated under ambient conditions. Under the optimized conditions of room temperature, reaction time (60 min), NaBH4 (2 g L−1), NiCl2 (0.1 g L−1) and stirring rate (300 rpm), the maximal desulfurization efficiency can reach about 86% when the initial S-content of gasoline is 400 ppm. It is highly efficient in removing OS in gasoline, especially for the thioether added in. Meanwhile, the produced Ni2B catalyst in the filtrate and waste aqueous solution can be reused for further desulfurization work. Furthermore, the environmentally friendly recycling of the Ni catalyst and aqueous solution will greatly decrease the expense of the desulfurization process. Significantly, the realization of the filtrate recycling, the evaluation of the hydrogen (H2) release and the exploration of the catalyst development will be further helpful in cost reduction in the future work.
In addition, the developed Ni–B/γ-Al2O3 supported amorphous catalyst shows excellent performance for hydrogen production from NaBH4 hydrolysis (improving the utilization ratio of NaBH4), and exhibits a high S-removal efficiency of gasoline by NaBH4 reduction. And the further employed EHDS can accomplish filtrate and boron (B) recycling for the first step, accordingly cutting down the secondary pollution and realizing economically sustainable development.
Acknowledgements
This work is financially supported by the National High Technology Research and Development Program (“863” program, Grant No. 2009AA062603). And the insightful comments and suggestions provided by reviewers and editor are greatly appreciated.
References
- T. Mochizuki, H. Itou, M. Toba, Y. Miki and Y. Yoshimura, Effects of acidic properties on the catalytic performance of CoMo sulfide catalysts in selective hydrodesulfurization of gasoline fractions [J]., Energy Fuels, 2008, 22(3), 1456–1462 CrossRef CAS.
- E. Rodríguez-Castellón, A. Jiménez-López and D. Eliche-Quesada, Nickel and cobalt promoted tungsten and molybdenum sulfide mesoporous catalysts for hydrodesulfurization [J]., Fuel, 2008, 87(7), 1195–1206 CrossRef.
-
A. Avidan, M. Cullen, paper AM-01-55, presented at the National Petroleum and Refiners Association Annual Meeting, Washington, DC, 18 to 20 March 2001.
- T. Yang Ralph, J. Herna′ndez-Maldonado Arturo and H. Yang Frances, Desulfurization of Transportation Fuels with Zeolites Under Ambient Conditions [J]., Science, 2003, 301, 79–80 CrossRef.
-
B. C. Gates, J. R. Katzer, G. C. A. Schuit, Chemistry of Catalytic Processes (McGraw-Hill, New York, 1979), chap. 5 Search PubMed.
- A. Sattler and G. Parkin, Carbon-Sulfur Bond Cleavage and Hydrodesulfurization of Thiophenes by Tungsten [J]., J. Am. Chem. Soc., 2011, 133, 3748–3751 CrossRef CAS.
- Y. Fan, H. Xiao, G. Shi, H. Liu and X. Bao, A novel approach for modulating the morphology of supported metal nanoparticles in hydrodesulfurization catalysts [J]., Energy Environ. Sci., 2011, 4, 572–582 CAS.
- M. C. Capel-Sanchez, J. M. Campos-Martin and J. L. G. Fierro, Removal of refractory organosulfur compoundsviaoxidation with hydrogen peroxide on amorphous Ti/SiO2catalysts [J]., Energy Environ. Sci., 2010, 3, 328–333 CAS.
- Y. F. Shen, T. H. Sun and J. P. Jia, Novel desulfurization method of sodium borohydride reduction for coal water slurry [J]., Energy Fuels, 2011, 25, 2963–2967 CrossRef CAS.
-
C. Sudhakar, G. G. Sandford, A. K. Bhattacharya, Selective hydrodesulfurization of naphtha using spent resid catalyst., US Patent: 1995, 5, 423, 975 Search PubMed.
- E. Lissner, W. F. de Souza, B. Ferrera and J. Dupont, Oxidative Desulfurization of Fuels with Task-specific Ionic Liquids [J]., ChemSusChem, 2009, 2, 962–964 CrossRef CAS.
- F. Li, R. Liu, J. Wen, D. Zhao, Z. Sun and Y. Liu, Desulfurization of Dibenzothiophene by Chemical Oxidation and Solvent Extraction with Me3NCH2C6H5Cl3·2ZnCl2 Ionic Liquid [J]., Green Chem., 2009, 11, 883–888 RSC.
- W. Zhu, H. Li, X. Jiang, Y. Yan, J. Lu and J. Xia, Oxidative Desulfurization of Fuels Catalyzed by Peroxotungsten and Peroxomolybdenum Complexes in Ionic Liquids [J]., Energy Fuels, 2007, 21(5), 2514–2516 CrossRef CAS.
- D. Zhao, Y. Wang and E. Duan, Oxidative Desulfurization of Fuel Oil by Pyridinium-based Ionic Liquids [J]., Molecules, 2009, 14(11), 4351–4357 CrossRef CAS.
- W. H. Lo, H. Y. Yang and G. T. Wei, One-pot Desulfurization of Light Oils by Chemical Oxidation and Solvent Extraction with Room Temperature Ionic Liquids [J]., Green Chem., 2003, 5, 639–642 RSC.
