One-pot crystalline ZnO nanorod growth in mineralizing peptide gels†
Luona
Anjia
,
Zengyan
Wei
and
Hiroshi
Matsui
*
Department of Chemistry and Biochemistry, City University of New York - Hunter College, 695 Park Avenue, New York, NY, USA 10065. E-mail: hmatsui@hunter.cuny.edu
First published on 22nd May 2012
Abstract
A new biomimetic approach for the one-pot synthesis of ZnO nanorods at neutral pH and room temperature is introduced; self-assembly of the ZnO-mineralzing peptides (CN111, PAGLQVGFAVEV-GGGSC) with Zn ions as bundles of nanofibers in the gel form leads to the growth of ZnO nanorod (NRD) inside the gel. This new nanomaterial synthesis may open a new strategy by using the catalytic peptide gels as nanomaterial reactors.
Zinc oxide, a semiconductor with a large direct band gap, possesses unique optical, acoustic, and electronic properties. ZnO is one of the most widely studied metal oxides for use in solar cells,1 sensors,2 ultraviolet nanolasers,3 and blue-light emitting diodes (LEDs).4 A wide variety of applications require the fabrication of ZnO nanostructures in distinct morphology and functionality. There is a growing interest in attempts to grow such inorganic nanoparticles in a controlled morphology and structure by using biomolecular templates. One of the research motivations for this combination is to take advantage of the catalytic activity for the room-temperature material growth and the ability of self-assembly to control structures on a large scale.5–9 Numerous peptides exhibiting high binding affinities for various inorganic materials were engineered using combinatorial library approaches such as phage display and cell-surface display, and some of these peptides were capable to mediate the formation of specific inorganic materials on templates incorporating these peptides.10–15 However, in many cases the coating of these mineralizing peptides on nanoscale templates and the morphology control on resulting peptide-coated templates were not straightforward for the subsequent crystal growth.
In this paper, we developed a new approach for the one-pot synthesis of ZnO nanorods at neutral pH and room temperature; by self-assembling peptides which possess the catalytic mineralization function for the specific metal oxide into gels consisting of bundles of nanofibers, ZnO nanorods (NRDs) are formed inside the gels where Zn ions are contained. Once pH and precursor concentration of the growth solution were optimized in the gelation step and the mineralization step respectively for the growth of highly crystalline ZnO NRDs, the one-pot synthesis of ZnO NRDs was demonstrated in the best growth conditions. A possible non-classical crystallization route is proposed for the ZnO-peptide NRD formation through amorphous Zn(OH)2 gels confined by the peptide bundles. This new ZnO nano-structure synthesis may open a new strategy to design peptides as building blocks of inorganic nanomaterials.
Previously, a ZnO-binding peptide ZnO-1 (EAHVMHKVAPRP) shows ZnO mineralization function at room temperature after adding a five amino acid tail (GGGSC) to the C-terminal.11 Without the tail, ZnO-1 could not dehydrate Zn(OH)2 to produce crystalline ZnO. There is another ZnO-binding peptide, CN111 (PAGLQVGFAVEV),12 which is more hydrophobic and negatively charged around neutral pH.16 Such properties of the CN111 peptide should be more appropriate to enhance the affinity to zinc ions and the potential of self-assembly in aqueous solution. Therefore, CN111 with the dehydration tail of GGGSC (CN111-GGGSC peptide) has the potential to be assembled into fibers and then attract zinc ions for crystalline ZnO growth through the dehydration process of intermediate Zn(OH)2. To examine this hypothesis, first the CN111-GGGSC peptide was dissolved in 0.01 M phosphate buffer solution at different pH (pH = 3.0, 7.0 and 10, respectively) to observe its assembling property. After mixing and shaking, small bubbles were formed and then these solutions became opalescent, indicating the formation of self-assembled nanostructures in the solutions. After an overnight incubation, nanofibers with an average diameter of 3 nm with lengths ranging from hundreds of nanometers to several micrometers were observed in transmission electron micrographs (TEMs) (Fig. 1). Since no fibers were observed in a pH 3.0 solution where the peptide has no net charge at this pH, the balance between the hydrophobic interaction and electrostatic interaction seems to be important for the formation of peptide nanofibers.
