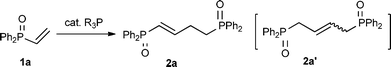
Umpolung catalyzed by organophosphines: efficient β,β-dimerization of vinylphosphonates affording linear dimers†
Xiang-Bo
Wang
a,
Yuta
Saga
b,
Ruwei
Shen
a,
Hiroyoshi
Fujino
b,
Midori
Goto
a and
Li-Biao
Han
*a
aNational Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Ibaraki 305-8565, Japan. E-mail: libiao-han@aist.go.jp; Fax: + 81 29-861-4855
bKatayama Chemical Industries Co., Ltd, Amagasaki, Hyogo 660-0892, Japan
First published on 6th June 2012
Abstract
Trimethylphosphine efficiently catalyzed the dimerization reaction of vinylphosphorus compounds to selectively afford the linear non-branched tail-to-tail β,β-dimerization dimers via umpolung of the substrates.
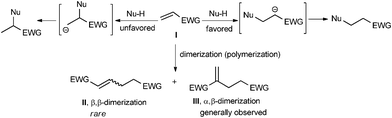
A base catalyzed Michael-type addition of Nu–H to an α,β-unsaturated alkene I bearing an electron-withdrawing group (EWG) takes place by the addition of Nu to the β-carbon of the alkene.1 An addition with the reversed regioselectivity (umpolung) is electronically unfavored and usually difficult to achieve (Scheme 1).
 | ||
| Scheme 1 A base initiated nucleophilic addition and dimerization of an electron-deficient alkene. | ||
This is also true for a base initiated dimerization (polymerization) of an electron-deficient alkene I, affording the synthetically important functional dimers II and III which are widely used in organic synthesis and industrial polymer chemistry. Two kinds of dimers, the linear one IIvia the β,β-dimerization and the branched one IIIvia the α,β-dimerization are possible from the dimerization of I. Whereas the formation of the branched IIIvia the α,β-dimerization of I is well established,3 the generation of the linear IIvia the umpolung of I is difficult to achieve. Thus, it is well known that the Rauhut–Currier reaction can efficiently produce III by the base-mediated dimerization of I.2 Although transition metal catalyzed dimerization of I to afford II is well studied,3 efficient transformation catalyzed by an organic catalyst is rare.2,4,5 Very recently, the N-heterocyclic carbene mediated β,β-dimerization of methyl methacrylate was disclosed by the groups of Matsuoka and Glorius.5
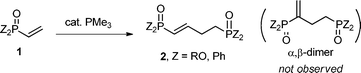
Herein we report an efficient β,β-dimerization of vinylphosphorus compounds 1via the umpolung of 1 catalyzed by a simple trimethylphosphine PMe3 [eqn (1)].4,6,7 We found this reaction accidentally during a study on the catalytic preparation of 1.8 We were surprised to find that the expected α,β-dimer 2′ from the Rauhut–Currier reaction could not be detected. This linear dimer 2 is a direct synthetic precursor for the preparation of 1,4-bisphophino compounds, which are important ligands widely used in metal catalysts.9 As shown in Table 1, when PMe3 (5 mol%) was added to a toluene solution of diphenylvinylphosphine oxide (0.8 M) at room temperature (run 1), white precipitation of the β,β-dimerization product 2a (2a′) gradually precipitated out. After 36 h, the 1-butene isomer 2a was obtained in 29% yield as a trans isomer together with a trace amount of the 2-butene isomer 2a′ (30% total yield of the β,β-dimerization product; 2a/2a′ = 97/3). Another possible isomer via the common α,β-dimerization could not be detected at all. By using more PMe3 catalyst or by conducting the reaction at a higher temperature, the yield of the dimer 2a (2a′) increased. Thus, 43% yield of the dimer was obtained with 10 mol% PMe3 catalyst (run 2), and with 5 mol% PMe3 catalyst at 60 °C, the β,β-dimer 2a was obtained in 47%, 56% and 77% yield, respectively, after 12 h, 24 h, and 48 h (runs 3–5). By using 10 mol% of the catalyst, 85% yield of the β,β-dimerization products was obtained upon heating a toluene solution at 60 °C (run 6). The reaction also proceeded efficiently in THF to give the β,β-dimers in 84% yield after heating at 60 °C for 12 h (run 7). Interestingly, the concentration of the substrates not only affected the reaction rates but also affected the selectivity for the products (2a/2a′). Thus, under similar conditions, when the reaction was conducted in a higher concentration solution (1.6 M in THF), the dimers were obtained in 87% yield with a 95/5 selectivity (run 8), while when the reaction was conducted in a dilute solution (0.4 M) only 35% yield of the dimers was obtained with a selectivity of 2a/2a′ = 66/34 (run 9). In addition to PMe3, phosphines such as n-Bu3P and PPhMe2 only sluggishly initiate this dimerization. Other phosphines such as t-Bu3P, PPh3 and Ph2PMe did not catalyze this dimerization under similar reaction conditions (runs 14–16).
