Novel synthesis route for Ag@SiO2 core–shell nanoparticles via micelle template of double hydrophilic block copolymer†
Bishnu Prasad
Bastakoti
a,
Sudhina
Guragain
a,
Shin-ichi
Yusa
b and
Kenichi
Nakashima
*a
aDepartment of Chemistry, Faculty of Science and Engineering, Saga University, 1 Honjo-machi, Saga 840-8502, Japan. E-mail: nakashik@cc.saga-u.ac.jp
bDepartment of Materials Science and Chemistry, University of Hyogo, 2167 Shosha, Himeji 671-2280, Japan
First published on 9th May 2012
Abstract
We report a new strategy for synthesizing core–shell nanoparticles of Ag@SiO2 templated by a complex micelle of silver ions and a double hydrophilic block copolymer, poly[3-(methacryloylamino) propyl trimethylammonium chloride]-block-[2-(dimethylamino) ethyl methacrylate] (PMAPTAC-b-PDMAEMA). The obtained Ag@SiO2 core–shell nanoparticles have been successfully applied as a catalyst for the reduction of p-nitrophenol.
There has been increased interest in silica-coated metal nanoparticles for diverse applications, especially for catalysis, diagnostics, imaging, surface enhanced Raman scattering, etc.1 The silica shell can alter the functionality, reactivity, and stability of the core. Furthermore, silica is an inert, robust and optically transparent material. It is also important for practical applications that the silica shells are easily labelled with various functional groups.2
Nanoparticles of noble metals, such as silver and gold were considered not to be suitable for direct silica coating due to their low chemical affinity to silica. Thus, prior to the silica coating, numerous surface attachments onto the metal nanoparticles are needed to protect noble metal nanoparticles from coalescence and to facilitate the hydrolysis/condensation of silica precursors. Mulvaney et al.3 and Matijevic et al.4 reported that nanoparticles of noble metals, such as Ag and Au could be coated with a silica shell using a silane coupling agent. However, these silica-coating procedures usually involved a multistep process. Graf et al.5 developed a new method to coat noble metal colloids with silica by adsorbing poly(vinyl pyrrolidone) onto the colloidal surface without using silane coupling agents. A surfactant,6 polyelectrolyte,7 enzyme,8 and gelatin9 are used for the synthesis of metal@silica core–shell nanoparticles. The synthesis of metal@silica core–shell nanoparticles were also reported without using any silane coupling agents or stabilizers.10 However, these methods can only be used for coating large colloidal particles (>50 nm) of noble metals because the smaller particles are unstable and tend to aggregate during the Stöber process.11
We now present a facile method to synthesize Ag@SiO2 core–shell nanoparticles templated by the complex micelle of silver ions and poly[3-(methacryloylamino) propyl trimethylammonium chloride]-block poly[2-(dimethylamino) ethyl methacrylate] (PMAPTAC-b-PDMAEMA) block copolymers (see Fig. S1† for the polymer structure). The synthesis route of the Ag@SiO2 core–shell nanoparticles is shown in Fig. 1. The significant feature of our method is that the polymer plays multiple roles: (i) nano-reactors for the precursors of Ag and silica, (ii) reducing agent for Ag+ ions, (iii) templates for the Ag core and SiO2 shell, and (iv) stabilizer for nano-colloids. The tertiary amines of the PDMAEMA block provides the site for the Ag+ ions to be adsorbed and serves as an electron donor for reducing the Ag+ ions to form Ag nanoparticles. The PMAPTAC block provides colloidal stability and a reaction site for the silica precursor which forms the silica shell around the Ag nanoparticles. As the PDMAEMA and PMAPTAC blocks template the Ag core and silica shell, respectively, the Ag core size and silica shell thickness are expected to be tuned by the chain length of each block. Unlike previous methods, neither an external reducing agent nor stablizing agent was required. We have applied the Ag@SiO2 core–shell nanoparticles as a nano-catalyst to the reduction of p-nitrophenol, and proved that the present nanoparticles work as an excellent catalyst.
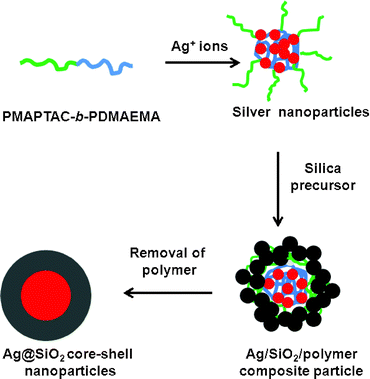 | ||
| Fig. 1 Schematic diagram of synthesis of Ag@SiO2 core–shell nanoparticles. | ||
The PMAPTAC-b-PDMAEMA was synthesized by controlled radical polymerization based on reversible addition–fragmentation chain transfer reaction. The degree of polymerization of PMATAC and PDMAEMA is 56 and 74, respectively (Fig. S1†). The molecular weight and molecular weight distribution for the PMAPTAC-b-PDMAEMA estimated by GPC are 1.95 × 104 and 1.06, respectively. The detailed synthesis procedure can be found in the Supporting Information.† AgNO3 (Wako), tetramethoxy silane (TMOS) (Shin Etsu), p-nitrophenol (TCI) and NaBH4 (Aldrich) were used without further purification.
