Well-controlled synthesis of boronic-acid functionalised poly(lactide)s: a versatile platform for biocompatible polymer conjugates and sensors†
Alison J.
Cross
,
Matthew G.
Davidson
*,
Daniel
García-Vivó
and
Tony D.
James
*
Centre for Sustainable Chemical Technologies, Department of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: m.g.davidson@bath.ac.uk; t.d.james@bath.ac.uk
First published on 4th May 2012
Abstract
We report the controlled synthesis of boronic-acid terminated polylactides via the use of a protected boronic acid-functionalised ring-opening polymerisation initiator and the subsequent deprotection of the resultant polymer. We have demonstrated the utility of this approach to biocompatible polymer conjugates of biologically-relevant diols using an indicator-displacement assay.
Boronic acids interact strongly and selectively as receptors (sensors) for a wide variety of species including carbohydrates, Lewis bases and anions1 and they are also versatile synthetic intermediates for mild and selective elaboration via cross-coupling reactions.2 Given the well-established utility of boronic acids in both supramolecular and synthetic chemistry, we reasoned that a straightforward and general route to boronic-acid terminated poly(lactide) (PLA) would provide access to new functional and biocompatible materials with a host of potential sensing and drug delivery applications.
Although boronic acid groups have previously been covalently attached to different polymeric materials with interesting results (i.e., formation of star-polymer assemblies),3,4 to our knowledge, these type of materials have never been exploited for the conjugation of biologically relevant molecules which could lead to quantitative polymer-drug conjugates. With this aim in mind, herein we report a route (Scheme 1) to simple boronic-acid terminated PLA materials, 4 (BA–PLA), and an initial demonstration of the conjugation of biologically relevant molecules, as determined using an Alizarin Red S-based indicator-displacement assay.5,6
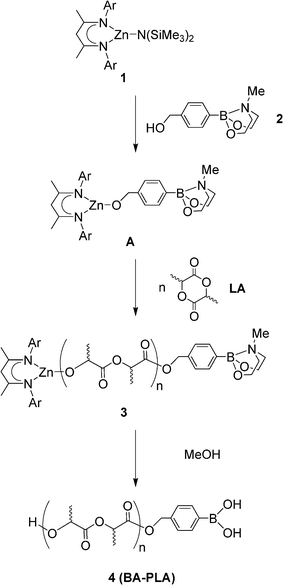 | ||
| Scheme 1 Synthesis of boronic acid terminated PLA's (Ar = 2,6-diisopropylphenyl). | ||
PLA is an aliphatic polyester that is of interest both as a renewable commodity polymer and for use in biomedical applications.7 The well-controlled synthesis of PLA is typically achieved by the ring-opening polymerisation (ROP) of lactide (LA) using well-defined metal alkoxide initiators (LnM–OR), which yield polymers with the OR group attached to the polymer terminus as an ester functionality. Therefore, using a protected boronic acid as the OR group of the initiator, would result in the incorporation of a boronic acid functionality. A similar strategy has been recently used to incorporate a range of other functional molecules; i.e., difluoroboron-dyes,8 nanoparticles,9 metal complexes10 and bioactive molecules.11
For preparation of the boronic acid-containing PLA we chose to employ the pre-initiator developed by Coates et al. based on the Zn amido complex [(BDI)Zn{N(SiMe3)2}] (1) [(BDI) = 2-((2,6-diisopropylphenyl)amido)-4-((2,6-diisopropylphenyl)-imino)-2-pentene].12 The N(SiMe3)2 group of this complex can be easily replaced by an alkoxide OR group upon reaction with an alcohol and the Zn alkoxide complex thus generated can act as initiator for the ring-opening polymerisation of LA (Scheme 1). The utility of these Zn complexes for functionalisation of PLA with bioactive molecules has been recently and elegantly demonstrated by Tong and Cheng.11a For our initial study, we chose the simplest possible and commercially available unit, 4-(hydroxymethyl)phenylboronic acid. However, in order to overcome its low solubility in the typical organic solvents required to carry out the polymerisation reaction, and also to avoid a possible side reaction between 1 and the boronic OH groups, we used its MIDA (N-methyldiethanolamine) protected derivative (2, Scheme 1),13 which is conveniently susceptible to concurrent deprotection with MeOH under the conditions commonly employed to quench PLA prepared with a metal alkoxide initiator.