 | (1) |
| Run | R3P (mol%) | Conditions | Yield (%)b | 2a/2a′ [%]c |
|---|---|---|---|---|
| a Unless otherwise noted, a 0.8 M solution of diphenylvinylphosphine oxide was used. b Combined yields of 2a and 2a′. c Ratio of 2a/2a′ determined by 1H NMR spectroscopy of the reaction mixture. The amount of cis-2a is tiny and ignorable. Ratios in parentheses refer to the cis/trans ratios of 2a′ estimated by 31P NMR spectroscopy. | ||||
| 1 | PMe3 (5) | Tol., 25 °C, 36 h | 30 | 97/3 |
| 2 | (10) | 44 | 98/2 | |
| 3 | PMe3 (5) | Tol., 60 °C, 12 h | 49 | 96/4 |
| 4 | 24 h | 61 | 92/8 | |
| 5 | 48 h | 82 | 94/6 | |
| 6 | PMe3 (10) | Tol., 60 °C, 18 h | 85 | 94/6 |
| 7 | PMe3 (10) | THF, 60 °C, 12 h | 84 | 82/18 (22/78) |
| 8 | (1.6 M) | 87 | 95/5 | |
| 9 | (0.4 M) | 35 | 66/34 (38/62) | |
| 10 | n-Bu3P (10) | THF, 60 °C, 12 h | 22 | 91/9 |
| 11 | (100) | 79 | 92/8 | |
| 12 | PhMe2P (10) | THF, 60 °C, 12 h | 0 | 0 |
| 13 | (100) | 32 | 13/87 (23/77) | |
| 14 | t-Bu3P (10) | 0 | 0 | |
| 15 | Ph2MeP (10) | 0 | 0 | |
| 16 | Ph3P (10) | 0 | 0 | |
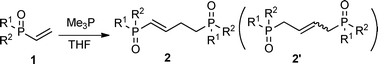
This PMe3 catalyzed selective β,β-dimerization can be applied to a variety of other vinylphosphoryl compounds, indicating that this is a general way for the preparation of the linear 1,4-bisphosphoryl compounds. As shown in Table 2, in addition to diphenylvinylphosphine oxide 1a, both vinylphosphonates (runs 1–7) and vinylphosphinates (runs 8–11) can afford the corresponding linear dimers in good yields. All these dimerizations could take place in the presence of a catalytic amount of PMe3 (10 mol%). As expected, under similar conditions, when more PMe3 (1.0 equiv.) was used, the dimerization was accelerated to produce the linear dimers in good yields in a short time (runs 2 and 4). The results in Table 2 show that the product selectivity is affected by the phosphine used, i.e. more phosphine gives rise to the formation of 2′ (Table 2, run 1 vs. 2, 3 vs. 4, 5 vs. 6, 8 vs. 9, 10 vs. 11). Worth noting is that this β,β-dimerization reaction could be successfully applied to the coupling of an enantiomerically pure P-chiral vinyl phosphinate to produce the corresponding chiral 1,4-bisphosphoryl compounds in good yields (entry 11). As exemplified by eqn (2), by using a simple hydrogenation process,10 these unsaturated 1,4-bisphosphoryl compounds could be transformed to the saturated 3 in a high yield which can be conveniently further converted to a variety of new chiral 1,4-diphosphine ligands by using known procedures.11
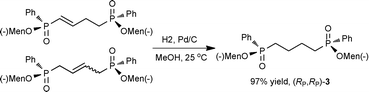 | (2) |
| Run | 1 | PMe3 (mol%) | Conditions | Yield (2/2′ (Z/E)) [%]a |
|---|---|---|---|---|
| a Ratio of 2/2′ determined by 1H NMR spectroscopy of the reaction mixture. The amount of cis-2 is tiny and ignorable. Ratios in parentheses refer to the cis/trans ratios of 2′ estimated by 13C NMR spectroscopy. b 0.8 M THF solution of vinylphosphorus compound was used. c 1.6 M THF solution of vinylphosphorus compound was used. d Men = menthyl. | ||||
| 1b |
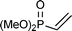
|
10 | 60 °C, 24 h | 59 (61/39 (47/53)) |
| 2b | 100 | 60 °C, 1.5 h | 80 (47/53 (40/60)) | |