The freshly prepared solution of AgNO3 was added to the polymer solution (1 g L−1) and stirred at room temperature for 3 h to obtain the Ag nanocolloids. The final concentration of Ag+ ions was 3 mM. The reduction of the Ag+ ions was monitored by UV-visible spectra measurements (Fig. S2†). The strong bands centered at around 410 nm confirmed the formation of the Ag nanoparticles. This band arises from the surface plasmon absorption of silver. The shape of these plasmon bands is almost symmetrical, suggesting that the nanoparticles are well dispersed and spherical in shape because silver nanoparticles are known to have characteristic size- and shape-dependent surface plasmon resonance bands in the UV-vis region. The tertiary amines from the PDMAEMA-block served as electron donors for reducing the Ag+ ions to form silver nanoparticles,12 but the quaternary amine of PMAPTAC could not efficiently reduce the Ag+ ions. This is evidenced by the fact that Ag nanoparticles were formed in the PDMAEMA homopolymer solutions (Fig. S3†), while they are not formed in the PMAPTAC homopolymer solution (Fig. S4†).
The morphology of the Ag nanoparticles was investigated by TEM measurements. The TEM image in Fig. 2a confirms that the Ag nanoparticles are spherical but quite polydisperse. The averaged diameter of Ag nanoparticles is about 15 nm. The hydrodynamic diameter of Ag nanoparticles was also determined using dynamic light scattering (DLS) experiment. The dimension from the TEM is somewhat smaller than that of DLS measurement (45 nm) indicating the formation of aggregates.
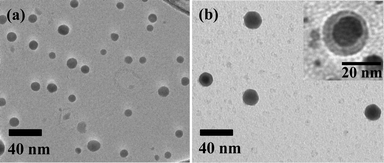 | ||
| Fig. 2 TEM images of (a) Ag nanoparticles and (b)Ag@SiO2 core–shell nanoparticles. | ||
The Ag nanocolloidal solution was stirred for 1 day after adding the desired amount of TMOS (the molar ratio of the quaternary amine of PMAPTAC to TMOS was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). The solution was then stored for 1 day without stirring to allow the silica network to be formed by the sol–gel reaction.13 After the sol–gel reaction was completed, the solution was dried at 50 °C for several hours to obtain the Ag@SiO2–polymer composites. The polymer template was then removed by calcination at 500 °C for 4 h. Fig. 2b shows the Ag@SiO2 core–shell nanoparticles. The 15 nm Ag core is encapsulated within a silica shell. It is worth stressing that all the nanoparticles have a Ag core and silica shell (i.e., no silica particles without a Ag core are found in the TEM image as shown in Fig. 2b and Fig. S5†). This indicates that the sol–gel reaction selectively proceeded in the Ag–polymer complex micelles. Fig. 3 shows the wide angle XRD of the silica coated Ag nanoparticles. All of the XRD diffraction peaks can be indexed to fcc silver crystal, which is in good agreement with the literature value (ICDD No 01–087–0719).
15). The solution was then stored for 1 day without stirring to allow the silica network to be formed by the sol–gel reaction.13 After the sol–gel reaction was completed, the solution was dried at 50 °C for several hours to obtain the Ag@SiO2–polymer composites. The polymer template was then removed by calcination at 500 °C for 4 h. Fig. 2b shows the Ag@SiO2 core–shell nanoparticles. The 15 nm Ag core is encapsulated within a silica shell. It is worth stressing that all the nanoparticles have a Ag core and silica shell (i.e., no silica particles without a Ag core are found in the TEM image as shown in Fig. 2b and Fig. S5†). This indicates that the sol–gel reaction selectively proceeded in the Ag–polymer complex micelles. Fig. 3 shows the wide angle XRD of the silica coated Ag nanoparticles. All of the XRD diffraction peaks can be indexed to fcc silver crystal, which is in good agreement with the literature value (ICDD No 01–087–0719).