 | ||
| Fig. 1 (a) TEM image of peptide nanofibers in pH 7.0. (b) TEM image of peptide nanofibers in pH 10. Scale bar = 100 nm. | ||
To investigate how the peptides interact with zinc ions and are assembled into certain structures, zinc acetate (Zn(Ac)2) was added and incubated in 0.1 mM peptide nanofiber-containing solution at pH 7.0. Here, pH 7.0 was chosen because under this condition the majority of zinc is in the ionic form on the basis of Ksp value (3 × 10−17 at room temperature) at a concentration of 1.0 mM Zn2+, and we are interested in observing how the peptide nanofibers are assembled under the influence of zinc ions prior to their conversion to ZnO. As shown in Fig. 2 a–c, a new structure of the spherical gel was observed in this solution. The energy dispersive spectroscopy (EDS) in Fig. 2d confirms the presence of Zn ions in the gel. Since the diameter of the network branches in the gel agrees with the diameter of peptide nanofibers, it supports our hypothesis that the gel consists of the bundles of peptide nanofibers. Fig. 2e–g are TEM images of nanofibers at various Zn ion concentrations in a higher magnification. Domains in the dark contrast in the background correspond to the aggregates of nanofibers that are not confined in the gels and spherical gels are not included in these images. As the concentration of Zn2+ is increased from 0.025 mM to 1.0 mM, the area of the dark background is decreased and thus it indicates that the amount of peptide nanofibers in the background is decreased with the increase of Zn2+ concentration. From this observation, we hypothesize that the interaction of peptide nanofibers with zinc is critical for the formation of the spherical gel. Since pH has a significant effect on the production of Zn(OH)2, to examine this hypothesis we changed the pH of the growth solution. At pH around 7.0, the gel structure is maintained for days for the sample in 1.0 mM of Zn2+ solution. However, Zn(OH)2 starts precipitating when the pH was increased to 8.0 (see Fig. S1 in supplementary information†).
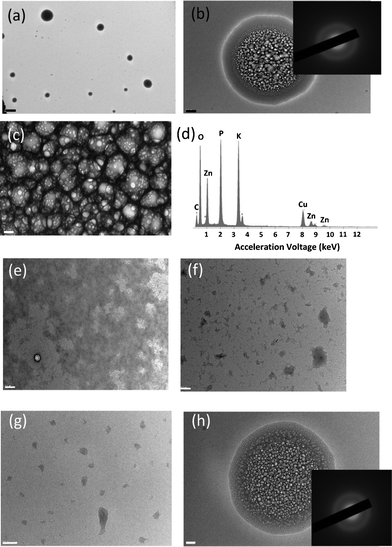 | ||
| Fig. 2 (a) TEM images of the Zn2+–peptide spherical gels observed in 0.1 mM peptide with 1.0 mM Zn(Ac)2 at pH 7.0. Scale bar = 2 μm. (b) Close view of the gel in (a). Inset of (b) is the SAED of the gel at pH 7.0. Scale bar = 200 nm. (c) Magnified image of the gel structure in (b). Scale bar = 20 nm. (d) EDS on the Zn2+–peptide spherical gels. (e–g) TEM images of the peptide nanofibers in various Zn ion concentrations: (e) 0.025 mM Zn2+, (f) 0.40 mM Zn2+, and (g) 1.0 mM Zn2+. Scale bar = 200 nm. (h) Representing TEM image and SAED of the Ca2+–peptide spherical gels observed in 0.1 mM peptide with Ca(NO3)2 in 1.0 mM at pH 7.0. Scale bar = 100 nm. | ||
To better understand the role of charged ions in the formation of the peptide-bundled gels, calcium nitrate (Ca(NO3)2) was used to examine whether Ca2+ can replace Zn2+ for the formation of the peptide gels. As shown in Fig. 2h, the spherical gel was produced in the solution containing peptide and Ca2+, and the similar diffraction pattern of the peptide assembly was observed. The existence of calcium in the gel was confirmed by EDS (Fig. S3†). It should be noted that monovalent cations such as K+ and Na+ show little influence on the peptide nanofiber structure, and no spherical gel can be formed in these samples. Although the exact formation mechanism of spherical gels is still not known yet, these observations indicate that both the pH and the charge of metal ions may play important roles in the generation of the gel structure.