In a typical polymer preparation (Scheme 1), addition of one equivalent of MIDA-protected phenylboronic acid to CH2Cl2 solutions of complex 1 generates in situ the Zn alkoxide complex A. Addition of rac-lactide (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer to initiator ratio, 25 °C and 15 min) affords polymer 3, which upon deprotection/quenching with MeOH and precipitation with hexane yields directly the desired BA-PLA, 4, as a colourless solid (see ESI† for further details). High conversions (typically >95% after 15 min) and well-controlled polymerisations [Mn(GPC) = 6107, Mn(theoretical) = 6983, Mw/Mn = 1.06]14 are indicative of rapid and quantitative formation of the zinc alkoxide initiator A and good control over the subsequent ring-opening polymerisation.12 Key proton resonances associated with the aromatic ring of the boronic acid end group were found in the 1H NMR of the isolated polymers [δ = 7.48, 7.12 (d, JHH = 8 Hz) ppm], further indicating that the strategy was successful.14
1 monomer to initiator ratio, 25 °C and 15 min) affords polymer 3, which upon deprotection/quenching with MeOH and precipitation with hexane yields directly the desired BA-PLA, 4, as a colourless solid (see ESI† for further details). High conversions (typically >95% after 15 min) and well-controlled polymerisations [Mn(GPC) = 6107, Mn(theoretical) = 6983, Mw/Mn = 1.06]14 are indicative of rapid and quantitative formation of the zinc alkoxide initiator A and good control over the subsequent ring-opening polymerisation.12 Key proton resonances associated with the aromatic ring of the boronic acid end group were found in the 1H NMR of the isolated polymers [δ = 7.48, 7.12 (d, JHH = 8 Hz) ppm], further indicating that the strategy was successful.14
Once the new polymeric materials had been prepared and characterised, we turned our attention to their potential for conjugation of biologically relevant species. We used an Alizarin Red S (ARS) indicator-displacement assay to monitor binding of hydroxyl-containing species taking advantage of the enhanced fluorescent properties of the boronic acid-complexed ARS, as well as the reversible nature of its binding with boronic acids (Scheme 2).1,5,6
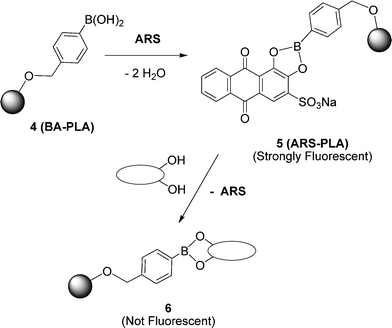 | ||
| Scheme 2 Schematic representation of the indicator-displacement strategy used. | ||
Coupling was readily accomplished by stirring CH2Cl2 solutions of polymer 4 with an excess of ARS overnight. After this time, careful filtration to remove any uncoupled ARS (insoluble in CH2Cl2), followed by reprecipitation from CH2Cl2 with MeOH yields ARS-conjugated polymer 5 as a fluorescent orange solid. Incorporation of the ARS is indicated by an orange coloration of the polymer and confirmed by signals due to ARS in 1H NMR spectra. Analysis of 5 by GPC confirms that the coupling process does not degrade the polymer backbone since values for Mn and PDI are similar to those of the parent polymer 4. To further confirm specific and well-defined covalent binding of ARS to the B(OH)2, end group of the polymer, unfunctionalised (OiPr-ended) polymers were treated with ARS and purified in a similar fashion to that described above, resulting in an uncoloured, non-fluorescent material. The fluorescence spectra of the ARS-coupled boronic acid polymers (strong fluorescence) and the OiPr-ended ‘blanks’ (no fluorescence) are shown in Fig. 1.