- Z. Sun, F. Li, R. Liu and H. Sha, Oxidative Desulfurization of Thiophene Catalyzed by (C4H9)4NBr3 · 2C6H11NO Coordinated Ionic Liquid [J]., Energy Fuels, 2008, 22, 3065–3069 CrossRef.
- W. Zhu, H. Li, Q. Gu, P. Wu, G. Zhu, Y. Yan and G. Chen, Kinetics and Mechanism for Oxidative Desulfurization of Fuels Catalyzed by Peroxo-molybdenum Amino Acid Complexes in Water-immiscible Ionic Liquids [J]., J. Mol. Catal. A: Chem., 2011, 336, 16–22 CrossRef CAS.
- J. Zhang, A. Wang, X. Li and X. Ma, Oxidative Desulfurization of Dibenzothiophene and Diesel over [Bmim]3PMo12 O40 [J]., J. Catal., 2011, 279, 269–275 CrossRef CAS.
- L. He, H. Li, W. Zhu, J. Guo, X. Jiang, J. Lu and Y. Yan, Deep Oxidative Desulfurization of Fuels using Peroxophosphomolybdate Catalysts in Ionic Liquids [J]., Ind. Eng. Chem. Res., 2008, 47(18), 6890–6895 CrossRef CAS.
- L. Wang, D. Zhao and K. Li, Oxidative Desulfurization of Dibenzothiophene using Ozone and Hydrogen Peroxide in Ionic Liquid [J]., Energy Fuels, 2010, 24, 2527–2529 CrossRef.
- D. Zhao, R. Liu, J. Wang and B. Liu, Photochemical Oxidation-Ionic Liquid Extraction Coupling Technique in Deep Desulphurization of Light Oil [J]., Energy Fuels, 2008, 22, 1100–1103 CrossRef CAS.
- G. Yu, J. Zhao, D. Song, C. Asumana, X. Zhang and X. Chen, Deep Oxidative Desulfurization of Diesel Fuels by Acidic Ionic Liquids [J]., Ind. Eng. Chem. Res., 2011, 50(20), 11043–11048 Search PubMed.
- S. Wang, L. Zhou, W. Su and Y. Sun, Deep Desulfurization of Transportation Desulfurization Fuels by Characteristic Reaction Resided in Adsorbents [J]., AIChE J., 2009, 55, 1872–1881 CrossRef CAS.
- Z. Li, T. Sun and J. Jia, An extremely rapid, convenient and mild coal desulfurization new process: Sodium borohydride reduction [J]., Fuel Process. Technol., 2010, 91, 1162–1167 CrossRef CAS.
- Q. H. Zeng, Sulfides characteristics of intermediate fraction and deep hydrotreated products [J]., Foreign Oilfield Engineering, 1994,(1), 8–14 Search PubMed.
- S. Schouten, D. Pavlović, J. S. Sinninghe Damsté and J. W. de Leeuw, Nickel boride: an improved desulphurizing agent for sulphur-rich geomacromolecules in polar and asphaltene fractions [J]., Org. Geochem., 1993, 20, 901–909 CrossRef CAS.
- T. G. Back, J. K. Yang and H. R. Krouse, Desulfurization of benzo- and dibenzothiophenes with nickel boride [J]., J. Org. Chem., 1992, 57, 1986–1990 CrossRef CAS.
- J. C. Walter, A. Zurawski, D. Montgomery, M. Thornburg and S. Revankar, Sodium borohydride hydrolysis kinetics comparison for nickel, cobalt, and ruthenium boride catalysts [J]., J. Power Sources, 2008, 179, 335–339 CrossRef CAS.
- X. Guo, S. Li, C. Yue and X. Ni, Desulfurization of benzothiophene with new reduction method., J. Appl. Chem. (Chinese), 2006, 23, 982–987 CAS.
- Y. F. Shen, X. L. Yang, T. H. Sun and J. P. Jia, Innovative desulfurization process of coal water slurry under atmospheric condition via sodium metaborate electroreduction in the isolated slot [J]., Energy Fuels, 2011, 25, 5007–5014 CrossRef CAS.
- L. Trimm David, O. Y. Liu Irene and W. Cant Noel, The selective hydrogenation of acetylene over a Ni/SiO2 catalyst in the presence and absence of carbon monoxide [J]., Appl. Catal. A: General, 2010, 347, 58–64 Search PubMed.
- Y. Pan, P. Kuai, Y. Liu, Q. Ge and C. Liu, Promotion effect of Ga2O3 on CO2 adsorption and conversion over a SiO2 supported Ni catalyst [J]., Energy Environ. Sci., 2010, 3, 1322–1325 CAS.
- Z. Hong, M. Wei, T. Lan, L. Jiang and G. Cao, Additive-free synthesis of unique TiO2 mesocrystals with enhanced lithium-ion intercalation properties [J]., Energy Environ. Sci., 2012, 5, 5408–5413 CAS.
-
Hal. B. H. Cooper, Electrolytic process for the production of alkali metal borohydride [P].US Pat: 3734842, 1973 - 05 - 32 Search PubMed.
-
Amendola Steven, Electroconversion Cell [P].
US Pat: 6497973, 2002 - 12 - 24 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622
622![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975
975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434
434![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111
111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382
382![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634
634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410
410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902
902![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995
995![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 981
981![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798
798![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030
030![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349
349![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631
631![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046
046![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519
519![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051
051![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124
124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276
276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 062
062