Our goal is to convert Zn ions on the peptide fibers in the peptide-bundled gel to ZnO for the formation of ZnO NRD templates on the mineralizing peptides. Based on the outcome from Fig. 2, to accomplish this aim we need to add NaOH to introduce the formation of Zn(OH)2 in the gel and slow dehydrolyzation of the Zn intermediates without triggering the complete decomposition of the gels. Therefore, to test this scheme ZnO was grown slowly for two months and the growth process was monitored by using TEM. After the solution containing 0.1 mM peptide and 1 mM Zn(Ac)2 was mixed with 3.5 mM NaOH and pH was carefully increased to 7.5 , the semi-rod structure of ZnO appears in the gel as shown in Fig. 3a. After four days, ZnO nanoparticles (NPs) were coated on the peptide nanofibers with a size distribution of 30–80 nm in width, 600 nm to micrometer size in length and The ZnO NPs diameters were between 3 nm and 10 nm (Fig. 3b). Since the dimension of NRDs is similar to the peptide nanofibers except the width, it is likely that the side-by-side bundled peptides template the ZnO NRD growth. The EDS shows the high zinc signal on the dark rod surface (Fig. 3c), and the SAED shows (100), (002), and (110) planes of ZnO (Fig. 3d). In the high-resolution transmission electron micrograph (HRTEM), ZnO (002) lattice fringe can be clearly observed (Fig. 3e). In a control experiment with much less [Zn(Ac)2], 0.025 mM, very few ZnO NPs were covered on the rod surface, and the rods look less rigid (Fig. 3f). This indicates that increased [Zn2+] will lead to increase the density of the ZnO NP coating and this increased coating coverage reinforces the resulting nanorod structure. In another control experiment grown in the same condition without the peptide, only Zn(OH)2 microcrystals are observed (see Fig. S2 in supplementary information†).
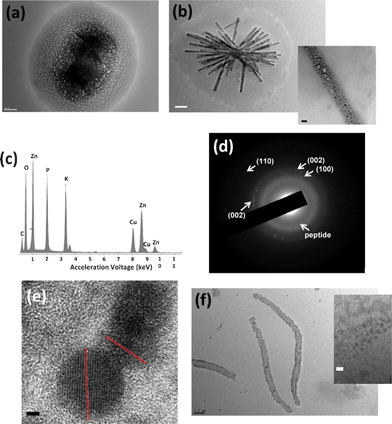 | ||
| Fig. 3 TEM images of (a) the ZnO–peptide NRDs in early growth stage (scale bar = 200 nm) (b) the ZnO–peptide NRDs formed in 1.0 mM Zn(Ac)2 (scale bar = 200 nm) after four days, inset is the magnified image of the NRD surface (Scale bar = 20 nm). (c) EDS of the ZnO NRDs after four days. (d) SAED of the ZnO-peptide NRDs after four days. (e) HRTEM of the ZnO NPs on ZnO–peptide NRDs after four days. Scale bar = 2 nm. (f) TEM image of the ZnO–peptide NRD formed in 0.025 mM Zn(Ac)2 after four days (scale bar = 500 nm). Inset is the magnified image on the NRD surface (scale bar = 5 nm). | ||
If our hypothesis about the conversion of amorphous Zn(OH)2 intermediates in the gel to ZnO is correct, Zn(OH)2 near the peptide nanofibers should be converted to crystalline ZnO and thus the volume of the gel should be reduced via this conversion with time. As compared with the gel reacted for four days (Fig. 4a), the gel incubated for two months reduced the size of gel around ZnO NRDs (Fig. 4b) in the same conditions. In addition, the surface area of the ZnO NPs coated on the NRDs (Fig. 4c) increases as the gel shrinks with incubation time (Fig. 4d and S4†) and after two months SAED of the gel surrounding the ZnO NRDs shows a diffraction pattern consisting of both peptide nanofibers and ZnO (Fig. 4e). This trend is consistent upto 60 days, and after 60 days the gel part completely disappears (Fig. S5 and S6†). All of these results indicate that Zn(OH)2 formed at pH 7.5 in the gel around peptide nanofibers is the precursor of the crystalline ZnO growth and the conversion occurs in the gel. All of these results indicate that the gel containing Zn(OH)2 and peptide is the source or reaction matrix of ZnO NRDs. It should be noted that crystalline Zn(OH)2 in the solution outgrows the peptide nanofiber-assembled gel at higher pH because of the reduction of available Zn ions for the gel formation (see Fig. S1 in supplementary information†).
