Highly-dispersed ultrafine Pt nanoparticles on graphene as effective hydrogenation catalysts†
Baoqiang
Sheng
a,
Lei
Hu
a,
Tingting
Yu
a,
Xueqin
Cao
*ab and
Hongwei
Gu
*a
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, China 215123. E-mail: hongwei@suda.edu.cn; xqcao@suda.edu.cn
bNational Engineering laboratory for Modern silk, Soochow University, Suzhou, Jiangsu Province 215123, P.R.China
First published on 25th April 2012
Abstract
Highly-dispersed ultrafine platinum (Pt) nanoparticles supported on graphene sheets were successfully prepared. Various unsaturated compounds were reduced in excellent yields under mild conditions. Higher pressure (4 atm) accelerated the reaction rate, and complete hydrogenation products were obtained in ten minutes.
Metal-catalyzed hydrogenation of organic compounds that contain carbon-carbon multiple bonds or aromatic nitro groups is of fundamental interest in chemical laboratories and industries.1,2 Widespread methods for catalytic hydrogenation of these unsaturated compounds involve heterogeneous catalysts including palladium,3–5 ruthenium,6–8 rhodium9–11 and nickel12–14 and use hydrogen as the reductant. Platinum nanoparticles (Pt NPs) have been proven to be highly active catalysts for hydrogenation,15 however, the nanoparticle catalyst often agglomerates in the reaction system, which leads to low catalytic activity after extended use. To overcome this disadvantage, inorganic16,17 and organic materials18,19 are employed as carriers to support the metal catalysts.
Nanostructure carbon materials such as carbon nanotubes (CNTs) and carbon nanofibers (CNFs) have been considered as promising metal-nanoparticle support materials because of their interesting electronic properties and micro- and macro-structural characteristics.20 Graphene nanosheets, structurally single-atom-thick two-dimensional layers of sp2-bonded carbon atoms, are also promising candidates for the development of high-performance nano-catalysts.21–23 Ravishankar's group24 has used Pt NPs supported on graphene nanosheets as electrocatalysts and has observed superior catalytic activity toward methanol electro-oxidation. Nakamura and Honma20 reported that Pt nanoparticles smaller than 0.5 nm in size on graphene nanosheets show unusually high activity for methanol electro-oxidation compared to Pt/carbon black catalyst. Wu and Li25 investigated the synergetic effect of the Pt/graphene composite using DFT calculations and found that when Pt particles were supported on graphene, electronic interactions between graphene and the Pt atoms led to electron transfer from graphene to Pt. The Ma and Bao group26 observed high catalytic activity using reduced graphene oxide to catalyze nitrobenzene reduction at room temperature with hydrazine hydrate as the hydrogen source. Very recently, Zhang and Yang groups27 synthesized Fe3O4-Pt/rGO nanocomposites and used them as the catalysts for benzyl alcohol oxidation in solution with a higher catalytic activity than Pt/CNT. Each of these results demonstrated that graphene not only acted as the supporter and stabilizer, but also played a direct role in regulating the Pt NPs electronic structure during reaction, which enhanced their electrocatalytic activity. Inspired by these results, we believe that graphene-supported Pt NPs will also display high activity for organic reactions under mild reaction conditions.
Herein, we adopt a simple route for obtaining a high dispersion of ultrafine Pt nanoparticles on graphene (G–Pt).24 The G–Pt composites were first used as catalysts in the hydrogenation of a series of unsaturated compounds using 1 bar initial pressure of hydrogen under mild conditions. The G–Pt catalysts showed outstanding catalytic activity.