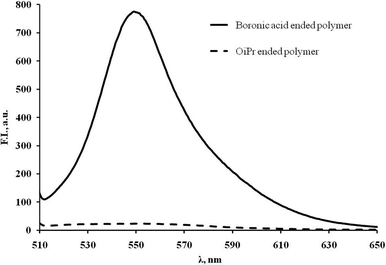 | ||
| Fig. 1 Fluorescence spectra of ARS-treated polymers in THF after MeOH precipitation: boronic acid-terminate (solid line) and OiPr-terminate (dashed line) (recorded from a solution of 20 mg of ARS-coupled materials in 2 mL of THF, λex = 495 nm). | ||
The results of the titration of the fluorescent polymer with various hydroxyl-containing species are given in the Table 1 (and described in further detail in the ESI†).15 Simple alcohols are not able to efficiently displace the ARS from the boronic acid, and therefore the decrease of the fluorescent intensity is modest. On the other hand, molecules containing 1,2-diol or α-hydroxyacid functionalities, which can be seen as representative models for a wide range of bioactive compounds, are much more efficient in the displacement of the ARS, accounting for large decreases in the fluorescence intensity. In particular, we observed significant decreases in the fluorescence intensity on addition of catechol (Fig. 2), L-DOPA16 and lactic acid, which can be ascribed to the formation of strong complexes between these species and the functionalised PLA.
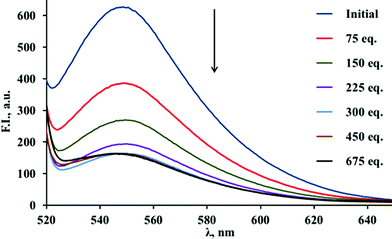 | ||
| Fig. 2 Fluorescence spectra of ARS–PLA in THF after the addition of catechol (recorded from successive additions of catechol to a solution of 10 mg of ARS–PLA in 2 mL of THF, λex = 495 nm, scan rate 60 nm min−1). | ||
| 150 eq. | 300 eq. | |
|---|---|---|
| Ethanol | 8 | 13 |
| Phenol | 10 | 15 |
| Pinacol | 33 | 36 |
| Catechol | 57 | 73 |
| L-DOPA | 31 | 68 |
| D/L-Lactic Acid | 51 | — |
In conclusion, boronic-acid terminated PLA (BA–PLA) was prepared in a well-controlled fashion using a well-defined boronic acid-functionalised ring-opening polymerisation initiator. The reversible formation of a well-defined fluorescent ARS–PLA conjugate was used to follow the binding of a range of hydroxyl-containing species to the BA–PLA polymer. Displacement of the ARS and selectivity for diols over simple alcohols was observed. The potential for formation of strong complexes between BA–PLA and hydroxyl-containing therapeutic agents suggests a versatile and highly specific route to quantitative polymer–drug conjugates and nanoconjugates, which have become a key target as drug delivery vehicles.11 We are currently investigating these and other synthetic and materials aspects of boronic acid-terminated PLA.
Acknowledgements
We thank the University of Bath (A.J.C.) and D.G.-V. thanks the European Commission for financial aid under the Marie Curie Intra-European Postdoctoral Fellowship program (PIEF-2009-235173).References
- (a) T. D. James and S. Shinkai, Top. Curr. Chem., 2002, 218, 159–200 CrossRef CAS; (b) W. Wang, X. Gao and B. Wang, Curr. Org. Chem., 2002, 6, 1285–1317 CrossRef CAS; (c) S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963–972 CrossRef CAS; (d) T. D. James, M. D. Phillips and S. Shinkai, in Boronic acids in saccharide recognition, RSC Publishing, Cambridge, UK, 2006 Search PubMed; (e) R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 1106–1123 CAS.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544–4568 CrossRef CAS; (c) V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, ChemSusChem, 2010, 3, 502–522 CrossRef CAS; (d) C. M. Crudden, B. W. Glasspoole and C. J. Lata, Chem. Commun., 2009, 6704–6716 RSC; (e) D. G. Hall, in Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2005 Search PubMed.