| 3c |
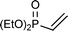
|
10 | 60 °C, 18 h. | 72 (67/33 (32/68)) |
| 4c | 100 | 60 °C, 2 h | 85 (18/82 (45/55)) | |
| 5b |
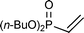
|
10 | 60 °C, 24 h | 55 (69/31 (67/33)) |
| 6c | 100 | RT, 12 h | 69 (20/80 (42/58)) | |
| 7b |
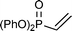
|
10 | RT, 1 h | 64 (69/31 (18/82)) |
| 8b |
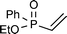
|
10 | 60 °C, 24 h | 62 (21/79 (20/80)) |
| 9b | 100 | RT, 12 h | 84 (17/83 (27/73)) | |
| 10bd |
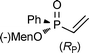
|
20 | 60 °C, 48 h | 51 (45/55 (57/43)) |
| 11cd | 100 | 60 °C, 12 h | 79 (30/70 (45/55)) | |
A proposed mechanism for this PMe3-catalyzed β,β-dimerization reaction of vinylphosphorus compounds 1 is depicted in Scheme 2. Firstly, PMe3 adds to the vinylphosphorus compound 1a to give phosphonium species 4.12 An ylide species 5 should be produced via a proton shift.4 It is expected that the rapid formation of the species 5 is crucial for the consequent β,β-dimerization of 1. Thus, 5 adds to 1 to produce 6 which can give either 7 or 8via the proton shift, from which the β,β-dimerization products 2 and 2′ are formed.13
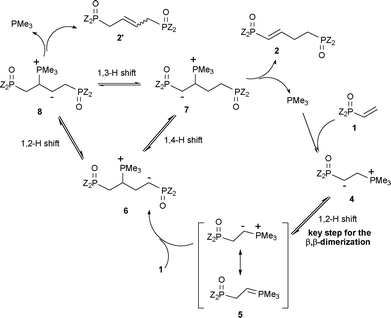 | ||
| Scheme 2 Possible mechanism of PMe3 catalyzed β,β-dimerization of vinylphosphorus compounds. | ||
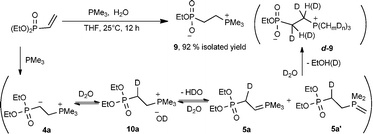
Firm evidence for the intermediary 4 involved in the catalytic cycle was obtained by carrying out a reaction of diethyl vinylphosphonate with PMe3 in the presence of water [eqn (3)]. Thus, at room temperature, when PMe3 (1.6 mmol) was added to a mixture of diethyl vinylphosphonate (1.6 mmol) and water (0.4 mL) in THF (4 mL), a phosphonium compound 9, with the loss of an ethoxy group, was obtained in high yield as a white solid. Good crystals suitable for X-ray analysis were obtained by recrystallization of 9 from hexane–methanol, undoubtedly confirming its structure (Fig. 1).
 | (3) |
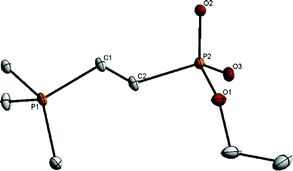 | ||
| Fig. 1 ORTEP drawing of 9. Selected bond lengths (Å) and angles (°): C(1)–C(2) = 1.540(2), P(1)–C(1) = 1.7889(18), P(2)–O(1) = 1.6177(14), P(2)–O(2) = 1.4975(13), P(2)–O(3) = 1.4905(13), O(1)–P(2)–O(2) = 104.29(8), O(1)–P(2)–O(3) = 110.43(8), O(2)–P(2)–O(3) = 119.54(8). | ||
Further mechanistic insight was obtained by carrying out a similar reaction in D2O, from which a deuterated compound d-9 was obtained [eqn (3)]. Interestingly, deuteration of the α,β carbons and the three methyl groups of PMe3 was detected. Since no such deuteration was observed between an isolated 9 in D2O under similar reaction conditions, it was assumed that d-9 was formed during the reaction of diethyl vinylphosphonate, rather than formed via H–D exchange of 9 with D2O. The result of this deuteration experiment is well explained by considering the participation of the phosphonium intermediate 4a and the ylide intermediates 5a/5a′ as depicted in eqn (3). Moreover, the inertness of 9 with D2O also showed that the hydrolysis of the diethylphosphoryl group took place predominantly via5a/5a′ rather than via4a or 10a, indicating a rapid conversion of 4a to 5a/5a′.