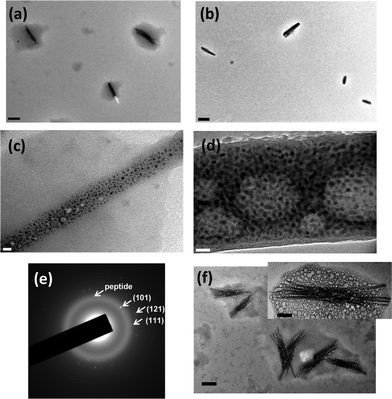 | ||
| Fig. 4 (a) TEM image of the gels right after synthesis, and (b) after two months. Scale bar = 400 nm. (c) High magnification TEM image of the surface of ZnO NRDs right after the synthesis, and (d) after two months. Scale bar = 20 nm. (e) SAED of the gels surrounding ZnO NRDs after two months. (f) ZnO NRDs formed by the one-pot synthesis at pH 7.5 after four days (scale bar = 200 nm). Inset is the ZnO NRD in higher magnification (scale bar = 60 nm). | ||
On the basis of a series of experiments we examined, ZnO NRDs are only observable through the formation of the Zn(OH)2–peptide gels as intermediates. Without the peptides, only crystalline Zn(OH)2 is formed, and when the pH of the growth solution is too high, overgrown Zn(OH)2 in solution consumes the majority of zinc ions necessary for the assembly of peptide nanofibers in the gel form and therefore there are less observed ZnO NRDs. The proper balance between certain pH and concentration ranges enables the growth of peptide nanofibers coated by densely packed ZnO nanocrystals via formation of the peptide–zinc ion gels.
When the peptide was incubated with Zn(Ac)2 at pH 7.5 for four days, this one-pot condition also enables growing ZnO NRDs in the same order of high crystallinity (Fig. 4f). The dimension of NRDs and the formation of gel media around the NRDs are quite similar to the ones in Fig. 3. This demonstration indicates that the optimized condition can be applied to grow highly crystalline ZnO NRDs in one step.
In summary, the ZnO-binding peptide CN111-GGGSC (PAGLQVGFAVEVGGGSC) is shown to assemble into nanofibers and its mineralization function enables the growth of crystalline ZnO NPs on these peptide nanofibers. These peptides are self-assembled into 3 nm wide, and the lengths range from hundreds of nanometers to several micrometers in neutral to basic (pH 10) water solutions. Addition of divalent metal ions such as Zn2+ and Ca2+ induces gelation of the peptide nanofibers due to the electrostatic interaction between positively charged metal ions and negatively charged peptides. After four days of incubation around neutral pHs, amorphous Zn(OH)2 inside the gel is dehydrated by the CN111-GGGSC peptide to produce a ZnO NP coating on the peptide nanofibers. The consumption of amorphous zinc derivatives in the gel is observed as the gel volume is reduced and ZnO coating area is increased in TEM images with time. The highly crystalline ZnO NRDs can also be grown in one-pot in the conditions optimized in the gelation and mineralization steps. A possible route for the ZnO–peptide NRD formation from the peptide nanofibers is proposed where the amorphous intermediate is an energetically efficient path to grow crystalline nanoparticles with high yield. In this work, we combine the self-assembly and the mineralization properties of peptides to synthesize ZnO NRDs without using preformed templates under mild and environmentally benign conditions—neutral pH and room temperature. This outcome shows that novel peptides, which possess both self-assembly and biomineralization functions, are very useful to generate desired materials controlled by peptide design.