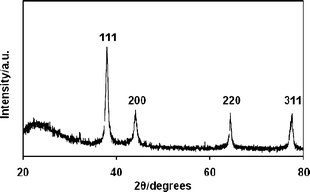 | ||
| Fig. 3 XRD pattern of Ag@SiO2 core–shell nanoparticles. | ||
It has been demonstrated that Ag nanoparticles have high catalytic activities in organic reactions. They can serve as an electron relay in the oxidant–reductant system so that they can find use in the catalytic reduction of aromatic nitrocompounds.14 However, Ag nanoparticles coalesce during the catalytic process because nanosized metal particles in the solution tend to aggregate as a result of high surface energy unless they are protected.15 To evaluate the catalytic activity of the Ag@SiO2 core–shell nanoparticles obtained in this study, the reduction of p-nitrophenol to p-aminophenol by NaBH4 was used as the model reaction.16 The reaction was spectrophotometrically monitored. It is known that the p-nitrophenol and p-nitrophenolate ions show absorption maxima at 317 and 400 nm, respectively, while the p-aminophenol and p-aminophenolate ions show absorption maxima at 295 and 315 nm, respectively. The solution of p-nitrophenol thus showed an absorption peak at 317 nm. However, after the addition of NaBH4, the band at 317 nm (p-nitrophenol) disappeared and a new band at 400 nm appeared due to the formation of the p-nitrophenolate ion. The p-nitrophenolate ion was reduced to p-aminophenol by NaBH4 in the presence of the Ag@SiO2 catalyst. Fig. 4a shows the spectral change in the reaction mixture during the reduction. The band at 400 nm (p-nitrophenolate ion) gradually decreased with time and a new band appeared at 295 nm (p-aminophenol) in the presence of the Ag@SiO2 core–shell nanoparticles. The complete conversion of the p-nitrophenolate ion to p-aminophenol could also be visually observed by the gradual change of the originally bright-yellow solution into a colorless one. The reactants, such as NaBH4 and p-nitrophenolate ion, diffuse through the silica shell onto the metal surface. The catalytic reduction is initiated by electron transfer from the BH4− to the substrate p-nitrophenolate ion. The product, p-aminophenol, desorbs from the silver catalyst surface and diffuses out of the silica shell.17 It should be noted here that the absorption bands of the p-nitrophenolate ion and Ag nanoparticles are significantly overlapped to each other. However, the amount of the added catalyst was so small that the absorption spectra of the p-nitrophenolate ion was hardly affected by the plasmon absorption of the Ag nanoparticles.
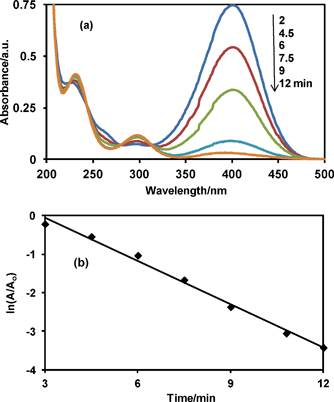 | ||
| Fig. 4 (a) Absorption spectra of p-nitrophenolate ion after catalytic reduction by NaBH4 in the presence of Ag@SiO2 core–shell nanoparticles, and (b) plot of ln(A/A0) versus reduction time. A0 and A are absorbance at 400 nm at time = 0 and corresponding time, respectively. | ||
The kinetics of the reduction reaction from the p-nitrophenolate ion to p-aminophenol was analyzed using the data in Fig. 4b. The concentration of NaBH4 selected for the present study was 1 × 10−2 M, which is very high compared with the concentration of p-nitrophenol (5 × 10−5 M) therefore, the reaction can be regarded as zero order with respect to the borohydride concentration. Therefore, pseudo-first order kinetics with respect to the p-nitrophenolate ion seemed be used to evaluate the catalytic rate. As expected, a linear relation is observed between ln(A/Ao) and time (Fig. 4b) giving the rate constant of 0.37 min−1. The advantage of the core–shell-type nanoreactors is their easy separation from the reaction mixture and easy dispersion in the next solvent for reuse. The nanoparticles could be separated from the reaction mixture by simple centrifugation. The outer silica shells are permeable and the Ag cores are accessed by the solvents and reactants for the next use.
In summary, a PMAPTAC-b-PDMAEMA diblock copolymer was found to be an excellent template for Ag@SiO2 nanoparticle formation. The advantage of our approach is that the polymer plays multiple roles in the synthesis. The tertiary amine containing the PDMAEMA block coordinately binds the Ag+ ions and reduces the Ag+ ions to a metallic Ag core. The PMAPTAC block stabilizes the Ag nanoparticles and provides a site for the sol–gel reaction to form the silica shell. Thus, the PDMAEMA and PMAPTAC blocks, respectively, template the Ag core and silica shell, so that the Ag core size and silica shell thickness can be tuned by the chain length of each block. This method is expected to be versatile and used for the synthesis of core–shell nanoparticles of various combinations of core and shell materials.