G–Pt catalysts were prepared by exploiting the nucleation of a metal precursor phase on GO surfaces, and the synthetic route is shown in Fig. 1A. The actual loading of Pt in this nanocomposite was 39.7 wt%, as determined by ICP. TEM images of G–Pt composites are shown in Fig. 1B. The Pt NPs are highly dispersed on the graphene sheet with average diameter 2–3 nm (Fig. 1B, inset). The visible lattice fringes in the high-resolution TEM image (Fig. 1B, inset) correspond to a spacing of 0.228 nm and agree with the accepted d-spacing of the Pt (111) plane.28 The X-ray powder diffraction (XRD) patterns of the GO and G–Pt are shown in Fig. 1C. The strong diffraction peak of GO near 12° is attributed to the introduction of oxygenated functional groups (carboxyl, carbonyl, hydroxyl, etc.) attached to the edges and sides of the graphene sheets.29 It has been suggested that these surface functional groups will subsequently act as anchors/nucleating centers for the controlled growth of nanoparticles.30 The peak at 26.5° in the GO sample indicates the existence of stacking structures.31 The diffraction peak at around 43° is associated with the (100) plane of the hexagonal carbon structure.32 From the G–Pt XRD pattern (Fig. 1C), no obvious GO diffraction peaks were observed because of nearly complete exfoliation of the GO sheets and their reduction during the composite formation.24 The strong diffraction peaks of Pt (111), Pt (200), and Pt (220) confirmed the formation of a fcc structure of Pt nanoparticles, and Pt (111) was the predominant plane.33 The average particle size was also calculated from the XRD result of G–Pt using the Scherrer equation. This gave an estimate for the Pt nanoparticle size of 2.2 nm, which is in good agreement with the TEM results. Comparing the EDS spectra of GO (Fig. S1†) and G–Pt catalyst (Fig. 1D), Pt was only observed in the catalyst, and the metal loading was determined to be 40.1 wt%, which agrees well with the ICP result.
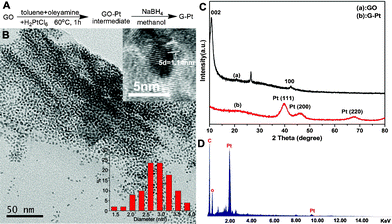 | ||
| Fig. 1 (A) Reaction scheme for the formation of G–Pt; (B) TEM image of the G–Pt (inset, above: high resolution TEM image; below: G–Pt particle diameter distribution); (C) XRD pattern for GO and G–Pt; (D) EDS analysis done on the hybrid G–Pt. | ||
The G–Pt nanomaterial was also characterized by X-ray photoelectron spectroscopy (XPS) (Fig. S2†), and the C 1s and Pt 4f core-level XPS spectra are shown in Fig. 2. Upon reduction of the GO and Pt using NaBH4, the –C–C– (sp2) component corresponding to graphene was largely restored and the binding energy of the –C–OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH groups decreased to lower values when compared with GO (Fig. S3, Table S1†). The XPS core level spectrum for the Pt 4f region shows values of 71.5 and 74.8 eV, respectively. These peak binding energies are consistent with Pt0 (4f7/2 BE 71.0 eV, 4f7/2 BE 72.6 eV). In addition, two different types of Pt2+ can be assigned, as indicated in Fig. 2, which suggested oxygen linkages between the Pt nanoparticles and the reduced GO surface and formation of oxide layers on the Pt surface. These results suggest a mechanistic view of G–Pt composite formation. The small Pt nanoparticles are formed by anchoring the Pt ion on the GO surface and by subsequent reduction to the fine particles, without any significant agglomeration.24
O and –COOH groups decreased to lower values when compared with GO (Fig. S3, Table S1†). The XPS core level spectrum for the Pt 4f region shows values of 71.5 and 74.8 eV, respectively. These peak binding energies are consistent with Pt0 (4f7/2 BE 71.0 eV, 4f7/2 BE 72.6 eV). In addition, two different types of Pt2+ can be assigned, as indicated in Fig. 2, which suggested oxygen linkages between the Pt nanoparticles and the reduced GO surface and formation of oxide layers on the Pt surface. These results suggest a mechanistic view of G–Pt composite formation. The small Pt nanoparticles are formed by anchoring the Pt ion on the GO surface and by subsequent reduction to the fine particles, without any significant agglomeration.24
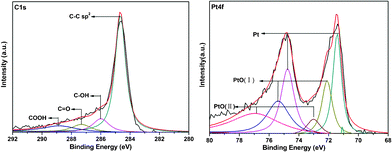 | ||
| Fig. 2 C 1s and Pt 4f core level XPS spectra of G–Pt. | ||
The catalytic activity of the G–Pt was first investigated with 1-decyne as the reactant under 1 bar hydrogen in different solvents. In CH3OH, the G–Pt catalyst shows outstanding catalytic activity with a high yield of 100% in 30 min (Table S2,† entry 1), and in THF, toluene, and 1,4-dioxane, (Table S2,† entries 2–4), it also shows good yields (> 99) in 1 h. When CHCl3, DMSO, and DMF were used as solvents, the partial hydrogenation product 1-decene was obtained with a conversion of 99% in 1 h (Table S2,† entry 5–7). A purely green process (water as the solvent) for 1-decyne hydrogenation was also tested, and the substrate was converted fully in 1 h, with 53.7% decane yield (Table S2,† entry 8). Higher decane yield can be obtained by prolonging the reaction time.