Experimental section
Preparation of peptide nanofibers
CN111-GGGSC peptide, PAGLQVGFAVEVGGGSC, was purchased from GenScript with 85% purity. 0.01 M phosphate buffer at pH 3.0, pH 7.0 and pH 10 were used separately to dissolve peptide powder and make 0.1 mM peptide solutions and incubate overnight at room temperature. Peptides were self-assembled into nanofibers in pH 7.0 and pH 10 buffer solutions. Almost no nanofibers were found in pH 3.0 buffer solution.Synthesis of ZnO NRDs
Typically, to generate ZnO NRDs, first 100 μl of CN111-GGGSC peptide (0.1 mM) in pH 7.0 phosphate buffer solution was prepared. Zinc acetate (Zn(Ac)2, Sigma-Aldrich) at different concentrations (0.025 mM–1.0 mM) was added and incubated with the peptide nanofiber solution overnight. Drops of 1 M NaOH were added and incubated with the nanofiber solution containing Zinc ions for four days to grow Zn(OH)2, and the extra amount of NaOH, besides forming Zn(OH)2, was added to adjust different incubation pH from 7.0 to pH 7.5 and higher pH. In the control experiment to examine the influence of divalent metal ions on the gelation of peptides, calcium nitrate (Ca(NO3)2, Sigma-Aldrich) was used instead of Zn(Ac)2, with exactly the same experiment conditions as using Zn(Ac)2.One-pot synthesis of ZnO NRDs from peptides
CN111-GGGSC peptide powder, 0.0011 g, was added into 7.0 ml pH 7.5 phosphate buffer solution (0.01 M), which makes the peptide concentration 0.1 mM, immediately followed by adding 0.0013 g Zn(Ac)2 to make the concentration 1.0 mM of zinc precursor. The solution was well mixed and incubated for four days.Characterization
The morphology of peptide nanofibers and ZnO coated structures were characterized by high-resolution transmission electron microscopy (HRTEM-JEOL JEM-2100). Peptide nanofiber specimens were negatively stained using 1% ammonium molybdate before using HRTEM. Peptide nanofibers with zinc/calcium ions specimens were tested without staining. Selected area electron diffraction (SAED) was used to study the structural nature of the peptide nanofiber, ZnO, and Zn(OH)2 in the gels. Energy dispersive spectroscopy (EDS, EDAX Inc.) was used to examine the existence of zinc or calcium elements in selected areas in the gels.Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-FG-02-01ER45935. Hunter College infrastructure is supported by the National Institutes of Health, the RCMI program (G12-RR003037-245476).References
- B. O'Regan, D. T. Schwarthz, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2000, 12, 1263 CrossRef CAS.
- H. M. Lin, S. J. Tzeng, P. J. Hsiau and W. L. Tsai, Nanostruct. Mater., 1998, 10, 465 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897 CrossRef CAS.
- A. Tsukazaki, A. Ohtomo, T. Onuma, M. Ohtani, T. Makino, M. Sumiya, K. Ohtani, S. F. Chichibu, S. Fuke, Y. Segawa, H. Ohno, H. Koinuma and M. Kawasaki, Nat. Mater., 2005, 4, 42 CrossRef CAS.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128 CrossRef CAS.
- R. Djalali, Y.-F. Chen and H. Matsui, J. Am. Chem. Soc., 2003, 125, 5873 CrossRef CAS.
- E. D. Sone and S. I. Stupp, J. Am. Chem. Soc., 2004, 126, 12756 CrossRef CAS.
- C. L. Chen, P. J. Zhang and N. L. Rosi, J. Am. Chem. Soc., 2008, 130, 13555 CrossRef CAS.
- S. Shekhar, L. Anjia, H. Matsui and S. I. Khondaker, Nanotechnology, 2011, 22, 095202 CrossRef.
- M. Sarikaya, C. Tamerler, A. K. Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577 CrossRef CAS.
- M. Umetsu, M. Mizuta, K. Tsumoto, S. Ohara, S. Takami, H. Watanabe, I. Kumagai and T. Adschiri, Adv. Mater., 2005, 17, 2571 CrossRef CAS.
- C. K. Thai, H. X. Dai, M. S. R. Sastry, M. Sarikaya, D. T. Schwartz and F. Baneyx, Biotechnol. Bioeng., 2004, 87, 129 CrossRef CAS.
- S. Brown, M. Sarikaya and E. Johnson, J. Mol. Biol., 2000, 299, 725 CrossRef CAS.
- Y. Huang, C. Y. Chiang, S. K. Lee, Y. Gao, E. L. Hu, J. De Yoreo and A. M. Belcher, Nano Lett., 2005, 5, 1429 CrossRef CAS.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885 CrossRef CAS.
- Obtained using the peptide property calculator at Innovagen, http://www.innovagen.se.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20333b |
| This journal is © The Royal Society of Chemistry 2012 |