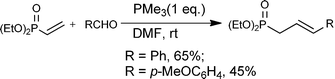
The presence of the intermediate 5a was further supported by carrying out the reaction of diethyl vinylphosphonate with trimethylphosphine in the presence of an aldehyde, from which the corresponding olefin via the Wittig-olefination reaction of 5a with the aldehyde was obtained [eqn (4)].
 | (4) |
In conclusion, we have found that a simple phosphine PMe3 can catalytically and selectively reverse the reactivity (umpolung) of vinylphosphorus compounds to successfully achieve unprecedented β,β-dimerizations which are difficult to realize by traditional methods. We expect that forthcoming studies on the reaction could lead to the establishment of a general method for the selective β,β-dimerizations of other electron-deficient α,β-unsaturated alkenes via similar umpolung catalyzed by organophosphines.14
Acknowledgements
This work was partially supported by the Canon Foundation.References
- March's Advanced Organic Chemistry: Reactions, Mechanism and Structure, ed. M. B. Smith and J. March, John Wiley & Sons, Inc., 6th edn, 2007 Search PubMed.
- C. E. Aroyan, A. Dermenci and S. J. Miller, Tetrahedron, 2009, 65, 4069 CrossRef CAS.
- G. W. Parshall and S. D. Ittel, Homogeneous Catalysis: the Applications and Chemistry of Catalysis by Soluble Transition Metal Complexes, John Wiley & Sons, Inc., 2nd edn, 1992, pp. 82 Search PubMed.
- (a) M. M. Baizer and J. D. Anderson, J. Org. Chem., 1965, 30, 1357 CrossRef CAS; (b) C. D. Hall, N. Lowther, B. R. Tweedy, A. C. Hall and G. Shaw, J. Chem. Soc., Perkin Trans. 2, 1998, 2047 RSC.
- (a) A. T. Biju, M. Padmanaban, N. E. Wurz and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 8412 CrossRef CAS; (b) S.-I. Matsuoka, Y. Ota, A. Washio, A. Katada, K. Ichioka, K. Takagi and M. Suzuki, Org. Lett., 2011, 13, 3722 CAS; (c) J. D. McClure, J. Org. Chem., 1970, 35, 3045 CrossRef CAS.
- Selected PMe3 catalyzed reactions: (a) J. Szeto, V. Sriramurthy and O. Kwon, Org. Lett., 2011, 13, 5420 CrossRef CAS; (b) I. C. Stewart, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2003, 125, 8696 CrossRef CAS; (c) S. A. Frank, D. J. Mergott and W. R. Roush, J. Am. Chem. Soc., 2002, 124, 2404 CrossRef CAS; (d) L.-C. Wang, A. L. Luis, K. Agapiou, H.-Y. Jang and M. J. Krische, J. Am. Chem. Soc., 2002, 124, 2402 CrossRef CAS; (e) M. E. Krafft and J. A. Wright, Chem. Commun., 2006, 2977 RSC; (f) J. L. Methot and W. R. Roush, Org. Lett., 2003, 5, 4223 CrossRef CAS.
- Other phosphine-mediated transformations: X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS.
- Q. Xu and L.-B. Han, J. Organomet. Chem., 2011, 696, 130 CrossRef CAS.
- (a) K. Gopalaiah and H. B. Kagan, Chem. Rev., 2011, 111, 4599 CrossRef CAS; (b) V. V. Grushin, Chem. Rev., 2004, 104, 1629 CrossRef CAS; (c) H. Fernandez-Perez, P. Etayo, A. Panossian and A. Vidal-Ferran, Chem. Rev., 2011, 111, 2119 CrossRef CAS; (d) P. W. N. M. Van Leeuwen, P. C. J. Kamer, J. N. H. Reek and P. Dierkes, Chem. Rev., 2000, 100, 2741 CrossRef CAS; (e) T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829 CrossRef CAS; (f) C. Nájera and J. M. Sansano, Chem. Rev., 2007, 107, 4584 CrossRef.
- A. A. Fokin, A. G. Yurchenko, V. N. Rodionov, P. A. Gunchenko, R. I. Yurchenko, A. Reichenberg, J. Wiesner, M. Hintz, H. Jomaa and P. R. Schreiner, Org. Lett., 2007, 9, 4379 CrossRef CAS.
- L. D. Quin, A Guide to Organophosphorus Chemistry, John Wiley & Sons, Inc., 2000 Search PubMed.
- Addition of R3P to α,β-unsaturated alkenes bearing an electron-withdrawing group: H. R. Hudson in The Chemistry of Organophosphorus Compounds, ed. F. R. Hartley, John Wiley & Sons, Inc., vol. 1, ch. 11, 1990 Search PubMed.
- It was confirmed that only a tiny change of the ratio of 2/2c′ was observed after heating PMe3 (0.32 mmol) and the isolated 2/2c′ (0.32 mmol; P(O)Z2 = P(O)(OEt)2; 2/2c′ = 55/45) in THF (0.4 mL). Therefore, an interconversion between 2 and 2′ might not be the path corresponding for their formation.
- Currently, other substrates such as methyl acrylate only produced α,β-dimers under similar conditions.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of new compounds. CCDC 873277. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20704d |
| This journal is © The Royal Society of Chemistry 2012 |