- (a) See for example: P. De, S. R. Gondi, D. Roy and B. S. Sumerlin, Macromolecules, 2009, 42, 5614–5621 CrossRef CAS; (b) B. A. Deore, I. Yu, J. Woodmass and M. S. Freund, Macromol. Chem. Phys., 2008, 209, 1094–1105 CrossRef CAS; (c) A. Sezgin, U. Akbey, M. R. Hansen, R. Graf, A. Bozkurt and A. Baykal, Polymer, 2008, 49, 3859–3864 CrossRef CAS; (d) J. N. Cambre, D. Roy, S. R. Gondi and B. S. Sumerlin, J. Am. Chem. Soc., 2007, 129, 10348–10349 CrossRef CAS; (e) T. R. Jackson, J. S. Springall, D. Rogalle, N. Masumoto, H. Ching Li, F. D'Hooge, S. P. Perera, T. A. Jenkins, T. D. James, J. S. Fossey and J. M. H. Van den Elsen, Electrophoresis, 2008, 29, 4185–4191 CrossRef CAS; (f) F. D'Hooge, D. Rogalle, M. J. Thatcher, S. P. Perera, J. M. H. Van den Elsen, T. A. Jenkins, T. D. James and J. S. Fossey, Polymer, 2008, 49, 3362–3365 CrossRef CAS; (g) M. P. Pereira Morais, J. D. Mackay, S. K. Bhamra, J. G. Buchanan, T. D. James, J. S. Fossey and J. M. H. Van den Elsen, Proteomics, 2009, 10, 48–58 CrossRef.
- (a) For examples based on polyester derivatives see: A. L. Korich, A. R. Walker, C. Hincke, C. Stevens and P. M. Iovine, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5767–5774 CrossRef CAS; (b) C. Guillaume, N. Ajellal, J.-F. Carpentier and S. M. Guillaume, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 907–917 CrossRef CAS.
- (a) S. C. McCleskey, P. N. Floriano, S. L. Wiskur, E. V. Anslyn and J. T. McDevitt, Tetrahedron, 2003, 59, 10089–10092 CrossRef CAS; (b) A. Goodey, J. J. Lavigne, S. M. Savoy, M. D. Rodriguez, T. Curey, A. Tsao, G. Simmons, J. Wright, S. J. Yoo, Y. Sohn, E. V. Anslyn, J. B. Shear, D. P. Neikirk and J. T. McDevitt, J. Am. Chem. Soc., 2001, 123, 2559–2570 CrossRef CAS; (c) Y.-S. Sohn, A. Goodey, E. V. Anslyn, J. T. McDevitt, J. B. Shear and D. P. Neikirk, Biosens. Bioelectron., 2005, 21, 303–312 CrossRef CAS; (d) A. P. Umali, E. V. Anslyn, A. T. Wright, C. R. Blieden, C. K. Smith, T. Tian, J. A. Truong, C. E. Crumm, J. E. Garcia, S. Lee, M. Mosier and C. P. Nguyen, J. Chem. Educ., 2010, 87, 832–835 CrossRef CAS; (e) B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118–3127 CrossRef CAS; (f) W. M. J. Ma, M. P. Pereira Morais, F. D'Hooge, J. M. H. Van den Elsen, J. P. L. Cox, T. D. James and J. S. Fossey, Chem. Commun., 2009, 532–534 RSC.
- R. Villamil-Ramos and A. K. Yatsimirsky, Chem. Commun., 2011, 47, 2694–2696 RSC and references therein.