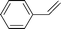
With the optimized conditions in hand, the hydrogenation of a range of unsaturated hydrocarbons was investigated in CH3OH under 1 bar initial hydrogen pressure using G–Pt as the catalyst. G–Pt shows excellent catalytic activity in the reduction of substrates with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds to the corresponding C–C saturated compounds with excellent yields (> 99%) (Table 1, entry 1–5). Functionalized anilines are important intermediates in the preparation of polymers, urethanes, dyes, pharmaceuticals, and other industrially-important chemical products.34 A simple method for the production of aromatic amines is catalytic hydrogenation of nitroarenes over heterogeneous catalysts. Our group has reported that nitroarenes can be quickly converted to the corresponding anilines using Pt nanowires as the catalyst.35 Inspired by the excellent catalytic activity of G–Pt for C
C bonds to the corresponding C–C saturated compounds with excellent yields (> 99%) (Table 1, entry 1–5). Functionalized anilines are important intermediates in the preparation of polymers, urethanes, dyes, pharmaceuticals, and other industrially-important chemical products.34 A simple method for the production of aromatic amines is catalytic hydrogenation of nitroarenes over heterogeneous catalysts. Our group has reported that nitroarenes can be quickly converted to the corresponding anilines using Pt nanowires as the catalyst.35 Inspired by the excellent catalytic activity of G–Pt for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
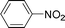
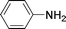
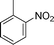
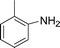
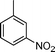
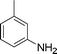
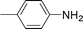
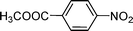
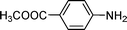
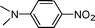
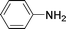
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C hydrogenation, we believed that G–Pt would also show high catalytic activity in nitroarenes reduction. The catalytic reduction of various nitroarenes (Table 1, entries 6–15) were investigated using G–Pt catalyst in CH3OH under 1 bar initial hydrogen pressure. The reduction of o-, m-, and p-methyl nitrobenzenes (Table 1, entries 7–9) showed good regioselectivity, and the corresponding aniline yields were higher than 99%. The presence of electron-donating or electron-withdrawing groups (Table 1, entries 10 to 14) did not influence nitroarene reduction significantly because of the good chemo-selectivity. All of these reactions were completed within 1 h which are faster than that of our previously reported Pt-nanowire catalyst.35 Hydrogenation of m-dinitrobenzene to the corresponding dianiline (Table 1, entry 15) also gave excellent yields, with 99% completion in 4 h under 1 bar initial hydrogen pressure.
C hydrogenation, we believed that G–Pt would also show high catalytic activity in nitroarenes reduction. The catalytic reduction of various nitroarenes (Table 1, entries 6–15) were investigated using G–Pt catalyst in CH3OH under 1 bar initial hydrogen pressure. The reduction of o-, m-, and p-methyl nitrobenzenes (Table 1, entries 7–9) showed good regioselectivity, and the corresponding aniline yields were higher than 99%. The presence of electron-donating or electron-withdrawing groups (Table 1, entries 10 to 14) did not influence nitroarene reduction significantly because of the good chemo-selectivity. All of these reactions were completed within 1 h which are faster than that of our previously reported Pt-nanowire catalyst.35 Hydrogenation of m-dinitrobenzene to the corresponding dianiline (Table 1, entry 15) also gave excellent yields, with 99% completion in 4 h under 1 bar initial hydrogen pressure.