Acknowledgements
The authors thank Mr Toshimi Tabata for his help with the TEM measurements. The present study was supported by a Grant-in-Aid for Scientific Research (20 310 054) from the Japan Society for the Promotion of Science (JSPS).References
- (a) Y. Lu, Y. Yin, Z. Li and Y. Xia, Nano Lett., 2002, 2, 785 CrossRef CAS; (b) S. C. Jaricot, M. Darbandia and T. Nann, Chem. Commun., 2007, 2031 RSC; (c) S. Liu and M. Y. Han, Chem. Asian J., 2010, 5, 36 CAS; (d) S. K. Rastogi, V. J. Rutledge, C. Gibson, D. A. Newcombe, J. R. Branen and A. L. Branen, Nanomed.: Nanotechnol., Biol. Med., 2011, 7, 305 CrossRef CAS; (e) C. Xue, X. Chen, S. J. Hurst and C. A. Mirkin, Adv. Mater., 2007, 19, 4071 CrossRef CAS.
- (a) S. Santra, R. Tapec, N. Theodoropoulou, J. Dobson, A. Hebard and W. Tan, Langmuir, 2001, 17, 2900 CrossRef CAS; (b) L. Levy, Y. Sahoo, K. S. Kim, E. J. Bergey and P. N. Prasad, Chem. Mater., 2002, 14, 3715 CrossRef CAS.
- L. M. Liz-Marzan, M. Giersig and P. Mulvaney, Langmuir, 1996, 12, 4329 CrossRef CAS.
- V. V. Hardikar and E. Matijevic, J. Colloid Interface Sci., 2000, 221, 133 CrossRef CAS.
- C. Graf, D. L. J. Vossen, A. Imhof and A. Blaaderen, Langmuir, 2003, 19, 6693 CrossRef CAS.
- K. Landfester, Angew. Chem., Int. Ed., 2009, 48, 4488 CrossRef CAS.
- I. Pastoriza-Santos, J. Prez-Juste and L. M. Liz-Marzan, Chem. Mater., 2006, 18, 2465 CrossRef CAS.
- S. M. Kang, K. B. Lee, D. J. Kim and J. S. Choi, Nanotechnology, 2006, 17, 4719 CrossRef CAS.
- S. H. Liu, Z. H. Zhang and M. Y. Han, Adv. Mater., 2005, 17, 1862 CrossRef CAS.
- L. Shuhua and M. Han, Adv. Funct. Mater., 2005, 15, 961 CrossRef.
- W. Stober, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- (a) M. A. Nash, J. J. Lai, A. S. Hoffman, P. Yager and P. S. Stayton, Nano Lett., 2010, 10, 85 CrossRef CAS; (b) Y. T. Li, A. E. Smith, B. S. Lokitz and C. L. McCormick, Macromolecules, 2007, 40, 8524 CrossRef CAS; (c) H. Sun, Z. Gao, L. Yang, L. Gao and X. Lv, Colloid Polym. Sci., 2010, 288, 1713 CrossRef CAS.
- A. Khanal, Y. Inoue, M. Yada and K. Nakashima, J. Am. Chem. Soc., 2007, 129, 1534 CrossRef CAS.
- (a) Y. Lu, Y. Mei, M. Drechsler and M. Ballauff, Angew. Chem., Int. Ed., 2006, 45, 813 CrossRef CAS; (b) S. Wunder, Y. Lu, M. Albrecht and M. Ballauff, ACS Catal., 2011, 1, 908 CrossRef CAS; (c) S. Tang, S. Vongehr and X. Meng, J. Phys. Chem. C, 2010, 114, 977 CrossRef CAS; (d) M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750 CrossRef CAS.
- (a) Z. Wang, X. Chen, M. Chen and L. Wu, Langmuir, 2009, 25, 7646 CrossRef CAS; (b) J. Kim, W. Bryan and T. R. Lee, Langmuir, 2008, 24, 11147 CrossRef CAS; (c) Z. Liang, A. S. Susha and F. Caruso, Adv. Mater., 2002, 14, 1160 CrossRef CAS.
- H. L. Jiang, T. Akita, T. Ishida, M. Haruta and Q. Xu, J. Am. Chem. Soc., 2011, 133, 1304 CrossRef CAS.
- P. Narayan, P. Anjali and P. Tarasankar, Colloids Surf., A, 2002, 196, 247 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: synthesis of polymer, UV-visible absorption spectra, TEM images. See DOI: 10.1039/c2ra20316b |
| This journal is © The Royal Society of Chemistry 2012 |