- (a) For recent reviews, see: P. J. Dijkstra, H. Z. Du and J. Feijen, Polym. Chem., 2011, 2, 520–527 RSC; (b) M. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC; (c) J. Wu, T.-L. Yu, C.-T. Chen and C.-C. Lin, Coord. Chem. Rev., 2006, 250, 602–626 CrossRef CAS; (d) M. Vert, Biomacromolecules, 2005, 6, 538–546 CrossRef CAS; (e) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS; (f) A.-C. Albertsson and I. K. Varma, Biomacromolecules, 2003, 4, 1466–1486 CrossRef CAS.
- (a) See for example: G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS; (b) G. Zhang, R. E. Evans, K. A. Campbell and C. L. Fraser, Macromolecules, 2009, 42, 8627–8633 CrossRef CAS; (c) A. Pfister, G. Zhang, J. Zareno, A. F. Horwitz and C. L. Fraser, ACS Nano, 2008, 2, 1252–1258 CrossRef CAS; (d) G. Zhang, J. Chen, S. J. Payne, S. E. Kooi, J. N. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 8942–8943 CrossRef CAS.
- F. Chen, Q. Gao, G. Hong and J. Ni, J. Magn. Magn. Mater., 2008, 320, 1921–1927 CrossRef CAS.
- (a) J. Chen, J. L. Gorczynski, G. Zhang and C. L. Fraser, Macromolecules, 2010, 43, 4909–4920 CrossRef CAS; (b) K. Saatchi and U. O. Haefeli, Dalton Trans., 2007, 4439–4445 RSC.
- (a) R. Tong and J. Cheng, J. Am. Chem. Soc., 2009, 131, 4744–4754 CrossRef CAS; (b) R. Tong and J. Cheng, Bioconjugate Chem., 2010, 21, 111–121 CrossRef CAS; (c) R. Tong and J. Cheng, Angew. Chem., Int. Ed., 2008, 47, 4830–4834 CrossRef CAS; (d) Z. Zhang and S.-S. Feng, Biomaterials, 2006, 27, 262–270 CrossRef CAS.
- B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229–3238 CrossRef CAS.
- E. P. Gillis and M. D. Burke, J. Am. Chem. Soc., 2008, 130, 14084–14085 CrossRef CAS.
- For some of the NMR samples of polymers we observed an additional set of signals in the aromatic region of the 1H NMR spectra which were attributed to the presence of small amounts of the anhydride cyclic trimer (PLA-OCH2PhB)3(μ–O)3. The formation of these anhydrides is also supported by the appearance of a minor second band in GPC curves of these polymers (see ESI†). We must note that similar anhydrides have been observed previously for other boronic acid functionalised polymers (ref. 3a, 4) and for p-tolueneboronic acid: T. M. B. Islam, K. Yoshino, H. Nomura, T. Mizuno and A. Sasane, Anal. Sci., 2002, 18, 363–366 CrossRef CAS.
- Initially, the fluorescence of a sample was monitored as function of time observing a slow decrease in intensity (ca. 10% of decrease after 10 min and 18% after 30 min), caused by hydrolysis of the ARS-PLA due to adventitious water. However, for all subsequent experiments the displacement reaction with other analytes was found to be much faster, rendering such hydrolysis insignificant. All the experiments were carried out at ambient conditions using “wet” THF as solvent. We used ca. 10 mg of the ARS-PLA dissolved in 2 mL of THF, to which we did consecutive additions of the analyte under study recording the fluorescence spectrum after 1 min of the addition (see ESI†).
- For a recent example of fluorescence response to dopamine by polymers with pendant boronic acids see: C. Xue, F. Cai and H. Liu, Chem.–Eur. J., 2008, 14, 1648–1653 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Full experimental details including spectroscopic data. See DOI: 10.1039/c2ra20373a/ |
| This journal is © The Royal Society of Chemistry 2012 |