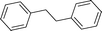
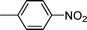
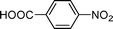
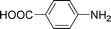
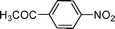
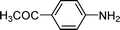
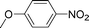
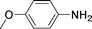
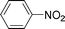
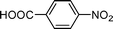
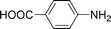
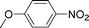
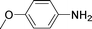
High pressure will accelerate the reaction rate, and we chose five substrates to investigate the catalytic activity of the G–Pt catalyst (Table 2) under increased pressure. 1-Decyne, 1-(cyclohex-3-enyl)benzene, nitrobenzene, and 4-nitrobenzoic acid were reduced to the corresponding reduction products in 10 min under 4 bar initial hydrogen pressure. A substrate with electron-donating character (1-methoxy-4-nitrobenzene, Table 2, entry 5) could also be reduced, but only 46.1% of the corresponding amine was obtained in the same reaction time, demonstrating that electron-donating groups will decrease the reaction activity of aromatic nitro groups. Similarly, the 5 min results (Table 2) demonstrate that the catalytic activity of nitrobenzene hydrogenation was decreased by the presence of electron-donating groups, and increased by electron-withdrawing groups, a trait which was also investigated by Keane using Au and Ag catalysts.36
| Entry | Substrate | Product | Time (min) | Yield (%)b | TOF (min−1) |
|---|---|---|---|---|---|
| a Reaction conditions: 1.5 mg G–Pt, 1 mmol substrate, 4 mL CH3OH at 60 °C under 4 bar initial hydrogen pressure. b GC yield. | |||||
| 1 |

|

|
5 | 17.3 | 11.3 |
| 10 | > 99 | 32.7 | |||
| 2 |
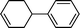
|
|
5 | 23.7 | 15.5 |
| 10 | > 99 | 32.7 | |||
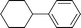
| 3 |
|
|
5 | 35.0 | 22.9 |
| 10 | > 99 | 32.7 | |||
| 4 |
|
|
5 | 49.5 | 32.4 |
| 10 | > 99 | 32.7 | |||
| 5 |
|
|
5 | 12.6 | 8.3 |
| 10 | 46.1 | 15.1 | |||
In conclusion, we applied a simple method to prepare highly-dispersed ultrafine Pt nanoparticles supported by graphene. We used these nanocomposites as catalysts for the reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) =C to the corresponding alkanes in excellent yields. The G–Pt catalyst was also used for nitroarene hydrogenation to the corresponding anilines, and showed outstanding catalytic activity for nitroarenes bearing one or two nitro groups. Nanosheet/metal-nanoparticle hybrids could be promising architectures for other catalytic reactions as high-activity heterogeneous catalysts.
=C to the corresponding alkanes in excellent yields. The G–Pt catalyst was also used for nitroarene hydrogenation to the corresponding anilines, and showed outstanding catalytic activity for nitroarenes bearing one or two nitro groups. Nanosheet/metal-nanoparticle hybrids could be promising architectures for other catalytic reactions as high-activity heterogeneous catalysts.
H. W. G. acknowledges financial support from the National Natural Science Foundation of China (no. 21003092), the Key Project of Chinese Ministry of Education (no. 211064), the Priority Academic Program Development of Jiangsu Higher Education Institutions; X. Q. C. gratefully thanks the financial support from the National Engineering Laboratory for Modern Silk, Soochow University, China.
References
- J. L. Serrano, S. B. Duckett, S. Aiken, K. Q. A. Lenero, E. Drent, J. P. Dunne, D. Konya and A. C. Whitwood, J. Am. Chem. Soc., 2007, 129, 6513 CrossRef.
- S. D. Kushch, N. S. Kujunko and B. P. Tarasov, Russ. J. Gen. Chem., 2009, 79, 706–710 CrossRef CAS.
- J. Huang, T. Jiang, H. X. Gao, B. X. Han, Z. M. Liu, W. Z. Wu, Y. H. Chang and G. Y. Zhao, Angew. Chem., Int. Ed., 2004, 43, 1397–1399 CrossRef CAS.
- Y. H. Niu, L. K. Yeung and R. M. Crooks, J. Am. Chem. Soc., 2001, 123, 6840–6846 CrossRef CAS.
- J. Huang, T. Jiang, B. X. Han, H. X. Gao, Y. H. Chang, G. Y. Zhao and W. Z. Wu, Chem. Commun., 2003, 1654–1655 RSC.
- R. Kuwano and M. Kashiwabara, Org. Lett., 2006, 8, 2653–2655 CrossRef CAS.
- P. J. Dyson, D. J. Ellis, D. G. Parkerc and T. Weltonb, Chem. Commun., 1999, 25–26 RSC.
- P. Kluson and L. Cerveny, Appl. Catal., A, 1995, 128, 13–3l CrossRef CAS.
- Y. Kuroki, Y. Sakamaki and K. Iseki, Org. Lett., 2001, 3, 457–459 CrossRef CAS.
- M. V. D. Berg, A. J. Minnaard, E. P. Schudde, J. V. Esch, A. H. M. D. Vries, J. G. D. Vries and B. L. Feringa, J. Am. Chem. Soc., 2000, 122, 11539–11540 CrossRef.
- D. Pena, A. J. Minnaard, J. G. D. Vries and B. L. Feringa, J. Am. Chem. Soc., 2002, 124, 14552–14553 CrossRef CAS.
- F. Alonso, P. Riente and M. Yus, Tetrahedron, 2009, 65, 10637–10643 CrossRef CAS.
- J. H. Wang, Z. L. Yuan, R. F. Nie, Z. Y. Hou and X. M. Zheng, Ind. Eng. Chem. Res., 2010, 49, 4664–4669 CrossRef CAS.
- H. Adkins and H. I. Cramer, J. Am. Chem. Soc., 1930, 52, 4349–4358 CrossRef CAS.
- E. Schmidt, A. Vargas, T. Mallat and A. Baiker, J. Am. Chem. Soc., 2009, 131, 12358–12367 CrossRef CAS.
- K. Nishijima, T. Fukahori, N. Murakami, T. A. Kamai, T. Tsubota and T. Ohno, Appl. Catal., A, 2008, 337, 105–109 CrossRef CAS.
- S. Chytil, W. R. Glomm, E. Vollebekk, H. Bergem, J. Walmsley, J. Sjoblom and E. A. Blekkan, Microporous Mesoporous Mater., 2005, 86, 198–206 CrossRef CAS.
- R. M. Crooks, M. Q. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS.
- L. W. Beakley, S. E. Yost, R. Cheng and B. D. Chandler, Appl. Catal., A, 2005, 292, 124–129 CrossRef CAS.
- E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura and I. Honma, Nano Lett., 2009, 9, 2255–2259 CrossRef CAS.
- X. Huang, X. Y. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- X. Huang, Z. Y. Yin, S. X. Wu, X. Y. Qi, Q. Y. He, Q. C. Zhang, Q. Y. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876–1902 CrossRef CAS.
- H. J. Jiang, Small, 2011, 7, 2413–2427 CAS.
- C. Nethravathi, E. A. Anumol, M. Rajamathi and N. Ravishankar, Nanoscale, 2011, 3, 569–571 RSC.
- W. Qin and X. Li, J. Phys. Chem. C, 2010, 114, 19009–19015 CAS.
- Y. J. Gao, D. Ma, C. L. Wang, J. Guan and X. H. Bao, Chem. Commun., 2011, 47, 2432–2434 RSC.
- S. Wu, Q. He, C. Zhou, X. Qi, X. Huang, Z. Yin, Y. Yang and H. Zhang, Nanoscale, 2012, 4, 2478–2483 RSC.
- Y. M. Li, L. H. Tang and J. H. Li, Electrochem. Commun., 2009, 11, 846–849 CrossRef CAS.
- H. K. Jeong, Y. P. Lee, R. J. W. E. Lahaye, M. H. Park, K. H. An, I. J. Kim, C. W. Yang, C. Y. Park, R. S. Ruoff and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 1362–1366 CrossRef CAS.
- C. C. Chien and K. T. Jeng, Mater. Chem. Phys., 2006, 99, 80–87 CrossRef CAS.
- J. Bian, M. Xiao, S. J. Wang, Y. X. Lu and Y. Z. Meng, Catal. Commun., 2009, 10, 1529–1533 CrossRef CAS.
- J. J. Niu and J. N. Wang, Electrochim. Acta, 2008, 53, 8058–8063 CrossRef CAS.
- M. Carmo, V.A. Paganina, J. M. Rosolen and E. R. Gonzalez, J. Power Sources, 2005, 142, 169–176 CrossRef CAS.
- V. Pandarus, R. Ciriminna, F. Beland and M. Pagliaro, Adv. Synth. Catal., 2011, 353, 1306–1316 CrossRef CAS.
- M. Li, L. Hu, X. Q. Cao, H. Y. Hong, J. M. Lu and H. W. Gu, Chem.–Eur. J., 2011, 17, 2763–2768 CrossRef CAS.
- F. C. Lizana, Z. M. D. Pedro, S. G. Quero and M. A. Keane, J. Mol. Catal. A: Chem., 2010, 326, 48–54 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures and results, XPS results of GO and G–Pt. See DOI: 10.1039/c2ra20400b/ |
| This journal is © The Royal Society of Chemistry 2012 |